Comprehensive Resource Utilization of Waste Using the Black Soldier Fly (Hermetia illucens (L.)) (Diptera: Stratiomyidae)
Abstract
Simple Summary
Abstract
1. Introduction
2. Biological Morphology
2.1. Morphological and Biological Characteristics
2.2. Ecological Characteristics and Environmental Stressors of Hermetia illucens
2.3. Research on Artificial Breeding on Hermetia illucens
3. Resource Value of Hermetia illucens
3.1. Resource Value of Hermetia illucens Proteins and Minerals
3.2. Resource Value of Hermetia illucens Grease
3.3. Other Hermetia illucens Resource Values
3.4. Ecological Value of the Treatment of Organic Waste by Hermetia illucens
3.4.1. Research on the Treatment of Excrement by Hermetia illucens
3.4.2. Research on the Treatment of Other Organic Waste by Hermetia illucens
3.4.3. Ecological Value of Hermetia illucens for the Treatment of Organic Waste
4. Conclusions and Prospects
Author Contributions
Funding
Conflicts of Interest
References
- James, M.T. The genus Hermetia in the United States (Dipt, Stratiomyidae). Bull. Brooklyn Entomol. Soc. 1935, 30, 165–170. [Google Scholar]
- Benelli, G.; Canale, A.; Raspi, A.; Fornaciari, G. The death scenario of an Italian Renaissance princess can shed light on a zoological dilemma: Did the black soldier fly reach Europe with Columbus? J. Archaeol. Sci. 2014, 49, 203–205. [Google Scholar] [CrossRef]
- Bradley, S.W.; Sheppard, D.C. House fly oviposition inhibition by larvae of Hermetia illucens, the black soldier fly [Musca domestica, allomone]. J. Chem. Ecol. 1984, 10, 853–859. [Google Scholar] [CrossRef]
- Rocha Oliveira, F.; Doelle, K.; Smith, R.P. External morphology of Hermetia illucens Stratiomyidae: Diptera (L.1758) based on Electron microscopy. Annu. Res. Rev. Biol. 2016, 9, 1–10. [Google Scholar] [CrossRef]
- Cranshaw, W.; Shetlar, D. Garden Insects of North. America: The Ultimate Guide to Backyard Bugs; Princeton University Press: Princeton, NJ, USA, 2018. [Google Scholar] [CrossRef]
- Bondari, K.; Sheppard, D.C. The soldier fly Hermetia illucens L., as feed for channel catfish, Ictalurus punctatus (Rafinesque), and blue tilapia, Oreochromis aureus (Steindachner). Aquac. Res. 1987, 18, 209–220. [Google Scholar] [CrossRef]
- Erickson, M.C.; Islam, M.; Sheppard, C.; Liao, J.; Doyle, M.P. Reduction of Escherichia coli O157:H7 and Salmonella enterica serovar Enteritidis in chicken manure by larvae of the black soldier fly. J. Food Prot. 2004, 67, 685–690. [Google Scholar] [CrossRef]
- Liu, Q.; Tomberlin, J.K.; Brady, J.A.; Sanford, M.R.; Yu, Z. Black soldier fly (Diptera: Stratiomyidae) larvae reduce Escherichia coli in dairy manure. Env. Entomol. 2008, 37, 1525–1530. [Google Scholar] [CrossRef]
- Lalander, C.; Diener, S.; Magri, M.E.; Zurbrügg, C.; Lindström, A.; Vinnerås, B. Faecal sludge management with the larvae of the black soldier fly (Hermetia illucens)—From a hygiene aspect. Sci. Total Environ. 2013, 458–460, 312–318. [Google Scholar] [CrossRef]
- Sheppard, D.C.; Tomberlin, J.K.; Joyce, J.A.; Kiser, B.C.; Sumner, S.M. Rearing methods for the black soldier fly (Diptera: Stratiomyidae). J. Med. Entomol. 2002, 39, 695–698. [Google Scholar] [CrossRef]
- Tomberlin, J.K.; Sheppard, D.C. Factors influencing mating and oviposition of black soldier flies (Diptera: Stratiomyidae) in a colony. J. Entomol. Sci. 2002, 37, 345–352. [Google Scholar] [CrossRef]
- Diener, S.; Zurbrügg, C.; Tockner, K. Conversion of organic material by black soldier fly larvae: Establishing optimal feeding rates. Waste Manag. Res. J. Int. Solid Wastes Public Clean. Assoc. Iswa 2009, 27, 603–610. [Google Scholar] [CrossRef]
- Oonincx, D.G.A.B.; van Broekhoven, S.; van Huis, A.; van Loon, J.J.A. Feed conversion, survival and development, and composition of four insect species on diets composed of food by-products. PLoS ONE 2015, 10, e0144601. [Google Scholar] [CrossRef]
- Gobbi, P.; Martinez-Sanchez, A.; Rojo, S. The effects of larval diet on adult life-history traits of the black soldier fly, Hermetia illucens (Diptera: Stratiomyidae). EJE 2013, 110, 461–468. [Google Scholar] [CrossRef]
- Zheng, L.Y.; Li, Q.; Zhang, J.B.; Yu, Z.N. Double the biodiesel yield: Rearing black soldier fly larvae, Hermetia illucens, on solid residual fraction of restaurant waste after grease extraction for biodiesel production. Renew. Energy 2012, 41, 75–79. [Google Scholar] [CrossRef]
- Zheng, L.Y.; Hou, Y.F.; Wu, L.; Yang, S.; Li, Q.; Yu, Z.N. Biodiesel production from rice straw and restaurant waste employing black soldier fly assisted by microbes. Energy 2012, 47, 225–229. [Google Scholar] [CrossRef]
- Sheppard, D.C.; Newton, G.L.; Thompson, S.A.; Savage, S. A value added manure management system using the black soldier fly. Bioresour. Technol. 1994, 50, 275–279. [Google Scholar] [CrossRef]
- Li, Q.; Zheng, L.; Qiu, N.; Cai, H.; Tomberlin, J.K.; Yu, Z. Bioconversion of dairy manure by black soldier fly (Diptera: Stratiomyidae) for biodiesel and sugar production. Waste Manag. 2011, 31, 1316–1320. [Google Scholar] [CrossRef]
- Spranghers, T.; Ottoboni, M.; Klootwijk, C.; Ovyn, A.; Deboosere, S.; De Meulenaer, B.; Michiels, J.; Eeckhout, M.; De Clercq, P.; De Smet, S. Nutritional composition of black soldier fly (Hermetia illucens) prepupae reared on different organic waste substrates. J. Sci. Food Agric. 2017, 97, 2594–2600. [Google Scholar] [CrossRef]
