Arachidonic Acid Regulation of Intracellular Signaling Pathways and Target Gene Expression in Bovine Ovarian Granulosa Cells
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Cells Culture and Treatment
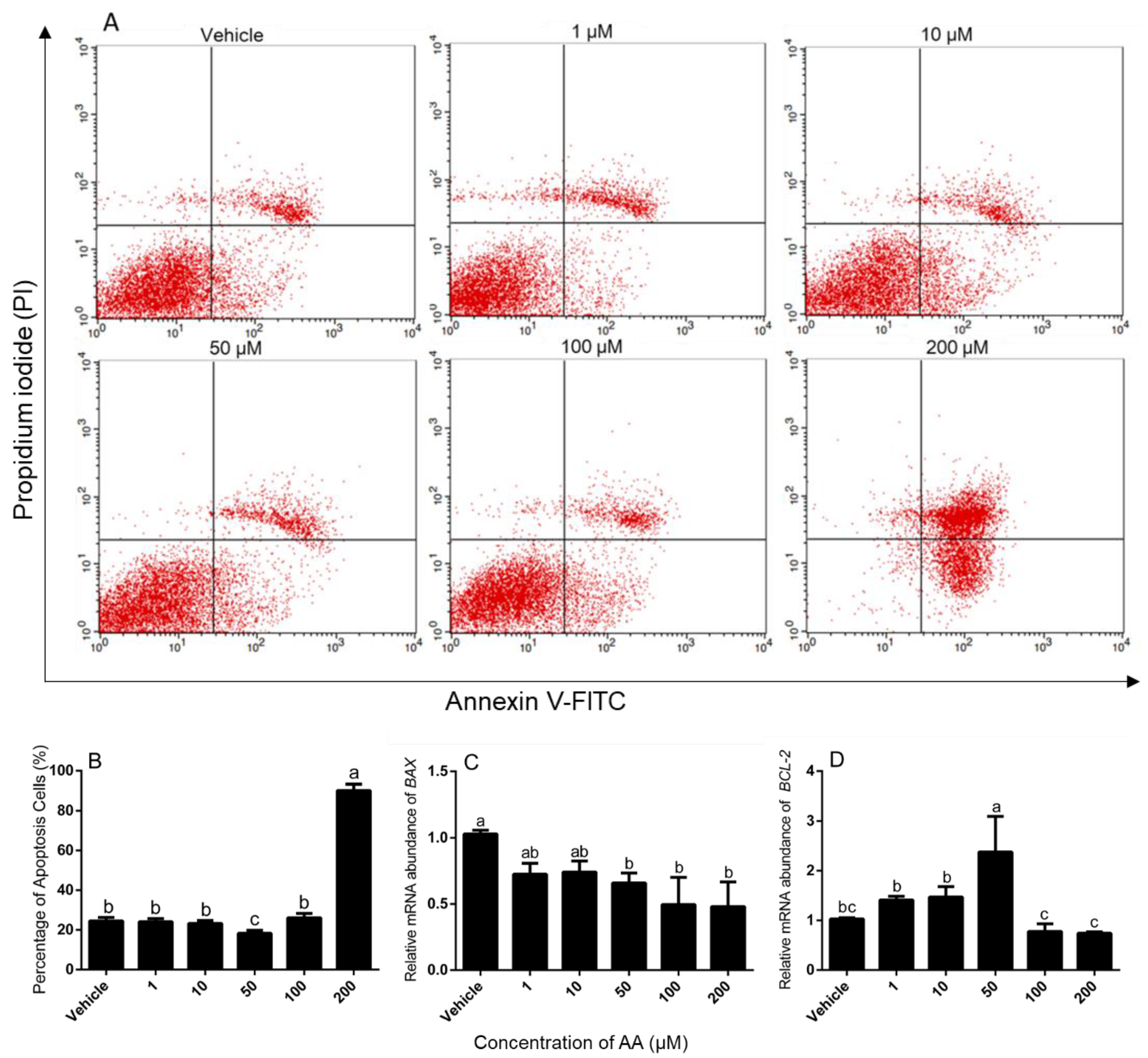
2.3. Assessment of Cell Survival and Apoptosis
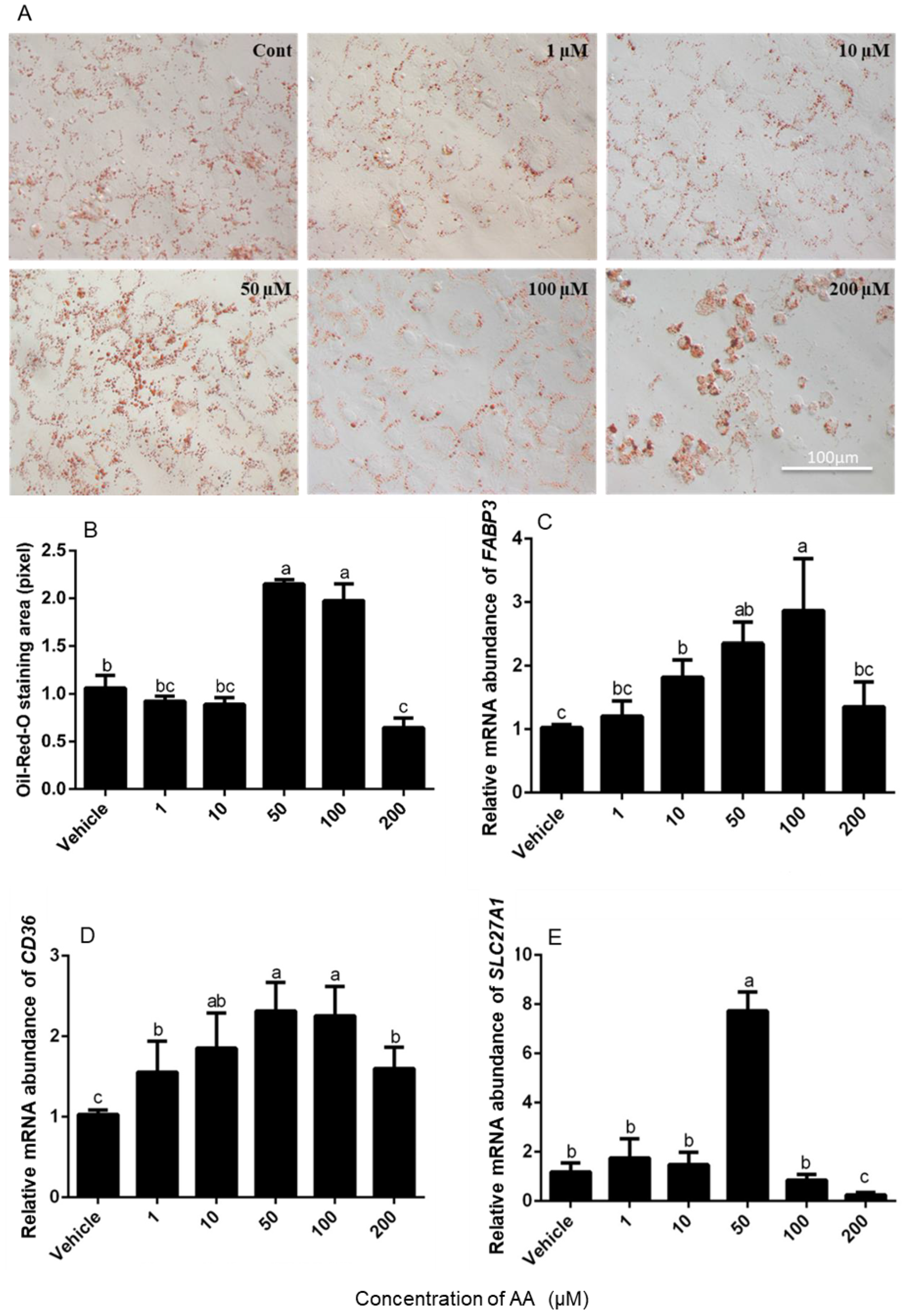
2.4. Oil Red O Staining
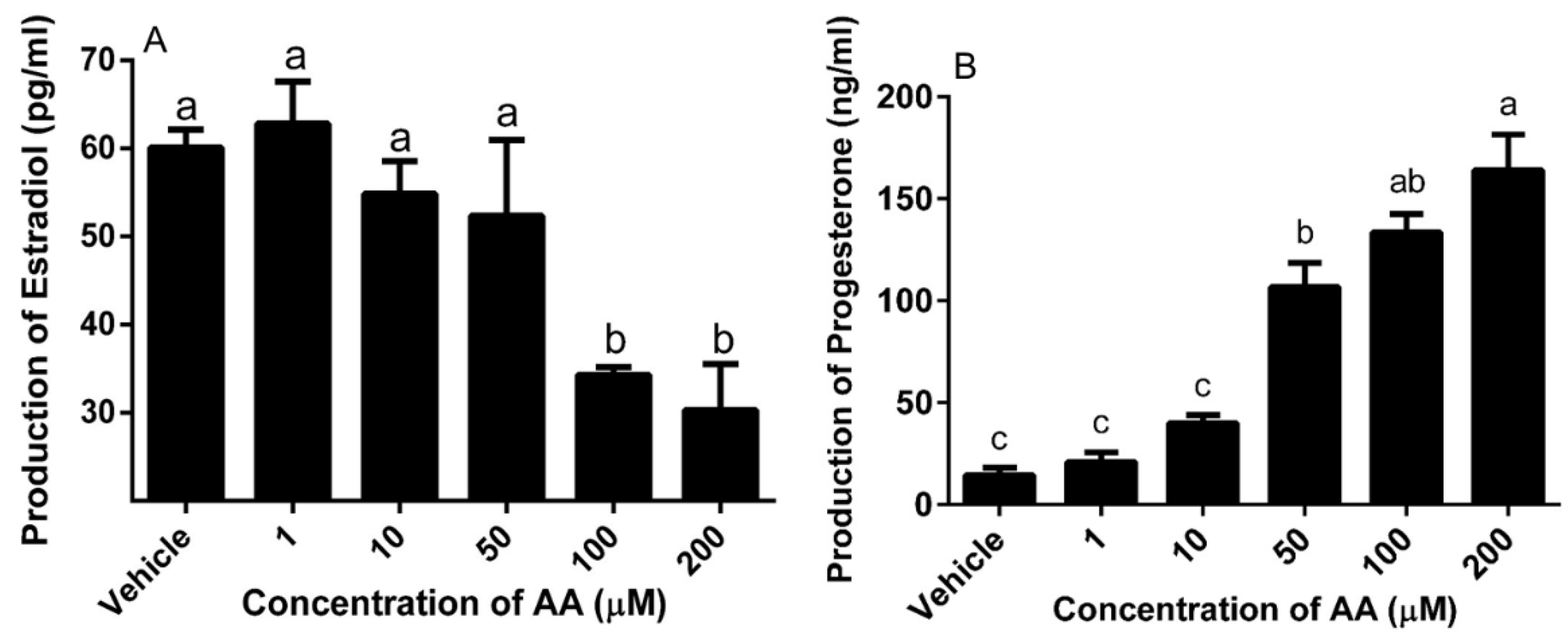
2.5. Steroid Production
2.6. RNA Extraction and Real-Time PCR
2.7. Western Blotting
2.8. Statistical Analysis
3. Results
3.1. Effects of AA on Survival and Apoptosis of Granulosa Cells
3.2. Effects of AA on Fatty Acid Absorption by Granulosa Cells
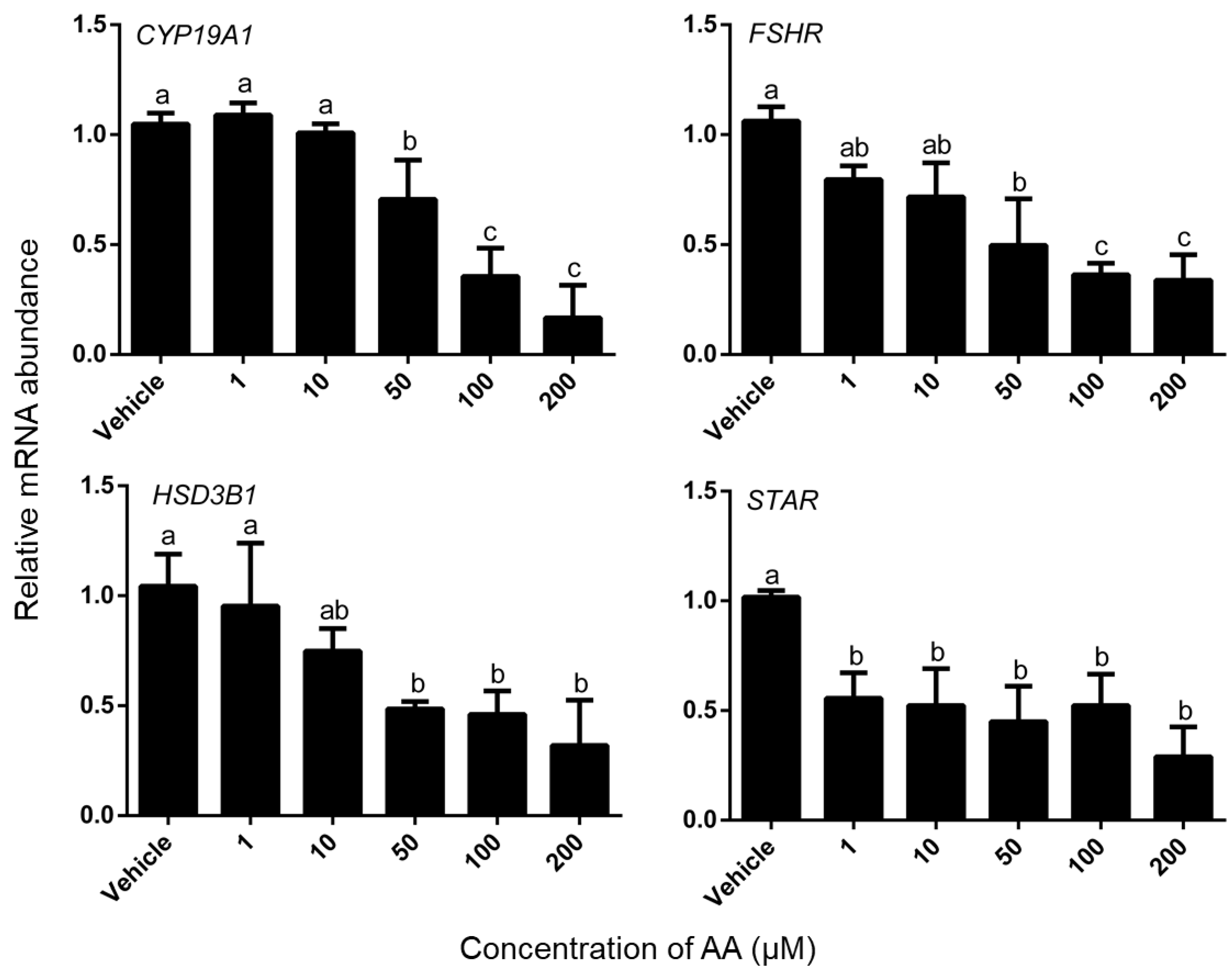
3.3. Effects of AA on Steroidogenesis
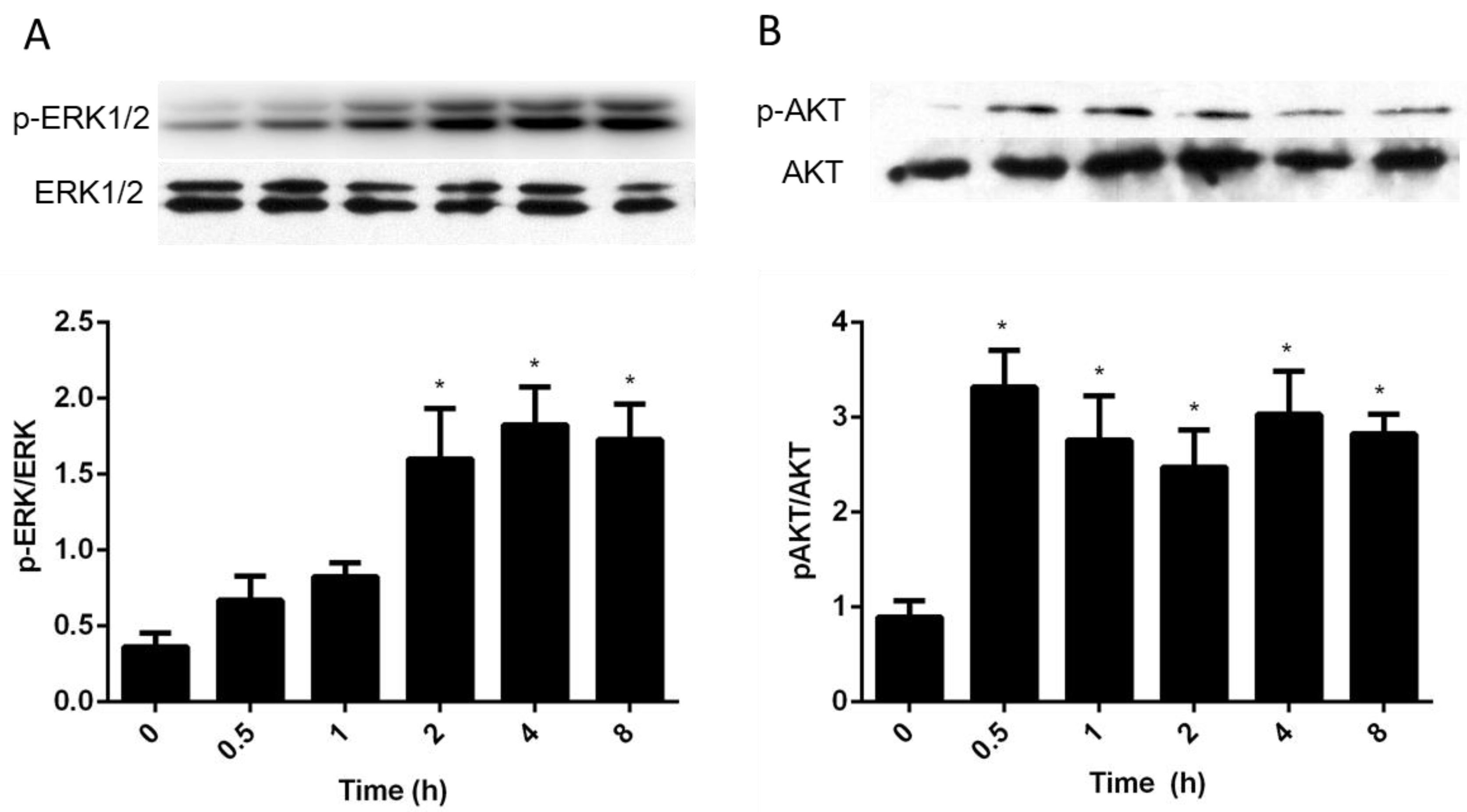
3.4. Intracellular Pathways Activated by AA in Granulosa Cells
4. Discussion
4.1. Effects of AA on Survival and Apoptosis of Granulosa Cells
4.2. Effects of AA on Lipid Droplet Accumulation in Granulosa Cells
4.3. AA Down-Regulated mRNA Expression Level of Steroidogenesis-Related Gene
4.4. Intracellular Signaling Pathways Activated by AA
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Khajeh, M.; Rahbarghazi, R.; Nouri, M.; Darabi, M. Potential role of polyunsaturated fatty acids, with particular regard to the signaling pathways of arachidonic acid and its derivatives in the process of maturation of the oocytes: Contemporary review. Biomed. Pharmacother. 2017, 94, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Sorbera, L.A.; Asturiano, J.F.; Carrillo, M.; Zanuy, S. Effects of polyunsaturated fatty acids and prostaglandins on oocyte maturation in a marine teleost, the European sea bass (Dicentrarchus labrax). Biol. Reprod. 2001, 64, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Marei, W.F.; Wathes, D.C.; Fouladi-Nashta, A.A. The effect of linolenic acid on bovine oocyte maturation and development. Biol. Reprod. 2009, 81, 1064–1072. [Google Scholar] [CrossRef] [PubMed]
