The Change of Metallothionein and Oxidative Response in Gills of the Oreochromis niloticus after Exposure to Copper
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Fish Acclimation
2.2. Determination 96-h LC50 of Copper
2.3. Treatment with Sublethal Cu
2.4. Water Quality
2.5. Determination of Cu
2.6. Determination of MT, SOD, CAT and MDA
2.7. Statistical Analysis
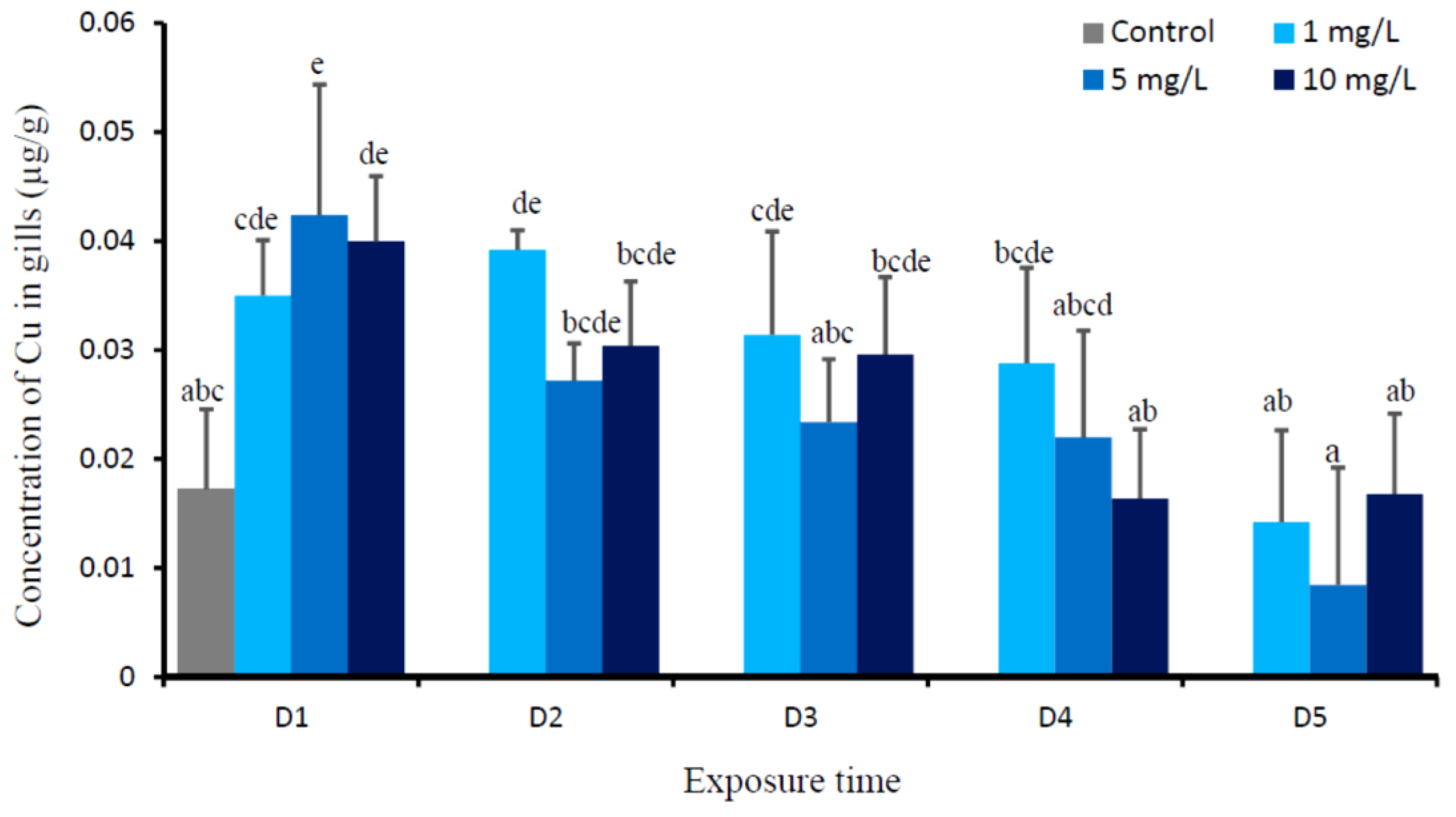
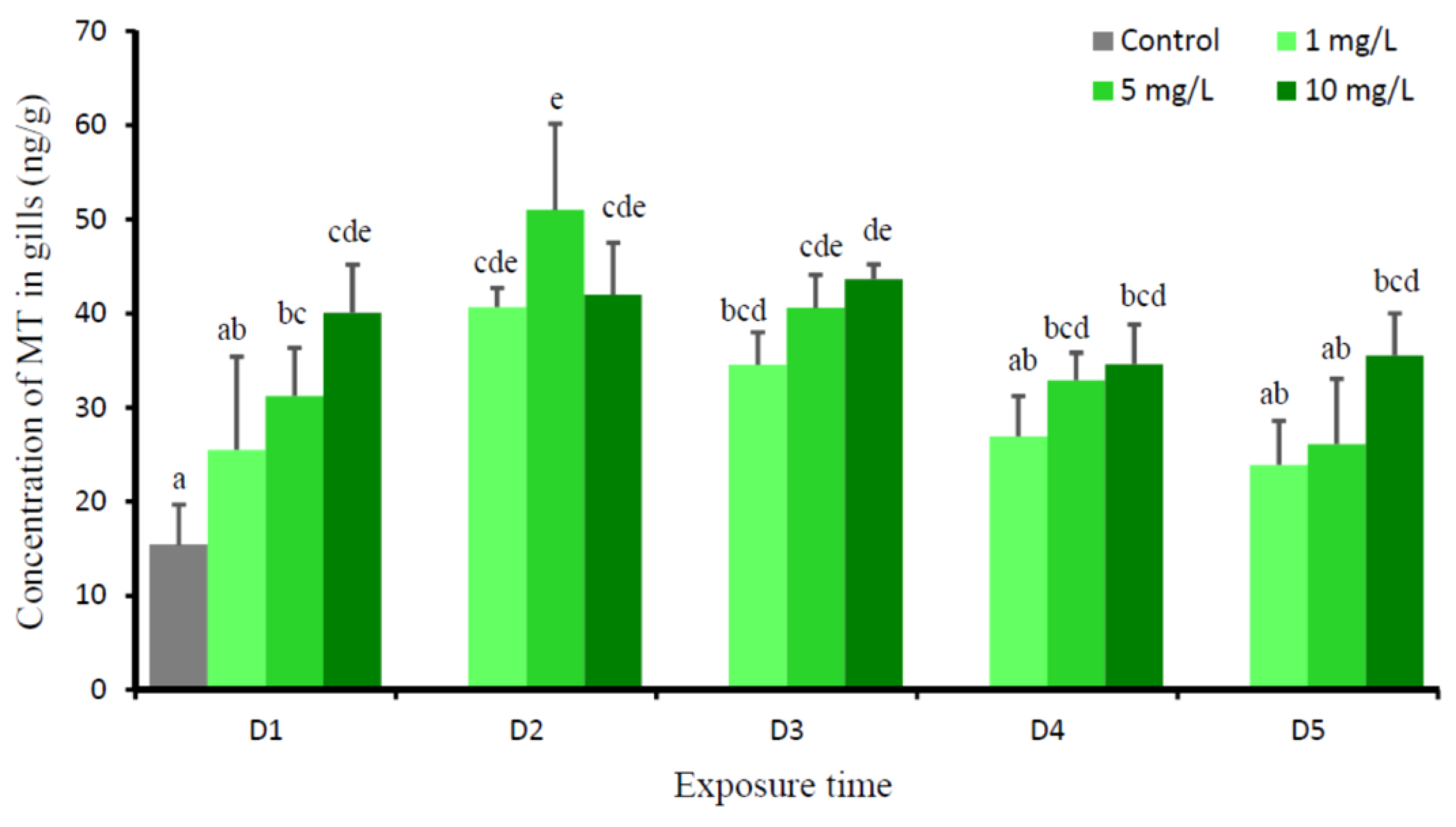
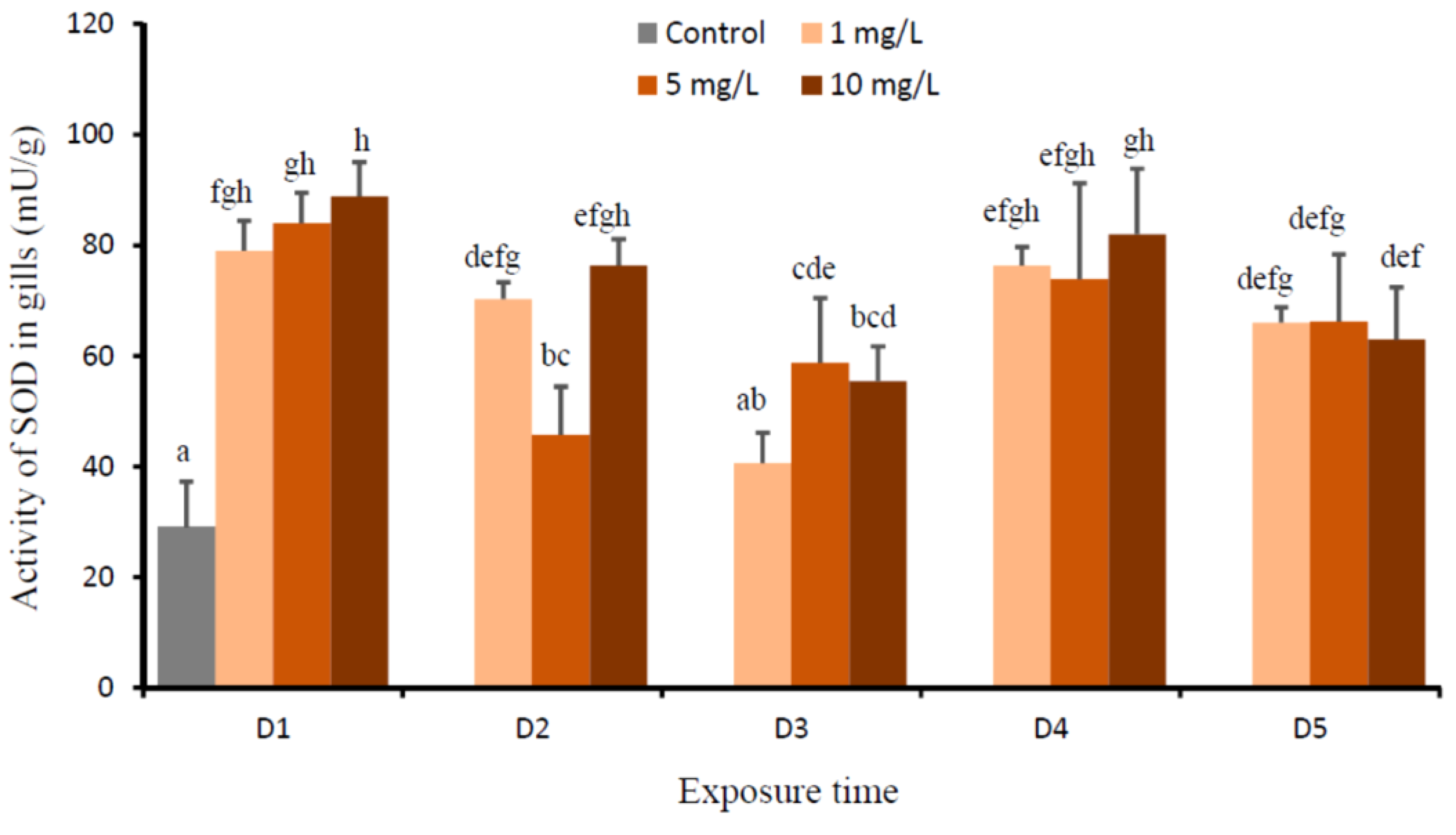
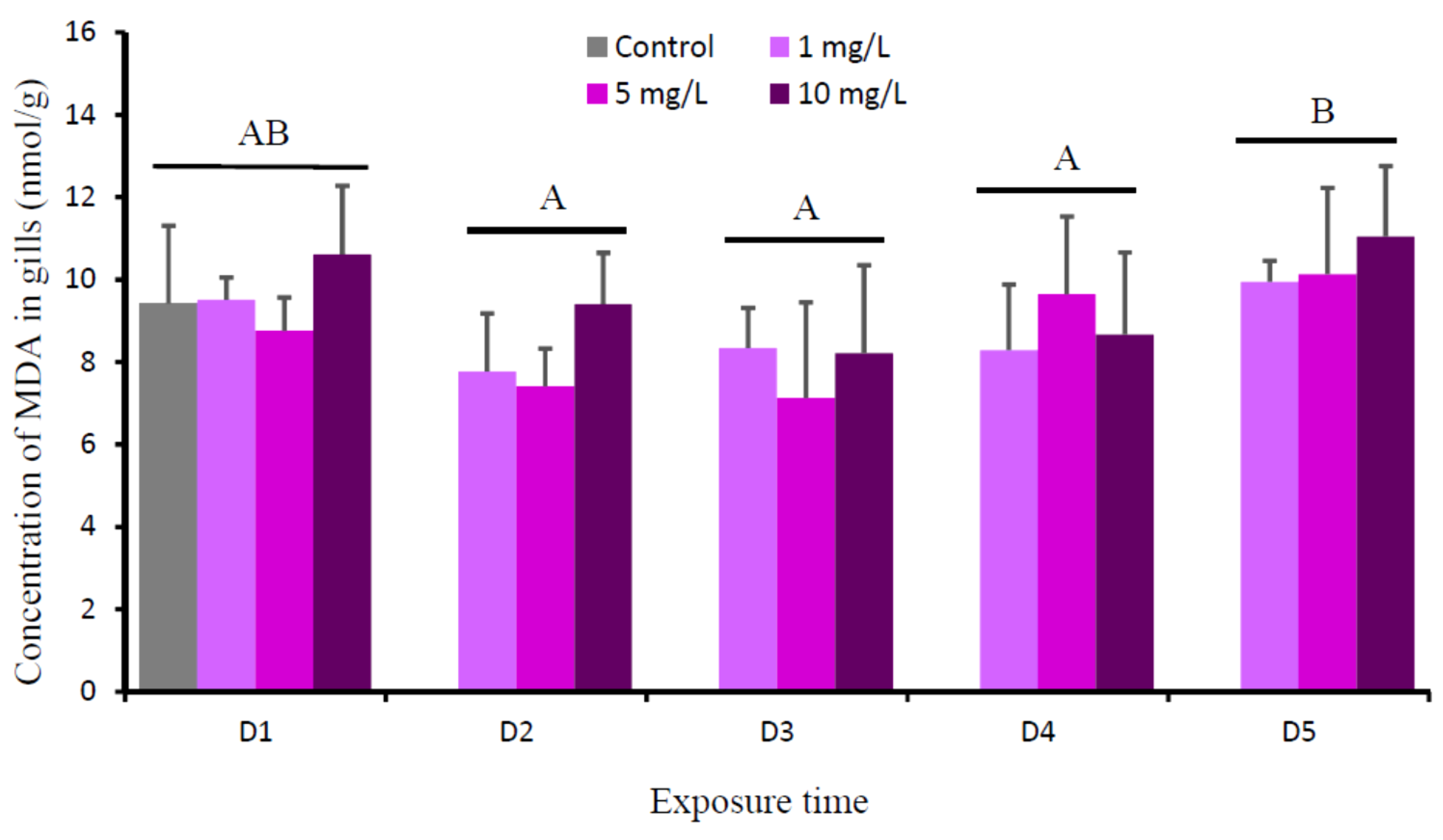
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- USEPA. Aquatic Life Ambient Freshwater Quality Criteria—Copper; EPA-822-R-07-001; 2007 revision; US Environmental Protection Agency: Washington, DC, USA, 2007.