- Jucker, C.; Erba, D.; Leonardi, M.G.; Lupi, D.; Savoldelli, S. Assessment of vegetable and fruit substrates as potential rearing media for Hermetia illucens (Diptera: Stratiomyidae) larvae. Environ. Entomol. 2017, 46, 1415–1423. [Google Scholar] [CrossRef]
- Mahanta, S.; Khanikor, B.; Sarma, R. Potentiality of essential oil from Citrus grandis (Sapindales: Rutaceae) against Culex quinquefasciatus Say (Diptera: Culicidae). J. Entomol. Zool. Stud. 2017, 5, 803–809. [Google Scholar]
- Hem, S.; Toure, S.; Sagbla, C.; Legendre, M. Bioconversion of palm kernel meal for aquaculture: Experiences from the forest region (Republic of Guinea). Afr. J. Biotechnol. 2008, 7, 1192–1198. [Google Scholar] [CrossRef]
- Abduh, M.Y.; Manurung, R.; Faustina, A.; Affanda, E.; Siregar, I.R.H. Bioconversion of Pandanus tectorius using black soldier fly larvae for the production of edible oil and protein-rich biomass. J. Entomol. Zool. Stud. 2017, 5, 803–809. [Google Scholar]
- Abduh, M.Y.; Jamilah, M.; Istiandari, P.; Manurung, S.; Manurung, R. Bioconversion of rubber seeds to produce protein and oil-rich biomass using black soldier fly larva assisted by microbes. J. Entomol. Zool. Stud. 2017, 5, 591–597. [Google Scholar]
- Manurung, R.; Supriatna, A.; Esyanthi, R.R.; Putra, R.E. Bioconversion of rice straw waste by black soldier fly larvae (Hermetia illucens L.): Optimal feed rate for biomass production. J. Entomol. Zool. Stud. 2016, 4, 1036–1041. [Google Scholar]
- Makkar, H.P.S.; Tran, G.; Heuzé, V.; Ankers, P. State-of-the-art on use of insects as animal feed. Anim. Feed Sci. Technol. 2014, 197, 1–33. [Google Scholar] [CrossRef]
- Cummins, V.C.; Rawles, S.D.; Thompson, K.R.; Velasquez, A.; Kobayashi, Y.; Hager, J.; Webster, C.D. Evaluation of black soldier fly (Hermetia illucens) larvae meal as partial or total replacement of marine fish meal in practical diets for Pacific white shrimp (Litopenaeus vannamei). Aquaculture 2017, 473, 337–344. [Google Scholar] [CrossRef]
- Rui, M.; Sánchez-López, A.; Leal, R.S.; Martínez-Llorens, S.; Oliva-Teles, A.; Peres, H. Black soldier fly (Hermetia illucens) pre-pupae meal as a fish meal replacement in diets for European seabass (Dicentrarchus labrax). Aquaculture 2017, 476, 79–85. [Google Scholar] [CrossRef]
- Spranghers, T.; Michiels, J.; Vrancx, J.; Ovyn, A.; Eeckhout, M.; Clercq, P.D.; Smet, S.D. Gut antimicrobial effects and nutritional value of black soldier fly (Hermetia illucens L.) prepupae for weaned piglets. Anim. Feed Sci. Technol. 2017, 235, 33–42. [Google Scholar] [CrossRef]
- Park, S.I.; Chang, B.S.; Yoe, S.M. Detection of antimicrobial substances from larvae of the black soldier fly, Hermetia illucens (Diptera: Stratiomyidae). Entomol. Res. 2014, 44, 58–64. [Google Scholar] [CrossRef]
- Park, S.I.; Kim, J.W.; Yoe, S.M. Purification and characterization of a novel antibacterial peptide from black soldier fly (Hermetia illucens) larvae. Dev. Comp. Immunol. 2015, 52, 98–106. [Google Scholar] [CrossRef]
- Park, S.I.; Yoe, S.M. A novel cecropin-like peptide from black soldier fly, Hermetia illucens: Isolation, structural and functional characterization. Entomol. Res. 2017, 47, 115–124. [Google Scholar] [CrossRef]
- Vogel, H.; Müller, A.; Heckel, D.; Gutzeit, H.; Vilcinskas, A. Nutritional immunology: Diversification and diet-dependent expression of antimicrobial peptides in the black soldier fly Hermetia illucens. Dev. Comp. Immunol. 2017, 78, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Waśko, A.; Bulak, P.; Polak-Berecka, M.; Nowak, K.; Polakowski, C.; Bieganowski, A. The first report of the physicochemical structure of chitin isolated from Hermetia illucens. Int. J. Biol. Macromol. 2016, 92, 316–320. [Google Scholar] [CrossRef]
- Callan, E.M. Hermetia illucens (L.) (Diptera, Stratiomyidae), a cosmopolitan American species long established in Australia and New Zealand. Entomol. Mon. Mag. 1974, 109, 232–234. [Google Scholar]
- Yu, G.; Cheng, P.; Chen, Y.; Li, Y.; Yang, Z.; Chen, Y.; Tomberlin, J.K. Inoculating poultry manure with companion bacteria influences growth and development of black soldier fly (Diptera: Stratiomyidae) larvae. Env. Entomol. 2011, 40, 30–35. [Google Scholar] [CrossRef] [PubMed]
- May, B.M. The occurrence in New Zealand and the life-history of the soldier fly Hermetia illucens (L.) (Diptera: Stratiomyidae). Nzj Sci. 1961, 4, 55–65. [Google Scholar]
- Liland, N.S.; Biancarosa, I.; Araujo, P.; Biemans, D.; Bruckner, C.G.; Waagbo, R.; Torstensen, B.E.; Lock, E.J. Modulation of nutrient composition of black soldier fly (Hermetia illucens) larvae by feeding seaweed-enriched media. PLoS ONE 2017, 12, e0183188. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.T.; Bae, S.W.; Park, H.C.; Park, K.H.; Lee, S.B.; Choi, Y.C.; Han, S.M.; Koh, Y.H. The larval age and mouth morphology of the black soldier fly, Hermetia illucens (Diptera: Stratiomyidae). Int. J. Ind. Entomol. 2010, 21, 185–187. [Google Scholar]
- Belghit, I.; Liland, N.S.; Waagbø, R.; Biancarosa, I.; Pelusio, N.; Li, Y.; Krogdahl, Å.; Lock, E.-J. Potential of insect-based diets for Atlantic salmon (Salmo salar). Aquaculture 2018, 491, 72–81. [Google Scholar] [CrossRef]