- Renaville, B.; Bacciu, N.; Comin, A.; Motta, M.; Poli, I.; Vanini, G.; Prandi, A. Plasma and follicular fluid fatty acid profiles in dairy cows. Reprod. Domest. Anim. 2010, 45, 118–121. [Google Scholar] [CrossRef] [PubMed]
- Shaaker, M.; Rahimipour, A.; Nouri, M.; Khanaki, K.; Darabi, M.; Farzadi, L.; Shahnazi, V.; Mehdizadeh, A. Fatty acid composition of human follicular fluid phospholipids and fertilization rate in assisted reproductive techniques. Iran. Biomed. J. 2012, 16, 162–168. [Google Scholar] [PubMed]
- Prates, E.; Alves, S.; Marques, C.; Baptista, M.; Horta, A.; Bessa, R.; Pereira, R. Fatty acid composition of porcine cumulus oocyte complexes (coc) during maturation: Effect of the lipid modulators trans-10, cis-12 conjugated linoleic acid (t10, c12 cla) and forskolin. In Vitro Cell. Dev. Biol. Anim. 2013, 49, 335–345. [Google Scholar] [CrossRef]
- Lapa, M.; Marques, C.; Alves, S.; Vasques, M.; Baptista, M.; Carvalhais, I.; Silva Pereira, M.; Horta, A.; Bessa, R.; Pereira, R. Effect of trans-10 cis-12 conjugated linoleic acid on bovine oocyte competence and fatty acid composition. Reprod. Domest. Anim. 2011, 46, 904–910. [Google Scholar] [CrossRef] [PubMed]
- Mele, P.G.; Dada, L.A.; Paz, C.; Neuman, I.; Cymeryng, C.B.; Mendez, C.F.; Finkielstein, C.V.; Cornejo Maciel, F.; Podesta, E.J. Involvement of arachidonic acid and the lipoxygenase pathway in mediating luteinizing hormone-induced testosterone synthesis in rat leydig cells. Endocr. Res. 1997, 23, 15–26. [Google Scholar] [CrossRef]
- Johnson, A.L.; Tilly, J.L. Arachidonic acid inhibits luteinizing hormone-stimulated progesterone production in hen granulosa cells. Biol. Reprod. 1990, 42, 458–464. [Google Scholar] [CrossRef]
- McKeegan, P.J.; Sturmey, R.G. The role of fatty acids in oocyte and early embryo development. Reprod. Fertil. Dev. 2011, 24, 59–67. [Google Scholar] [CrossRef]