- Wong, C.K. Effects of cadmium, copper, nickel, and zinc on longevity and reproduction of the cladoceran Moina macrocopa. Bull. Environ. Contam. Toxicol. 1993, 50, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Saager, P.M.; De Baar, H.J.W.; De Jong, J.T.M.; Nolting, R.F.; Schijf, J. Hydrography and local sources of dissolved trace metals Mn, Ni, Cu, and Cd in the northeast Atlantic Ocean. Mar. Chem. 1997, 57, 195–216. [Google Scholar] [CrossRef]
- Soegianto, A.; Charmantier-Daures, M.; Trilles, J.-P.; Charmantier, G. Impact of copper on the structure of gills and epipodites of the shrimp Penaeus japonicus. J. Crust. Biol. 1999, 19, 209–223. [Google Scholar] [CrossRef]
- Boran, M.; Altinok, I. A review of heavy metals in water, sediment and living organisms in the Black Sea. Turk. J. Fish. Aquat. Sci. 2010, 10, 565–572. [Google Scholar] [CrossRef]
- Davis, A.; Ashenberg, D. The aqueous geochemistry of the Berkeley Pit, Butte, Montana, USA. Appl. Geochem. 1989, 4, 23–36. [Google Scholar] [CrossRef]
- Robins, R.G.; Berg, R.B.; Dysinger, D.K.; Duaime, T.E.; Metesh, J.J.; Diebold, F.E.; Twidwell, L.G.; Mitman, G.G.; Chatham, W.H.; Huang, H.H.; et al. Chemical, physical and biological interactions at the Berkeley Pit, Butte, Montana. In Proceedings of the Tailings and Mine Waste, Colorado State University, Fort Collins, CO, USA, 13–17 January 1997. [Google Scholar]
- Ogino, C.; Yang, G.Y. Requirements of carp and rainbow trout for dietary manganese and copper. Bull. Japan Soc. Sci. Fish. 1980, 46, 455–458. [Google Scholar] [CrossRef]
- Satoh, S.; Yamamoto, H.; Takeuchi, T. Effects on growth and mineral composition of carp of deletion of trace elements or magnesium from fish meal diet. Bull. Jpn. Soc. Sci. Fish. 1983, 49, 431–435. [Google Scholar] [CrossRef]
- Solomon, E.I.; Lowery, M.D. Electronic structure contributions to function in bioinorganic chemistry. Science 1993, 259, 1575–1581. [Google Scholar] [CrossRef]
- Pena, M.M.O.; Lee, J.; Thiele, D. A delicate balance: Homeostatic control of copper uptake and distribution. J. Nutr. 1999, 129, 1251–1260. [Google Scholar] [CrossRef]
- Harris, Z.I.; Gitlin, J.D. Genetic and molecular basis for copper toxicity. Am. J. Clin. Nutr. 1996, 63, 8365–8415. [Google Scholar] [CrossRef]
- Kamunde, C.; Clayton, C.; Wood, C.M. Waterborne vs. dietary copper uptake in rainbow trout and the effects of previous waterborne copper exposure. Am. J. Physio-Reg. I 2002, 283, R69–R78. [Google Scholar] [CrossRef] [PubMed]
- Bury, N.R.; Walker, P.A.; Glover, C.N. Nutritive metal uptake in teleost fish. J. Exp. Biol. 2003, 206, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, W.-X. Homeostatic regulation of copper in a marine fish simulated by a physiologically based pharmacokinetic model. Environ. Pollut. 2016, 218, 1245–1254. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, C.S.; Fernandes, M.N. Effect of copper on liver key enzymes of anaerobic glucose metabolism from freshwater tropical fish Prochilodus lineatus. Comp. Biochem. Physiol. 2008, 51A, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Grosell, M.; Wood, C.M. Copper uptake across rainbow trout gills: Mechanisms of apical entry. J. Exp. Biol. 2002, 205, 1179–1188. [Google Scholar] [PubMed]
- Soegianto, A.; Irawan, B.; Usman, N. Effects of sublethal copper concentrations on gills of white shrimp (Litopenaeus vanamei, Boone 1931). Bull. Environ. Contam. Toxicol. 2013, 91, 630–634. [Google Scholar] [CrossRef]