- Cammack, J.A.; Tomberlin, J.K. The Impact of Diet Protein and Carbohydrate on Select Life-History Traits of The Black Soldier Fly Hermetia illucens (L.) (Diptera: Stratiomyidae). Insects 2017, 8, 56. [Google Scholar] [CrossRef]
- Barragan-Fonseca, K.B.; Dicke, M.; Loon, J.J.A.v. Influence of larval density and dietary nutrient concentration on performance, body protein, and fat contents of black soldier fly larvae (Hermetia illucens). Entomol. Exp. Appl. 2018, 166, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Chia, S.Y.; Tanga, C.M.; Osuga, I.M.; Mohamed, S.A.; Khamis, F.M.; Salifu, D.; Sevgan, S.; Fiaboe, K.K.M.; Niassy, S.; van Loon, J.J.A.; et al. Effects of waste stream combinations from brewing industry on performance of Black Soldier Fly, Hermetia illucens (Diptera: Stratiomyidae). PeerJ 2018, 6, e5885. [Google Scholar] [CrossRef] [PubMed]
- Camenzuli, L.; Van Dam, R.; de Rijk, T.; Andriessen, R.; Van Schelt, J.; Van der Fels-Klerx, H.J.I. Tolerance and Excretion of the Mycotoxins Aflatoxin B₁, Zearalenone, Deoxynivalenol, and Ochratoxin A by Alphitobius diaperinus and Hermetia illucens from Contaminated Substrates. Toxins 2018, 10, 91. [Google Scholar] [CrossRef]
- Palma, L.; Ceballos, S.J.; Johnson, P.C.; Niemeier, D.; Pitesky, M.; VanderGheynst, J.S. Cultivation of black soldier fly larvae on almond byproducts: Impacts of aeration and moisture on larvae growth and composition. J. Sci. Food Agric. 2018, 98, 5893–5900. [Google Scholar] [CrossRef] [PubMed]
- Meneguz, M.; Gasco, L.; Tomberlin, J.K. Impact of pH and feeding system on black soldier fly (Hermetia illucens, L.; Diptera: Stratiomyidae) larval development. PLoS ONE 2018, 13, e0202591. [Google Scholar] [CrossRef] [PubMed]
- Bosch, G.; Fels-Klerx, H.J.v.d.; Rijk, T.C.d.; Oonincx, D.G.A.B. Aflatoxin B1 Tolerance and Accumulation in Black Soldier Fly Larvae (Hermetia illucens) and Yellow Mealworms (Tenebrio molitor). Toxins 2017, 9, 185. [Google Scholar] [CrossRef] [PubMed]
- Diener, S.; Zurbrügg, C.; Tockner, K. Bioaccumulation of heavy metals in the black soldier fly, Hermetia illucens and effects on its life cycle. J. Insects Food Feed 2015, 1, 261–270. [Google Scholar] [CrossRef]
- Trumble, J.T.; Jensen, P.D. Ovipositional response, developmental effects and toxicity of hexavalent chromium to megaselia scalaris, a terrestrial detritivore. Arch. Environ. Contam. Toxicol. 2004, 46, 372–376. [Google Scholar] [CrossRef]
- Diener, S.; Studt Solano, N.M.; Roa Gutiérrez, F.; Zurbrügg, C.; Tockner, K. Biological treatment of municipal organic waste using black soldier fly larvae. Waste Biomass Valoriz. 2011, 2, 357–363. [Google Scholar] [CrossRef]
- Nursita, A.I.; Singh, B.; Lees, E. The effects of cadmium, copper, lead, and zinc on the growth and reproduction of Proisotoma minuta Tullberg (Collembola). Ecotoxicol. Environ. Saf. 2005, 60, 306–314. [Google Scholar] [CrossRef]
- Xia, Q.; Dan, J.L.; Zhu, W.; Liao, Y.; Yu, G.H.; Chen, Y.F. Effects of Zinc on the growth and development of black soldier fly Hermetia illucens L. (Dipetra: Stratiomyidae). J. Environ. Entomol. 2013, 35, 294–299. [Google Scholar]
- Liu, H.Y. Accumulation of Zn2+ in the Hemolymph of Larvae of Hermetia Illucens L. and Its Preliminary Effects on the Enzyme Activity and Protein of Hemolymph; Zunyi Medical College: Zuiyi, China, 2016. [Google Scholar]
- Liu, L.; Xia, Q. Research progresses about the effect of copper pollution on the growth and reproduction of insects. J. Environ. Entomol. 2016, 38, 451–456. [Google Scholar]
- Shen, Y.; Xu, Q.Y.; An, X.C. The study of stress resistance for larva and pre-pupa stage of black soldier fly, Hermetia illucens. J. Environ. Entomol. 2012, 34, 240–242. [Google Scholar]
- Qiao, G.; Wang, X.; Wang, W.; Lei, C.; Zhu, F. Influences of chromium and cadmium on the development of black soldier fly larvae. Environ. Sci. Pollut. Res. Int. 2017, 24, 8637–8644. [Google Scholar] [CrossRef]
- Booth, D.C.; Sheppard, C. Oviposition of the Black Soldier Fly, Hermetia illucens (Diptera: Stratiomyidae): Eggs, Masses, Timing, and Site Characteristics. Environ. Entomol. 1984, 13, 421–423. [Google Scholar] [CrossRef]
- Shumo, M.; Khamis, F.M.; Tanga, C.M.; Fiaboe, K.K.M.; Subramanian, S.; Ekesi, S.; van Huis, A.; Borgemeister, C. Influence of Temperature on Selected Life-History Traits of Black Soldier Fly (Hermetia illucens) Reared on Two Common Urban Organic Waste Streams in Kenya. Animals (Basel) 2019, 9, 79. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Huang, L.; He, J.; Tomberlin, J.K.; Li, J.; Lei, C.; Sun, M.; Liu, Z.; Yu, Z. An artificial light source influences mating and oviposition of black soldier flies, Hermetia illucens. J. Insect Sci. 2010, 10, 202. [Google Scholar] [CrossRef] [PubMed]
- Heussler, C.D.; Walter, A.; Oberkofler, H.; Insam, H.; Arthofer, W.; Schlick-Steiner, B.C.; Steiner, F.M. Influence of three artificial light sources on oviposition and half-life of the Black Soldier Fly, Hermetia illucens (Diptera: Stratiomyidae): Improving small-scale indoor rearing. PLoS ONE 2018, 13, e0197896. [Google Scholar] [CrossRef]