- Sugimoto, Y.; Inazumi, T.; Tsuchiya, S. Roles of prostaglandin receptors in female reproduction. J. Biochem. 2015, 157, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Wathes, D.C.; Abayasekara, D.R.E.; Aitken, R.J. Polyunsaturated fatty acids in male and female reproduction. Biol. Reprod. 2007, 77, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Hanna, V.S.; Hafez, E.A.A. Synopsis of arachidonic acid metabolism: A review. J. Adv. Res. 2018, 11, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Ciepiela, P.; Baczkowski, T.; Drozd, A.; Kazienko, A.; Stachowska, E.; Kurzawa, R. Arachidonic and linoleic acid derivatives impact oocyte ICSI fertilization—A prospective analysis of follicular fluid and a matched oocyte in a ‘one follicle—One retrieved oocyte—One resulting embryo’ investigational setting. PLoS ONE 2015, 10, e0119087. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Sheldrick, E.L.; Marshall, E.; Wathes, D.C.; Abayasekara, D.R.; Flint, A.P. Control of cyclic amp concentration in bovine endometrial stromal cells by arachidonic acid. Reproduction 2007, 133, 1017–1026. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Hughes-Fulford, M. Prostaglandin e2 and the protein kinase a pathway mediate arachidonic acid induction of c-fos in human prostate cancer cells. Br. J. Cancer 2000, 82, 2000–2006. [Google Scholar] [PubMed]
- Zuccolo, E.; Dragoni, S.; Poletto, V.; Catarsi, P.; Guido, D.; Rappa, A.; Reforgiato, M.; Lodola, F.; Lim, D.; Rosti, V.; et al. Arachidonic acid-evoked Ca2+ signals promote nitric oxide release and proliferation in human endothelial colony forming cells. Vasc. Pharmacol. 2016, 87, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Hall, Z.; Ament, Z.; Wilson, C.H.; Burkhart, D.L.; Ashmore, T.; Koulman, A.; Littlewood, T.; Evan, G.I.; Griffin, J.L. Myc expression drives aberrant lipid metabolism in lung cancer. Cancer Res. 2016, 76, 4608–4618. [Google Scholar] [CrossRef]
- Hughes-Fulford, M.; Li, C.F.; Boonyaratanakornkit, J.; Sayyah, S. Arachidonic acid activates phosphatidylinositol 3-kinase signaling and induces gene expression in prostate cancer. Cancer Res. 2006, 66, 1427–1433. [Google Scholar] [CrossRef]