- Lauren, D.J.; McDonald, D.G. Acclimation to copper by rainbow trout, Salmo gairdneri: Biochemistry. Can. J. Fish. Aquat. Sci. 1987, 44, 105–111. [Google Scholar] [CrossRef]
- Reid, S.D.; McDonald, D.G. Effects of cadmium, copper and low pH on ion fluxes in the rainbow trout, Salmo gairdneri. Can. J. Fish. Aquat. Sci. 1988, 45, 244–253. [Google Scholar] [CrossRef]
- Cerqueira, C.C.C.; Fernandes, M.N. Gill tissue recovery after copper exposure and blood parameter responses in the tropical fish, Prochilodus scrofa. Ecotoxicol. Environ. Saf. 2002, 52, 83–89. [Google Scholar] [CrossRef]
- Monteiro, D.A.; Rantin, F.T.; Kalinin, A.L. Inorganic mercury exposure:toxicological effects, oxidative stress biomarkers and bioaccumulation in thetropical freshwater fish matrinxa, Brycon amazonicus (Spix and Agassiz, 1829). Ecotoxicology 2010, 19, 105–123. [Google Scholar] [CrossRef]
- Usman, N.; Irawan, B.; Soegianto, A. Effect of copper on survival and osmoregulation in different life stages of white shrimp Litopenaeus vannamei Bonne, 1931. Cah. Biol. Mar. 2013, 54, 191–197. [Google Scholar]
- Asih, A.Y.P.; Irawan, B.; Soegianto, A. Effect of copper on survival, osmoregulation, and gill structures of freshwater prawn (Macobrachium rosenbergii, de Man) at different development stages. Mar. Freshwat. Behav. Physiol. 2013, 46, 75–88. [Google Scholar] [CrossRef]
- Bervoets, L.; Knapen, D.; De Jonge, M.; Campenhout, K.V.; Blust, R. Differential hepatic metal and metallothionein levels in three feral fish species along a metal pollution gradient. PLoS ONE 2013, 8, e60805. [Google Scholar] [CrossRef] [PubMed]
- Nursanti, L.; Nofitasari, E.; Hayati, A.; Hariyanto, S.; Irawan, B.; Soegianto, A. Effects of cadmium on metallothionein and histology in gills of tilapia (Oreochromis niloticus) at di fferent salinities. Toxicol. Environ. Chem. 2017, 99, 926–937. [Google Scholar] [CrossRef]
- Elia, A.C.; Galarini, R.; Taticchi, M.I.; Dörr, A.J.M.; Mantilacci, L. Antioxidant responses and bioaccumulation in Ictalurus melas under mercury exposure. Ecotoxicol. Environ. Saf. 2003, 55, 162–167. [Google Scholar] [CrossRef]
- Atli, G.; Canli, M. Response of antioxidant system of freshwater fish Oreochromis niloticus to acute and chronic metal (Cd, Cu, Cr, Zn, Fe) exposures. Ecotoxicol. Environ. Saf. 2010, 73, 1884–1889. [Google Scholar] [CrossRef] [PubMed]
- Saglam, D.; Atli, G.; Dogan, Z.; Baysoy, E.; Gurler, C.; Eroglu, A.; Canli, M. Response of the antioxidant system of freshwater fish (Oreochromis niloticus) exposed to metals (Cd, Cu) in differing hardness. Turk. J. Fish. Aquat. Sci. 2014, 14, 43–52. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, P.; Shen, Q.; Wang, Q.; Liu, D.; Li, J.; Wang, L. The effects of cadmium exposure on the oxidative state and cell death in the gill of freshwater crab Sinopotamon henanense. PLoS ONE 2013, 8, e64020. [Google Scholar] [CrossRef]
- Shofiatun, S.; Handayani, K.S.; Suryani, H.; Irawan, B.; Soegianto, A. Oxidative stress responses in gills of white shrimp Litopenaeus vannamei Boone, 1931 following exposure to cadmium at different salinities. Cah. Biol. Mar. 2017, 58, 461–466. [Google Scholar] [CrossRef]