- Holmes, L.A.; Vanlaerhoven, S.L.; Tomberlin, J.K. Substrate effects on pupation and adult emergence of Hermetia illucens (Diptera: Stratiomyidae). Environ. Entomol. 2013, 42, 370–374. [Google Scholar] [CrossRef]
- Yu, G.H.; Niu, C.Y.; He, B.G.; Zhou, L.; Xia, Q.; CHeng, P. Isolation and identification of bacteria producing enzymes from gut and skin of black soldier fly. Chin. Bull. Entomol. 2010, 47, 889–894. [Google Scholar]
- Zheng, L.; Crippen, T.L.; Singh, B.; Tarone, A.M.; Dowd, S.; Yu, Z.; Wood, T.K.; Tomberlin, J.K. A survey of bacterial diversity from successive life stages of black soldier fly (Diptera: Stratiomyidae) by using 16S rDNA pyrosequencing. J. Med. Entomol. 2013, 50, 647–658. [Google Scholar] [CrossRef]
- Toth-Prestia, C.; Hirshfield, I.N. Isolation of plasmid-harboring serratia plymuthica from facultative gut microflora of the tobacco hornworm, manduca sexta. Appl. Environ. Microb. 1988, 54, 1855–1857. [Google Scholar]
- Yoshiyama, M.; Kimura, K. Bacteria in the gut of Japanese honeybee, Apis cerana japonica, and their antagonistic effect against Paenibacillus larvae, the causal agent of American foulbrood. J. Invertebr. Pathol. 2009, 102, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Bradley, S.W.; Booth, D.C.; Sheppard, D.C. Parasitism of the black soldier fly by trichopria sp. (Hymenoptera: Diapriidae) in poultry houses. Environ. Entomol. 1984, 13, 451–454. [Google Scholar] [CrossRef]
- Varotto Boccazzi, I.; Ottoboni, M.; Martin, E.; Comandatore, F.; Vallone, L.; Spranghers, T.; Eeckhout, M.; Mereghetti, V.; Pinotti, L.; Epis, S. A survey of the mycobiota associated with larvae of the black soldier fly (Hermetia illucens) reared for feed production. PLoS ONE 2017, 12, e0182533. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.; Park, S.; Choi, J.; Jeong, G.; Lee, S.B.; Choi, Y.; Lee, S.J. The intestinal bacterial community in the food waste-reducing larvae of Hermetia illucens. Curr. Microbiol. 2011, 62, 1390–1399. [Google Scholar] [CrossRef]
- De Smet, J.; Wynants, E.; Cos, P.; Van Campenhout, L. Microbial Community Dynamics during Rearing of Black Soldier Fly Larvae (Hermetia illucens) and Impact on Exploitation Potential. Appl. Environ. Microbiol. 2018, 84. [Google Scholar] [CrossRef]
- Lee, Y.S.; Seo, S.H.; Yoon, S.H.; Kim, S.Y.; Hahn, B.S.; Sim, J.S.; Koo, B.S.; Lee, C.M. Identification of a novel alkaline amylopullulanase from a gut metagenome of Hermetia illucens. Int. J. Biol. Macromol. 2016, 82, 514–521. [Google Scholar] [CrossRef]
- Lee, C.M.; Lee, Y.S.; Seo, S.H.; Yoon, S.H.; Kim, S.J.; Hahn, B.S.; Sim, J.S.; Koo, B.S. Screening and characterization of a novel cellulase gene from the gut microflora of Hermetia illucens using metagenomic library. J. Microbiol. Biotechnol. 2014, 24, 1196–1206. [Google Scholar] [CrossRef]
- Park, D.S.; Oh, H.W.; Jeong, W.J.; Kim, H.; Park, H.Y.; Bae, K.S. A culture-based study of the bacterial communities within the guts of nine longicorn beetle species and their exo-enzyme producing properties for degrading xylan and pectin. J. Microbiol. 2007, 45, 394–401. [Google Scholar]
- Warnecke, F.; Luginbuhl, P.; Ivanova, N.; Ghassemian, M.; Richardson, T.H.; Stege, J.T.; Cayouette, M.; McHardy, A.C.; Djordjevic, G.; Aboushadi, N.; et al. Metagenomic and functional analysis of hindgut microbiota of a wood-feeding higher termite. Nature 2007, 450, 560–565. [Google Scholar] [CrossRef] [PubMed]
- Tomberlin, J.K.; Sheppard, D.C.; Joyce, J.A. Selected life-history traits of black soldier flies (Diptera: Stratiomyidae) reared on three artificial diets. Ann. Entomol. Soc. Am. 2002, 95, 379–386. [Google Scholar] [CrossRef]
- Xu, Q.Y.; Long, J.C.; Ye, M.Q.; An, X.C.; Han, S.C. Development rate and food conversion efficiency of black soldier fly. J. Environ. Entomol. 2014, 36, 561–564. [Google Scholar]
- DeFoliart, G.R. Edible insects as minilivestock. Biodivers. Conserv. 1995, 4, 306–321. [Google Scholar] [CrossRef]
- Veldkamp, T.; Bosch, G. Insects: A protein-rich feed ingredient in pig and poultry diets. Anim. Front. 2015, 5, 45–50. [Google Scholar] [CrossRef]
- Salomone, R.; Saija, G.; Mondello, G.; Giannetto, A.; Fasulo, S.; Savastano, D. Environmental impact of food waste bioconversion by insects: Application of Life Cycle Assessment to process using Hermetia illucens. J. Clean. Prod. 2017, 140, 890–905. [Google Scholar] [CrossRef]
- Popa, R.; Green, T.R. Using black soldier fly larvae for processing organic leachates. J. Econ. Entomol. 2012, 105, 374–378. [Google Scholar] [CrossRef] [PubMed]
- Myers, H.M.; Tomberlin, J.K.; Lambert, B.D.; Kattes, D. Development of black soldier fly (Diptera: Stratiomyidae) larvae fed dairy manure. Environ. Entomol. 2008, 37, 11–15. [Google Scholar] [CrossRef]
- Kim, W.; Bae, S.; Park, K.; Lee, S.; Choi, Y.; Han, S.; Koh, Y. Biochemical characterization of digestive enzymes in the black soldier fly, Hermetia illucens (Diptera: Stratiomyidae). J. Asia Pac. Entomol. 2011, 14, 11–14. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Tomberlin, J.K.; Vanlaerhoven, S. Ability of Black Soldier Fly (Diptera: Stratiomyidae) Larvae to Recycle Food Waste. Environ. Entomol. 2015, 44, 406–410. [Google Scholar] [CrossRef]