- Paine, E.; Palmantier, R.; Akiyama, S.K.; Olden, K.; Roberts, J.D. Arachidonic acid activates mitogen-activated protein (map) kinase-activated protein kinase 2 and mediates adhesion of a human breast carcinoma cell line to collagen type iv through a p38 map kinase-dependent pathway. J. Biol. Chem. 2000, 275, 11284–11290. [Google Scholar] [CrossRef]
- Khan, W.A.; Blobe, G.C.; Hannun, Y.A. Arachidonic acid and free fatty acids as second messengers and the role of protein kinase C. Cell. Signal. 1995, 7, 171–184. [Google Scholar] [CrossRef]
- Moran, L.J.; Tsagareli, V.; Noakes, M.; Norman, R. Altered preconception fatty acid intake is associated with improved pregnancy rates in overweight and obese women undertaking in vitro fertilisation. Nutrients 2016, 8, 10. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Ripamonte, P.; Buratini, J.; Portela, V.; Price, C. Fibroblast growth factor-2 regulation of sprouty and nr4a genes in bovine ovarian granulosa cells. J. Cell. Physiol. 2011, 226, 1820–1827. [Google Scholar] [CrossRef] [PubMed]
- Burdge, G. Α-linolenic acid metabolism in men and women: Nutritional and biological implications. Curr. Opin. Clin. Nutr. Metab. Care 2004, 7, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Wonnacott, K.E.; Kwong, W.Y.; Hughes, J.; Salter, A.M.; Lea, R.G.; Garnsworthy, P.C.; Sinclair, K.D. Dietary omega-3 and -6 polyunsaturated fatty acids affect the composition and development of sheep granulosa cells, oocytes and embryos. Reproduction (Camb. Engl.) 2010, 139, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Bender, K.; Walsh, S.; Evans, A.C.; Fair, T.; Brennan, L. Metabolite concentrations in follicular fluid may explain differences in fertility between heifers and lactating cows. Reproduction (Camb. Engl.) 2010, 139, 1047–1055. [Google Scholar] [CrossRef] [PubMed]