- Rodrıguez-Ariza, A.; Alhama, J.; Dıaz-Mendez, F.M.; Lopez Barea, J. Content of 8-oxodG in chromosomal DNA of Sparus aurata fish as biomarker of oxidative stress and environmental pollution. Mutat. Res. 1999, 438, 97–107. [Google Scholar] [CrossRef]
- Valko, M.; Rhodes, C.J.; Moncol, J.; Izakovic, M.; Mazur, M. Free radicals, metals and antioxidants in oxidative stressinduced cancer. Chem.-Biol. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Putranto, T.W.C.; Andriani, R.; Munawwaroh, A.; Irawan, B.; Soegianto, A. Effect of cadmium on survival, osmoregulation and gill structure of the Sunda prawn, Macrobrachium sintangense (De Man), at different salinities. Mar. Freshwat. Behav. Physiol. 2014, 47, 349–360. [Google Scholar] [CrossRef]
- Soegianto, A.; Adhim, M.A.; Zainuddin, A.; Putranto, T.W.C.; Irawan, B. Effect of different salinity on serum osmolality, ion levels and hematological parameters of East Java strain tilapia Oreochromis niloticus. Mar. Freshwat. Behav. Physiol. 2017, 50, 105–113. [Google Scholar] [CrossRef]
- Ruaeny, T.A.; Hariyanto, S.; Soegianto, A. Contamination of copper, zinc, cadmium and lead in fishs pecies captured from Bali Strait, Indonesia, and potential risks to human health. Cah. Biol. Mar. 2015, 56, 89–95. [Google Scholar]
- McDonald, D.G.; Wood, C.M. Branchial mechanisms of acclimation to metals in freshwater fish. In Fish Ecophysiology; Rankin, J.C., Jensen, F.B., Eds.; Chapman & Hall: London, UK, 1993; pp. 297–321. [Google Scholar]
- Mc Geer, J.C.; Szebedinszky, C.; McDonald, D.G.; Wood, C.M. Effects of chronic sublethal exposure to waterborne Cu, Cd or Zn in rainbow trout 2: Tissue specific metal accumulation. Aquat. Toxicol. 2000, 50, 247–258. [Google Scholar]
- Bradley, R.W.; DuQuesnay, C.; Sprague, J.B. Acclimation of rainbow trout, Salmo gairdneri Richardson, to zinc: Kinetics and mechanisms of enhanced tolerance induction. J. Fish Biol. 1985, 27, 367–379. [Google Scholar] [CrossRef]
- Hogstrand, C.; Wood, C.M. The physiology and toxicology of zinc in fish. In Toxicology of Aquatic Pollution: Physiological, Cellular and Molecular Approaches; Taylor, E.W., Ed.; Cambridge University Press: Cambridge, UK, 1996; pp. 61–84. [Google Scholar]
- Hogstrand, C.; Haux, C. Binding and detoxification of heavy metals in lower vertebrates with references to metallothionein. Comp. Biochem. Physiol. 1991, 100C, 137–141. [Google Scholar] [CrossRef]
- Brown, D.A.; Parsons, T.R. Relationship between cytoplasmic distribution of mercury and toxic effects to zooplankton and chum salmon (Oncorhynchus keta) exposed to mercury in a controlled ecosystem. J. Fish. Res. Board Can. 1978, 35, 880–884. [Google Scholar] [CrossRef]
- Coyle, P.; Philcox, J.C.; Carey, L.C.; Rofe, A.M. Metallothionein: The multipurpose protein. Cell. Mol. Life Sci. 2002, 59, 627–647. [Google Scholar] [CrossRef]
- Di Giulio, R.T.; Meyer, J.N. Reactive oxygen species and oxidative stress. In The Toxicology of Fishes; Di Giulio, R.T., Hilton, D.E., Eds.; CRC Press: Boca Raton, FL, USA, 2008; pp. 273–326. [Google Scholar]
- Simonato, J.D.; Mela, M.; Doria, H.B.; Guiloski, I.C.; Randi, M.A.F.; Carvalho, P.S.M.; Meletti, P.C.; Silva de Assis, H.C.; Bianchini, A.; Martinez, C.B.R. Biomarkers of waterborne copper exposure in the Neotropical fish Prochilodus lineatus. Aquat. Toxicol. 2016, 170, 31–41. [Google Scholar] [CrossRef]