- Bruno, D.; Bonelli, M.; De Filippis, F.; Di Lelio, I.; Tettamanti, G.; Casartelli, M.; Ercolini, D.; Caccia, S. The Intestinal Microbiota of Hermetia illucens Larvae Is Affected by Diet and Shows a Diverse Composition in the Different Midgut Regions. Appl. Environ. Microbiol. 2019, 85, e01864-18. [Google Scholar] [CrossRef] [PubMed]
- Bonelli, M.; Bruno, D.; Caccia, S.; Sgambetterra, G.; Cappellozza, S.; Jucker, C.; Tettamanti, G.; Casartelli, M. Structural and Functional Characterization of Hermetia illucens Larval Midgut. Front. Physiol. 2019, 10, 204. [Google Scholar] [CrossRef]
- Bertinetti, C.; Samayoa, A.C.; Hwang, S.Y. Effects of Feeding Adults of Hermetia illucens (Diptera: Stratiomyidae) on Longevity, Oviposition, and Egg Hatchability: Insights into Optimizing Egg Production. J. Insect Sci. 2019, 19. [Google Scholar] [CrossRef] [PubMed]
- Wynants, E.; Frooninckx, L.; Van Miert, S.; Geeraerd, A.; Claes, J.; Van Campenhout, L. Risks related to the presence of Salmonella sp. during rearing of mealworms (Tenebrio molitor) for food or feed: Survival in the substrate and transmission to the larvae. Food Control 2019, 100, 227–234. [Google Scholar] [CrossRef]
- Defoliart, G.R. Insect fatty acids: Similar to those of poultry and fish in their degree of unsaturation, but higher in the polyunsaturates. Food Insects News Lett. 1991, 4, 1–4. [Google Scholar]
- Barragan-Fonseca, K.B.; Dicke, M.; Loon, J.J.A.V. Nutritional value of the black soldier fly (Hermetia illucens L.) and its suitability as animal feed. J. Insects Food Feed 2017, 3, 105–120. [Google Scholar] [CrossRef]
- Schiavone, A.; Marco, M.D.; Martínez, S.; Dabbou, S.; Renna, M.; Madrid, J.; Hernandez, F.; Rotolo, L.; Costa, P.; Gai, F. Nutritional value of a partially defatted and a highly defatted black soldier fly larvae (Hermetia illucens L.) meal for broiler chickens: Apparent nutrient digestibility, apparent metabolizable energy and apparent ileal amino acid digestibility. J. Anim. Sci. Biotechnol. 2017, 8, 51. [Google Scholar] [CrossRef]
- St-Hilaire, S.; Sheppard, C.; Tomberlin, J.K.; Irving, S.; Newton, L.; Mcguire, M.A.; Mosley, E.E.; Hardy, R.W.; Sealey, W. Fly Prepupae as a Feedstuff for Rainbow Trout, Oncorhynchus mykiss. J. World Aquac. Soc. 2007, 38, 59–67. [Google Scholar] [CrossRef]
- Nguyen, H.C.; Liang, S.H.; Doan, T.T.; Su, C.H.; Yang, P.C. Lipase-catalyzed synthesis of biodiesel from black soldier fly (Hermetica illucens): Optimization by using response surface methodology. Energy Convers. Manag. 2017, 145, 335–342. [Google Scholar] [CrossRef]
- Wang, C.; Qian, L.; Wang, W.; Wang, T.; Deng, Z.; Yang, F.; Xiong, J.; Feng, W. Exploring the potential of lipids from black soldier fly: New paradigm for biodiesel production (I). Renew. Energy 2017, 111, 749–756. [Google Scholar] [CrossRef]
- Sheppard, C. House fly and lesser fly control utilizing the black soldier fly in manure management systems for caged laying hens. Environ. Entomol. 1983, 12, 1439–1442. [Google Scholar] [CrossRef]
- Newton, G.L.; Booram, C.V.; Barker, R.W.; Hale, O.M. Dried Hermetia illucens larvae meal as a supplement for swine. J. Anim. Sci. 1977, 44, 395–400. [Google Scholar] [CrossRef]
- Wang, Y.P.; Liu, J.; Wu, Y.M.; Liu, L.E.; Lv, Q.J.; Wu, Y.J. Analysis of nutrition composition on silkworm pupa. J. Zhengzhou Univ. (Med. Sci.) 2009, 44, 638–641. [Google Scholar]
- Yu, G.H.; Chen, Y.H.; Yu, Z.N.; Cheng, P. Research progression on the larvae and prepupae of black soldier fly Hermetia illucens used as animal. Chin. Bull. Entomol. 2009, 46, 41–45. [Google Scholar]
- Yang, Z.; Lin, Y.; Chen, Y.; Wu, X. Nutritional components of the larvae of Tenebrio molitor L. and its control. Kun Chong Zhi Shi 1999, 36, 97–100. [Google Scholar]
- Fu, G.Y. Experiment on growing-finishing pigs fed on earthworm meal instead of fish meal. Hunan J. Anim. Sci. Vet. Med. 2006, 11–12. [Google Scholar] [CrossRef]
- Wu, J.W.; Chen, M.; Peng, W.F. Study on the nutritional value of the housefly larva fed with pig manure. J. Guiyang Med. Coll. 2001, 26, 377–379. [Google Scholar]
- Caligiani, A.; Marseglia, A.; Leni, G.; Baldassarre, S.; Maistrello, L.; Dossena, A.; Sforza, S. Composition of black soldier fly prepupae and systematic approaches for extraction and fractionation of proteins, lipids and chitin. Food Res. Int. 2018, 105, 812–820. [Google Scholar] [CrossRef]
- De Marco, M.; Martínez, S.; Hernandez, F.; Madrid, J.; Gai, F.; Rotolo, L.; Belforti, M.; Bergero, D.; Katz, H.; Dabbou, S.; et al. Nutritional value of two insect larval meals (Tenebrio molitor and Hermetia illucens) for broiler chickens: Apparent nutrient digestibility, apparent ileal amino acid digestibility and apparent metabolizable energy. Anim. Feed Sci. Technol. 2015, 209, 211–218. [Google Scholar] [CrossRef]
- Dalle Zotte, A.; Singh, Y.; Michiels, J.; Cullere, M. Black Soldier Fly (Hermetia Illucens) as Dietary Source for Laying Quails: Live Performance, and Egg Physico-Chemical Quality, Sensory Profile and Storage Stability. Animals 2019, 9, 115. [Google Scholar] [CrossRef]
- Kawasaki, K.; Hashimoto, Y.; Hori, A.; Kawasaki, T.; Hirayasu, H.; Iwase, S.-I.; Hashizume, A.; Ido, A.; Miura, C.; Miura, T.; et al. Evaluation of Black Soldier Fly (Hermetia illucens) Larvae and Pre-Pupae Raised on Household Organic Waste, as Potential Ingredients for Poultry Feed. Animals 2019, 9, 98. [Google Scholar] [CrossRef] [PubMed]