- Fayard, J.M.; Tessier, C.; Cohen, H.; Lagarde, M.; Pageaux, J.F.; Laugier, C. Phospholipase a2 inhibitors regulate the proliferation of normal uterine cells. Eur. J. Pharmacol. 1994, 251, 281–289. [Google Scholar] [CrossRef]
- Habbel, P.; Weylandt, K.H.; Lichopoj, K.; Nowak, J.; Purschke, M.; Wang, J.-D.; He, C.-W.; Baumgart, D.C.; Kang, J.X. Docosahexaenoic acid suppresses arachidonic acid-induced proliferation of ls-174t human colon carcinoma cells. World J. Gastroenterol. 2009, 15, 1079–1084. [Google Scholar] [CrossRef]
- Chan, T.A.; Morin, P.J.; Vogelstein, B.; Kinzler, K.W. Mechanisms underlying nonsteroidal antiinflammatory drug-mediated apoptosis. Proc. Natl. Acad. Sci. USA 1998, 95, 681–686. [Google Scholar] [CrossRef]
- Pompeia, C.; Lima, T.; Curi, R. Arachidonic acid cytotoxicity: Can arachidonic acid be a physiological mediator of cell death? Cell Biochem. Funct. 2003, 21, 97–104. [Google Scholar] [CrossRef]
- Aardema, H.; van Tol, H.T.A.; Wubbolts, R.W.; Brouwers, J.; Gadella, B.M.; Roelen, B.A.J. Stearoyl-coa desaturase activity in bovine cumulus cells protects the oocyte against saturated fatty acid stress. Biol. Reprod. 2017, 96, 982–992. [Google Scholar] [CrossRef] [PubMed]
- Vanholder, T.; Leroy, J.L.; Soom, A.V.; Opsomer, G.; Maes, D.; Coryn, M.; de Kruif, A. Effect of non-esterified fatty acids on bovine granulosa cell steroidogenesis and proliferation in vitro. Anim. Reprod. Sci. 2005, 87, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Mu, Y.-M.; Yanase, T.; Nishi, Y.; Tanaka, A.; Saito, M.; Jin, C.-H.; Mukasa, C.; Okabe, T.; Nomura, M.; Goto, K.; et al. Saturated FFAs, palmitic acid and stearic acid, induce apoptosis in human granulosa cells. Endocrinology 2001, 142, 3590–3597. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, F.; Inoue, N.; Manabe, N.; Ohkura, S. Follicular growth and atresia in mammalian ovaries: Regulation by survival and death of granulosa cells. J. Reprod. Dev. 2012, 58, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Yang, D.; Li, D.; Tang, B.; Yang, Y. Oleic acid induces smooth muscle foam cell formation and enhances atherosclerotic lesion development via cd36. Lipids Health Dis. 2011, 10, 53. [Google Scholar] [CrossRef]