- Lushchak, V.I. Environmentally induced oxidative stress in aquatic animals. Aquat. Toxicol. 2011, 101, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Tawwab, M.; Hamed, H.S. Effect of bisphenol A toxicity on growth performance, biochemical variables, and oxidative stress biomarkers of Nile tilapia, Oreochromis niloticus (L.). J. Appl. Ichthyol. 2018. [Google Scholar] [CrossRef]
- Carvalho, C.S.; Bernusso, V.A.; Fernandes, M.N. Copper levels and changes in pH induce oxidative stress in the tissue of curimbata (Prochilodus lineatus). Aquat. Toxicol. 2015, 167, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Wang, L.; Yan, B.; Li, Y.; Ye, F.; Li, J.; Wang, Q. Assessment of antioxidant defense system responses in the hepatopancreas of the freshwater crab Sinopotamon henanense exposed to lead. Hydrobiologia 2014, 741, 3–13. [Google Scholar] [CrossRef]
- Romeo, M.; Bennani, N.; Gnassia-Barelli, M.; Lafaurie, M.; Girard, J.P. Cadmium and copper display different responses towards oxidative stress in the kidney of the sea bass Dicentrarchus labrax. Aquat. Toxicol. 2000, 48, 185–194. [Google Scholar] [CrossRef]
- Verma, S.; Dubey, R.S. Lead toxicity induces lipid peroxidation and alters the activities of antioxidant enzymes in growing rice plants. Plant Sci. 2003, 164, 645–655. [Google Scholar] [CrossRef]
- Fatima, R.A.; Ahmad, M. Certain antioxidant enzymes of Allium cepa as biomarkers for the detection of toxic heavy metals in wastewater. Sci. Total Environ. 2005, 346, 256–273. [Google Scholar] [CrossRef]
- Wei, K.; Yang, J. Oxidative damage induced by copper andbeta-cypermethrin in gill of the freshwater crayfish Procambarus clarkia. Ecotoxicol. Environ. Saf. 2015, 113, 446–453. [Google Scholar] [CrossRef]
- Moghaddam, H.S.; Samarghandian, S.; Farkhondeh, T. Effect of bisphenol A on blood glucose, lipid profile and oxidative stress indices in adult male mice. Toxicol. Mech. Meth. 2015, 25, 507–513. [Google Scholar] [CrossRef]

| Copper Concentration (mg/L) | Number of Tanks Used | Treatment Day (Fish Sampled/Total Fish) | ||||
|---|---|---|---|---|---|---|
| D1 | D2 | D3 | D4 | D5 | ||
| 0 | 2 | 5/10 | - | - | - | - |
| 1 | 10 | 5/10 | 5/10 | 5/10 | 5/10 | 5/10 |
| 5 | 10 | 5/10 | 5/10 | 5/10 | 5/10 | 5/10 |
| 10 | 10 | 5/10 | 5/10 | 5/10 | 5/10 | 5/10 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma’rifah, F.; Saputri, M.R.; Soegianto, A.; Irawan, B.; Putranto, T.W.C. The Change of Metallothionein and Oxidative Response in Gills of the Oreochromis niloticus after Exposure to Copper. Animals 2019, 9, 353. https://doi.org/10.3390/ani9060353
Ma’rifah F, Saputri MR, Soegianto A, Irawan B, Putranto TWC. The Change of Metallothionein and Oxidative Response in Gills of the Oreochromis niloticus after Exposure to Copper. Animals. 2019; 9(6):353. https://doi.org/10.3390/ani9060353
Chicago/Turabian StyleMa’rifah, Faridlotul, Miftahul Rohmah Saputri, Agoes Soegianto, Bambang Irawan, and Trisnadi Widyaleksono Catur Putranto. 2019. "The Change of Metallothionein and Oxidative Response in Gills of the Oreochromis niloticus after Exposure to Copper" Animals 9, no. 6: 353. https://doi.org/10.3390/ani9060353
APA StyleMa’rifah, F., Saputri, M. R., Soegianto, A., Irawan, B., & Putranto, T. W. C. (2019). The Change of Metallothionein and Oxidative Response in Gills of the Oreochromis niloticus after Exposure to Copper. Animals, 9(6), 353. https://doi.org/10.3390/ani9060353