- Mwaniki, Z.; Neijat, M.; Kiarie, E. Egg production and quality responses of adding up to 7.5% defatted black soldier fly larvae meal in a corn-soybean meal diet fed to Shaver White Leghorns from wk 19 to 27 of age. Poult. Sci. 2018, 97, 2829–2835. [Google Scholar] [CrossRef]
- Wardhana, A.H. Black Soldier Fly (Hermetia illucens) as an Alternative Protein Source for Animal Feed. WARTAZOA. Indones. Bull. Anim. Vet. Sci. 2016, 26, 069–078. [Google Scholar] [CrossRef]
- Lee, J.; Kim, Y.M.; Park, Y.K.; Yang, Y.C.; Jung, B.G.; Lee, B.J. Black soldier fly (Hermetia illucens) larvae enhances immune activities and increases survivability of broiler chicks against experimental infection of Salmonella Gallinarum. J. Vet. Med. Sci. 2018, 80, 736–740. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Feng, W.; Xiong, J.; Wang, T.; Wang, W.; Wang, C.; Yang, F. Impact of drying method on the nutritional value of the edible insect protein from black soldier fly (Hermetia illucens L.) larvae: Amino acid composition, nutritional value evaluation, in vitro digestibility, and thermal properties. Eur. Food Res. Technol. 2019, 245, 11–21. [Google Scholar] [CrossRef]
- Leong, S.Y.; Kutty, S.R.; Malakahmad, A.; Tan, C.K. Feasibility study of biodiesel production using lipids of Hermetia illucens larva fed with organic waste. Waste Manag. 2016, 47, 84–90. [Google Scholar] [CrossRef]
- St-Hilaire, S.; Cranfill, K.; McGuire, M.A.; Mosley, E.E.; Tomberlin, J.K.; Newton, L.; Sealey, W.; Sheppard, C.; Irving, S. Fish Offal Recycling by the Black Soldier Fly Produces a Foodstuff High in Omega-3 Fatty Acids. J. World Aquac. Soc. 2007, 38, 309–313. [Google Scholar] [CrossRef]
- Henry, M.; Gasco, L.; Piccolo, G.; Fountoulaki, E. Review on the use of insects in the diet of farmed fish: Past and future. Animal Feed Sci. Technol. 2015, 203, 1–22. [Google Scholar] [CrossRef]
- Li, Q.; Zheng, L.; Cai, H.; Garza, E.; Yu, Z.; Zhou, S. From organic waste to biodiesel: Black soldier fly, Hermetia illucens, makes it feasible. Fuel 2011, 90, 1545–1548. [Google Scholar] [CrossRef]
- Canakci, M.; Sanli, H. Biodiesel production from various feedstocks and their effects on the fuel properties. J. Ind. Microbiol. Biotechnol. 2008, 35, 431–441. [Google Scholar] [CrossRef]
- Li, W.; Zheng, L.Y.; Li, Q.; Liu, X.L.; Li, M.S.; Zhang, Y.L.; Zhang, J.B.; Yu, Z.N. Conversion process and resource utilization of restaurant waste by black soldier fly. ChemBioEng Rev. 2014. [Google Scholar] [CrossRef]
- Sealey, W.M.; Gaylord, T.G.; Barrows, F.T.; Tomberlin, J.K.; McGuire, M.A.; Ross, C.; St-Hilaire, S. Sensory Analysis of Rainbow Trout, Oncorhynchus mykiss, Fed Enriched Black Soldier Fly Prepupae, Hermetia illucens. J. World Aquac. Soc. 2011, 42, 34–45. [Google Scholar] [CrossRef]
- Teotia, J.S. Fly pupae as a dietary ingredient for starting chicks. Poult. Sci. 1973, 52, 1830–1835. [Google Scholar] [CrossRef]
- Schiavone, A.; Cullere, M.; De Marco, M.; Meneguz, M.; Biasato, I.; Bergagna, S.; Dezzutto, D.; Gai, F.; Dabbou, S.; Gasco, L.; et al. Partial or total replacement of soybean oil by black soldier fly larvae (Hermetia illucens L.) fat in broiler diets: Effect on growth performances, feed-choice, blood traits, carcass characteristics and meat quality. Ital. J. Anim. Sci. 2017, 16, 93–100. [Google Scholar] [CrossRef]
- Schiavone, A.; Dabbou, S.; De Marco, M.; Cullere, M.; Biasato, I.; Biasibetti, E.; Capucchio, M.T.; Bergagna, S.; Dezzutto, D.; Meneguz, M.; et al. Black soldier fly larva fat inclusion in finisher broiler chicken diet as an alternative fat source. Anim. Int. J. Anim. Biosci. 2018, 12, 2032–2039. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Ji, H.; Zhang, B.; Tian, J.; Zhou, J.; Yu, H. Influence of black soldier fly (Hermetia illucens) larvae oil on growth performance, body composition, tissue fatty acid composition and lipid deposition in juvenile Jian carp (Cyprinus carpio var. Jian). Aquaculture 2016, 465, 43–52. [Google Scholar] [CrossRef]
- Li, W.; Li, M.; Zheng, L.; Liu, Y.; Zhang, Y.; Yu, Z.; Ma, Z.; Li, Q. Simultaneous utilization of glucose and xylose for lipid accumulation in black soldier fly. Biotechnol. Biofuels 2015, 8, 117. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, N.; Wang, J.; Yao, H.; Qiu, Q.; Chapman, S.J. High turnover rate of free phospholipids in soil confirms the classic hypothesis of PLFA methodology. Soil Biol. Biochem. 2019, 135, 323–330. [Google Scholar] [CrossRef]
- Wang, H.; Rehman, K.U.; Liu, X.; Yang, Q.; Zheng, L.; Li, W.; Cai, M.; Li, Q.; Zhang, J.; Yu, Z. Insect biorefinery: A green approach for conversion of crop residues into biodiesel and protein. Biotechnol. Biofuels 2017, 10, 304. [Google Scholar] [CrossRef]
- Xia, Q.; Zhao, Q.F.; Liao, Y.; Zhu, W.; Guo-Hui, Y.U.; Chen, Y.F.; Song, M.Y. Black soldier fly antimicrobial peptides induced conditions optimization and research of crude extracts activity. J. Environ. Entomol. 2013, 35, 44–48. [Google Scholar] [CrossRef]