- Dube, E.; Gravel, A.; Martin, C.; Desparois, G.; Moussa, I.; Ethier-Chiasson, M.; Forest, J.C.; Giguere, Y.; Masse, A.; Lafond, J. Modulation of fatty acid transport and metabolism by maternal obesity in the human full-term placenta. Biol. Reprod. 2012, 87, 14. [Google Scholar] [CrossRef]
- Yenuganti, V.R.; Viergutz, T.; Vanselow, J. Oleic acid induces specific alterations in the morphology, gene expression and steroid hormone production of cultured bovine granulosa cells. Gen. Comp. Endocrinol. 2016, 232, 134–144. [Google Scholar] [CrossRef]
- Turner, N.; Cooney, G.J.; Kraegen, E.W.; Bruce, C.R. Fatty acid metabolism, energy expenditure and insulin resistance in muscle. J. Endocrinol. 2014, 220, T61–T79. [Google Scholar] [CrossRef]
- Rohwedder, A.; Zhang, Q.; Rudge, S.A.; Wakelam, M.J. Lipid droplet formation in response to oleic acid in huh-7 cells is a fatty acid receptor mediated event. J. Cell Sci. 2014, 127, 3104–3115. [Google Scholar] [CrossRef]
- Regan, S.L.P.; Knight, P.G.; Yovich, J.L.; Leung, Y.; Arfuso, F.; Dharmarajan, A. Granulosa cell apoptosis in the ovarian follicle-a changing view. Front. Endocrinol. 2018, 9, 61. [Google Scholar] [CrossRef]
- Hsueh, A.J.; Rauch, R. Ovarian kaleidoscope database: Ten years and beyond. Biol. Reprod. 2012, 86, 192. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.J.; Dyson, M.T.; Jo, Y.; Eubank, D.W.; Stocco, D.M. Involvement of 5-lipoxygenase metabolites of arachidonic acid in cyclic amp-stimulated steroidogenesis and steroidogenic acute regulatory protein gene expression. J. Steroid Biochem. Mol. Biol. 2003, 85, 159–166. [Google Scholar] [CrossRef]
- Eimerl, S.; Orly, J. Regulation of steroidogenic genes by insulin-like growth factor-1 and follicle-stimulating hormone: Differential responses of cytochrome p450 side-chain cleavage, steroidogenic acute regulatory protein, and 3beta-hydroxysteroid dehydrogenase/isomerase in rat granulosa cells. Biol. Reprod. 2002, 67, 900–910. [Google Scholar] [PubMed]
- Kovo, M.; Kandli-Cohen, M.; Ben-Haim, M.; Galiani, D.; Carr, D.W.; Dekel, N. An active protein kinase a (PKA) is involved in meiotic arrest of rat growing oocytes. Reproduction (Camb. Engl.) 2006, 132, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Nader, N.; Courjaret, R.; Dib, M.; Kulkarni, R.P.; Machaca, K. Release from xenopus oocyte prophase I meiotic arrest is independent of a decrease in camp levels or PKA activity. Development 2016, 143, 1926–1936. [Google Scholar] [CrossRef] [PubMed]
- Maitra, S.; Das, D.; Ghosh, P.; Hajra, S.; Roy, S.S.; Bhattacharya, S. High camp attenuation of insulin-stimulated meiotic g2-m1 transition in zebrafish oocytes: Interaction between the camp-dependent protein kinase (PKA) and the mapk3/1 pathways. Mol. Cell. Endocrinol. 2014, 393, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Davis, W.J.; Lehmann, P.Z.; Li, W. Nuclear PI3K signaling in cell growth and tumorigenesis. Front. Cell Dev. Biol. 2015, 3, 24. [Google Scholar] [CrossRef] [PubMed]
- Andrade, G.M.; da Silveira, J.C.; Perrini, C.; Del Collado, M.; Gebremedhn, S.; Tesfaye, D.; Meirelles, F.V.; Perecin, F. The role of the PI3K-Akt signaling pathway in the developmental competence of bovine oocytes. PLoS ONE 2017, 12, e0185045. [Google Scholar] [CrossRef] [PubMed]
- Cseh, B.; Doma, E.; Baccarini, M. “Raf” neighborhood: Protein-protein interaction in the Raf/Mek/Erk pathway. FEBS Lett. 2014, 588, 2398–2406. [Google Scholar] [CrossRef]
- Evans, A.C.; Martin, F. Kinase pathways in dominant and subordinate ovarian follicles during the first wave of follicular development in sheep. Anim. Reprod. Sci. 2000, 64, 221–231. [Google Scholar] [CrossRef]

| Gene | (5′-3′) Forward Primer | (5′-3′) Reverse Primer | Accession No. |