- Elhag, O.; Zhou, D.; Song, Q.; Soomro, A.A.; Cai, M.; Zheng, L.; Yu, Z.; Zhang, J. Screening, Expression, Purification and Functional Characterization of Novel Antimicrobial Peptide Genes from Hermetia illucens (L.). PLoS ONE 2017, 12, e0169582. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.F. Preliminary Research on Functional Roles in Antimicrobial Peptides from Crude Extracting in Black Soldier Fly; Zunyi Medical College: Zunyi, China, 2012. [Google Scholar]
- Xia, Q.; Zhao, Q.F.; Liao, Y.; Zhu, W.; Yu, G.H.; Chen, Y.F. Biological stability observation of antibacterial peptides in black soldier fly. Shandong Med. J. 2013, 53, 91–92. [Google Scholar]
- Tharanathan, R.N.; Kittur, F.S. Chitin—The undisputed biomolecule of great potential. Crit. Rev. Food Sci. Nutr. 2003, 43, 61–87. [Google Scholar] [CrossRef] [PubMed]
- Gades, M.D.; Stern, J.S. Chitosan supplementation and fat absorption in men and women. J. Am. Diet. Assoc. 2005, 105, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Masuoka, K.; Ishihara, M.; Asazuma, T.; Hattori, H.; Matsui, T.; Takase, B.; Kanatani, Y.; Fujita, M.; Saito, Y.; Yura, H. The interaction of chitosan with fibroblast growth factor-2 and its protection from inactivation. Biomaterials 2005, 26, 3277–3284. [Google Scholar] [CrossRef] [PubMed]
- Nemtsev, S.V.; Zueva, O.Y.; Khismatullin, M.R.; Albulov, A.I.; Varlamov, V.P. Isolation of chitin and chitosan from honey bees. Appl. Biochem. Microbiol. 2004, 40, 39–43. [Google Scholar] [CrossRef]
- Abdou, E.S.; Nagy, K.S.; Elsabee, M.Z. Extraction and characterization of chitin and chitosan from local sources. Bioresour. Technol. 2008, 99, 1359–1367. [Google Scholar] [CrossRef]
- Aranaz, I.; Mengibar, M.; Harris, R.; Panos, I.; Miralles, B.; Acosta, N.; Galed, G.; Heras, A. Functional Characterization of Chitin and Chitosan. Curr. Chem. Biol. 2009, 3, 203–230. [Google Scholar] [CrossRef]
- Gold, M.; Tomberlin, J.K.; Diener, S.; Zurbrugg, C.; Mathys, A. Decomposition of biowaste macronutrients, microbes, and chemicals in black soldier fly larval treatment: A review. Waste Manag. 2018, 82, 302–318. [Google Scholar] [CrossRef]
- Lohri, C.R.; Diener, S.; Zabaleta, I.; Mertenat, A.; Zurbrügg, C. Treatment technologies for urban solid biowaste to create value products: A review with focus on low- and middle-income settings. Rev. Environ. Sci. Bio/Technol. 2017, 16, 81–130. [Google Scholar] [CrossRef]
- Zurbrügg, C.; Dortmans, B.; Fadhila, A.; Vertsappen, B.; Diener, S. From pilot to full scale operation of a waste-to-protein treatment facility. Detritus 2018, 1, 18–22. [Google Scholar] [CrossRef]
- Čičková, H.; Newton, G.L.; Lacy, R.C.; Kozánek, M. The use of fly larvae for organic waste treatment. Waste Manag. 2015, 35, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Chia, S.Y.; Tanga, C.M.; Khamis, F.M.; Mohamed, S.A.; Salifu, D.; Sevgan, S.; Fiaboe, K.K.M.; Niassy, S.; van Loon, J.J.A.; Dicke, M.; et al. Threshold temperatures and thermal requirements of black soldier fly Hermetia illucens: Implications for mass production. PLoS ONE 2018, 13, e0206097. [Google Scholar] [CrossRef] [PubMed]
- Smetana, S.; Schmitt, E.; Mathys, A. Sustainable use of Hermetia illucens insect biomass for feed and food: Attributional and consequential life cycle assessment. Resour. Conserv. Recycl. 2019, 144, 285–296. [Google Scholar] [CrossRef]
- Lohri, C.R.; Rajabu, H.M.; Sweeney, D.J.; Zurbrügg, C. Char fuel production in developing countries—A review of urban biowaste carbonization. Renewable and Sustainable Energy Reviews 2016, 59, 1514–1530. [Google Scholar] [CrossRef]
- Hu, J.R.; He, F.; Mo, W.Y.; Chen, X.Y.; Huang, Y.H.; Wang, G.X.; Sun, Y.P. The feeding value of black soldier fly Hermetia illucens larvae for feeding different organic wastes. China Feed 2017, 24–27. [Google Scholar] [CrossRef]
- Ali, N.; Khan, S.; Yao, H.; Wang, J. Biochars reduced the bioaccessibility and (bio)uptake of organochlorine pesticides and changed the microbial community dynamics in agricultural soils. Chemosphere 2019, 224, 805–815. [Google Scholar] [CrossRef]
- Li, Y.; Liao, H.; Yao, H. Prevalence of Antibiotic Resistance Genes in Air-Conditioning Systems in Hospitals, Farms, and Residences. Int. J. Environ. Res. Public Health 2019, 16, 683. [Google Scholar] [CrossRef]
- Shen, X.L. Characterization and Comparison Study on Energy and Fertilizer Related Properties of Animal Manure in China; China Agricultural University: Beijing, China, 2016. [Google Scholar]
- Hale, O.M. Dried Hermetia illucens larvae (Diptera: Stratiomyidae) as a feed additive for poultry. Ga Entomol. Soc. J. 1973, 8, 16–20. [Google Scholar]
- Newton, L.; Sheppard, C.; Watson, D.W.; Burtle, G.; Dove, R. Using the black soldier fly, Hermetia illucens, as a value-added tool for the management of swine manure. Anim. Poult. Waste Manag. Cent. North. Carol. State Univ. Raleigh NC 2005, 17. [Google Scholar]
- Newton, G.; Sheppard, D.; Watson, D.; Burtle, G.; Dove, C.; Tomberlin, J.; Thelen, E. The black soldier fly, Hermetia illucens, as a manure management/resource recovery tool. In Proceedings of the Symposium on the State of the Science of Animal Manure and Waste Management, San Antonio, TX, USA, 5–7 January 2005. [Google Scholar]
- Nyakeri, E.M.; Ogola, H.J.; Ayieko, M.A.; Amimo, F.A. An open system for farming black soldier fly larvae as a source of proteins for smallscale poultry and fish production. J. Insects Food Feed 2016, 3, 51–56. [Google Scholar] [CrossRef]