|---|---|---|---|
| H2AFZ | GAGGAGCTGAACAAGCTGTTG | TTGTGGTGGCTCTCAGTCTTC | NM_174809 |
| FABP3 | CAGGCAGGTGGGCAATATGA | GCGTCACGATGGACTTGACTT | NM_174313 |
| CD36 | TGAGGCAGACACAACAAGAGT | ATCAGTGGTAACCAGTTGGAAGTC | NM_001278621 |
| SLC27A1 | CCAGGTCAGCCAGGGAACAA | CTCGCATCCTAGAGACCCTGAAG | NM_001033625 |
| FSHR | AATTCATTTGTGCCAGCATCC | AGTTCGACCGCATCCCTG | NM_174061 |
| HSD3B1 | ACAATCTGACCGCATCGTCCT | CCACTTGCACCAGTGTCTTG | NM_174343 |
| CYP19A1 | GTGGACGTGTTGACCCTCAT | GGCACTTTCATCCAAGGGGA | NM_174305 |
| STAR | GCCCAGAAACCTCAGCTCTTA | AGCTTTCCTGCTCCTAAGCAA | NM_174189 |
| BAX | AACATGGAGCTGCAGAGGAT | CAGTTGAAGTTGCCGTCAGA | NM_173894 |
| BCL-2 | ATGACTTCTCTCGGCGCTAC | CTGAAGAGCTCCTCCACCAC | NM_001166486 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, N.; Wang, L.; Luo, G.; Tang, X.; Ma, L.; Zheng, Y.; Liu, S.; A. Price, C.; Jiang, Z. Arachidonic Acid Regulation of Intracellular Signaling Pathways and Target Gene Expression in Bovine Ovarian Granulosa Cells. Animals 2019, 9, 374. https://doi.org/10.3390/ani9060374
Zhang N, Wang L, Luo G, Tang X, Ma L, Zheng Y, Liu S, A. Price C, Jiang Z. Arachidonic Acid Regulation of Intracellular Signaling Pathways and Target Gene Expression in Bovine Ovarian Granulosa Cells. Animals. 2019; 9(6):374. https://doi.org/10.3390/ani9060374
Chicago/Turabian StyleZhang, Nina, Liqiang Wang, Guoya Luo, Xiaorong Tang, Lizhu Ma, Yuxin Zheng, Shujie Liu, Christopher A. Price, and Zhongliang Jiang. 2019. "Arachidonic Acid Regulation of Intracellular Signaling Pathways and Target Gene Expression in Bovine Ovarian Granulosa Cells" Animals 9, no. 6: 374. https://doi.org/10.3390/ani9060374
APA StyleZhang, N., Wang, L., Luo, G., Tang, X., Ma, L., Zheng, Y., Liu, S., A. Price, C., & Jiang, Z. (2019). Arachidonic Acid Regulation of Intracellular Signaling Pathways and Target Gene Expression in Bovine Ovarian Granulosa Cells. Animals, 9(6), 374. https://doi.org/10.3390/ani9060374