- Rehman, K.U.; Rehman, A.; Cai, M.; Zheng, L.; Xiao, X.; Somroo, A.A.; Wang, H.; Li, W.; Yu, Z.; Zhang, J. Conversion of mixtures of dairy manure and soybean curd residue by black soldier fly larvae (Hermetia illucens L.). J. Clean. Prod. 2017, 154, 366–373. [Google Scholar] [CrossRef]
- Oonincx, D.G.A.B.; Huis, A.v.; Loon, J.J.A.v. Nutrient utilisation by black soldier flies fed with chicken, pig, or cow manure. J. Insects Food Feed 2015, 1, 131–139. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Tomberlin, J.K.; Vanlaerhoven, S. Influence of resources on Hermetia illucens (Diptera: Stratiomyidae) larval development. J. Med. Entomol. 2013, 50, 898–906. [Google Scholar] [CrossRef] [PubMed]
- Nyakeri, E.M.; Ogola, H.J.O.; Ayieko, M.A.; Amimo, F.A. Valorisation of organic waste material: Growth performance of wild black soldier fly larvae (Hermetia illucens) reared on different organic wastes. J. Insects Food Feed 2017, 3, 193–202. [Google Scholar] [CrossRef]
- Banks, I.J.; Gibson, W.T.; Cameron, M.M. Growth rates of black soldier fly larvae fed on fresh human faeces and their implication for improving sanitation. Trop. Med. Int. Health 2014, 19, 14–22. [Google Scholar] [CrossRef]
- Dobermann, D.; Field, L.M.; Michaelson, L.V. Using Hermetia illucens to process Ugandan waragi waste. J. Clean. Prod. 2019, 211, 303–308. [Google Scholar] [CrossRef]
- An, X.C. Reliability Analysis about Technology for Using Black Soldier Fly on Bioconversion from Food Waste to Entomic Protein. Environ. Sustain. Dev. 2016, 41, 92–94. [Google Scholar]
- Tinder, A.C.; Puckett, R.T.; Turner, N.D.; Cammack, J.A.; Tomberlin, J.K. Bioconversion of sorghum and cowpea by black soldier fly (Hermetia illucens (L.)) larvae for alternative protein production. J. Insects Food Feed 2017, 3, 121–130. [Google Scholar] [CrossRef]
- Mohd-Noor, S.-N.; Wong, C.-Y.; Lim, J.-W.; Mah-Hussin, M.-I.-A.; Uemura, Y.; Lam, M.-K.; Ramli, A.; Bashir, M.J.K.; Tham, L. Optimization of self-fermented period of waste coconut endosperm destined to feed black soldier fly larvae in enhancing the lipid and protein yields. Renew. Energy 2017, 111, 646–654. [Google Scholar] [CrossRef]
- Green, T.R.; Popa, R. Enhanced Ammonia Content in Compost Leachate Processed by Black Soldier Fly Larvae. Appl. Biochem. Biotechnol. 2012, 166, 1381–1387. [Google Scholar] [CrossRef] [PubMed]
- Tschirner, M.; Simon, A. Influence of different growing substrates and processing on the nutrient composition of black soldier fly larvae destined for animal feed. J. Insects Food Feed 2015, 1, 249–259. [Google Scholar] [CrossRef]
- Wang, Y.S.; Shelomi, M. Review of Black Soldier Fly (Hermetia illucens) as Animal Feed and Human Food. Foods (Basel, Switzerland) 2017, 6, 91. [Google Scholar] [CrossRef] [PubMed]
- Lalander, C.; Senecal, J.; Gros Calvo, M.; Ahrens, L.; Josefsson, S.; Wiberg, K.; Vinneras, B. Fate of pharmaceuticals and pesticides in fly larvae composting. Sci. Total Environ. 2016, 565, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Van der Fels-Klerx, H.J.; Camenzuli, L.; van der Lee, M.K.; Oonincx, D.G. Uptake of Cadmium, Lead and Arsenic by Tenebrio molitor and Hermetia illucens from Contaminated Substrates. PLoS ONE 2016, 11, e0166186. [Google Scholar] [CrossRef] [PubMed]
- Purschke, B.; Scheibelberger, R.; Axmann, S.; Adler, A.; Jager, H. Impact of substrate contamination with mycotoxins, heavy metals and pesticides on the growth performance and composition of black soldier fly larvae (Hermetia illucens) for use in the feed and food value chain. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2017, 34, 1410–1420. [Google Scholar] [CrossRef] [PubMed]
- Skaldina, O.; Peräniemi, S.; Sorvari, J. Ants and their nests as indicators for industrial heavy metal contamination. Environ. Pollut. 2018, 240, 574–581. [Google Scholar] [CrossRef]
- Biancarosa, I.; Liland, N.S.; Biemans, D.; Araujo, P.; Bruckner, C.G.; Waagbø, R.; Torstensen, B.E.; Lock, E.-J.; Amlund, H. Uptake of heavy metals and arsenic in black soldier fly (Hermetia illucens) larvae grown on seaweed-enriched media. J. Sci. Food Agric. 2018, 98, 2176–2183. [Google Scholar] [CrossRef] [PubMed]
- Lalander, C.H.; Hill, G.B.; Vinnerås, B. Hygienic quality of faeces treated in urine diverting vermicomposting toilets. Waste Manag. 2013, 33, 2204–2210. [Google Scholar] [CrossRef]
- Lalander, C.H.; Fidjeland, J.; Diener, S.; Eriksson, S.; Vinnerås, B. High waste-to-biomass conversion and efficient Salmonella spp. reduction using black soldier fly for waste recycling. Agron. Sustain. Dev. 2015, 35, 261–271. [Google Scholar] [CrossRef]
- Charlton, A.J.; Dickinson, M.; Wakefield, M.E.; Fitches, E.; Kenis, M.; Han, R.; Zhu, F.; Kone, N.; Grant, M.; Devic, E.; et al. Exploring the chemical safety of fly larvae as a source of protein for animal feed. J. Insects Food Feed 2015, 1, 7–16. [Google Scholar] [CrossRef]
- Canteri de Souza, P.; Custódio Caloni, C.; Wilson, D.; Sergio Almeida, R. An Invertebrate Host to Study Fungal Infections, Mycotoxins and Antifungal Drugs: Tenebrio molitor. J. Fungi 2018, 4, 125. [Google Scholar] [CrossRef] [PubMed]
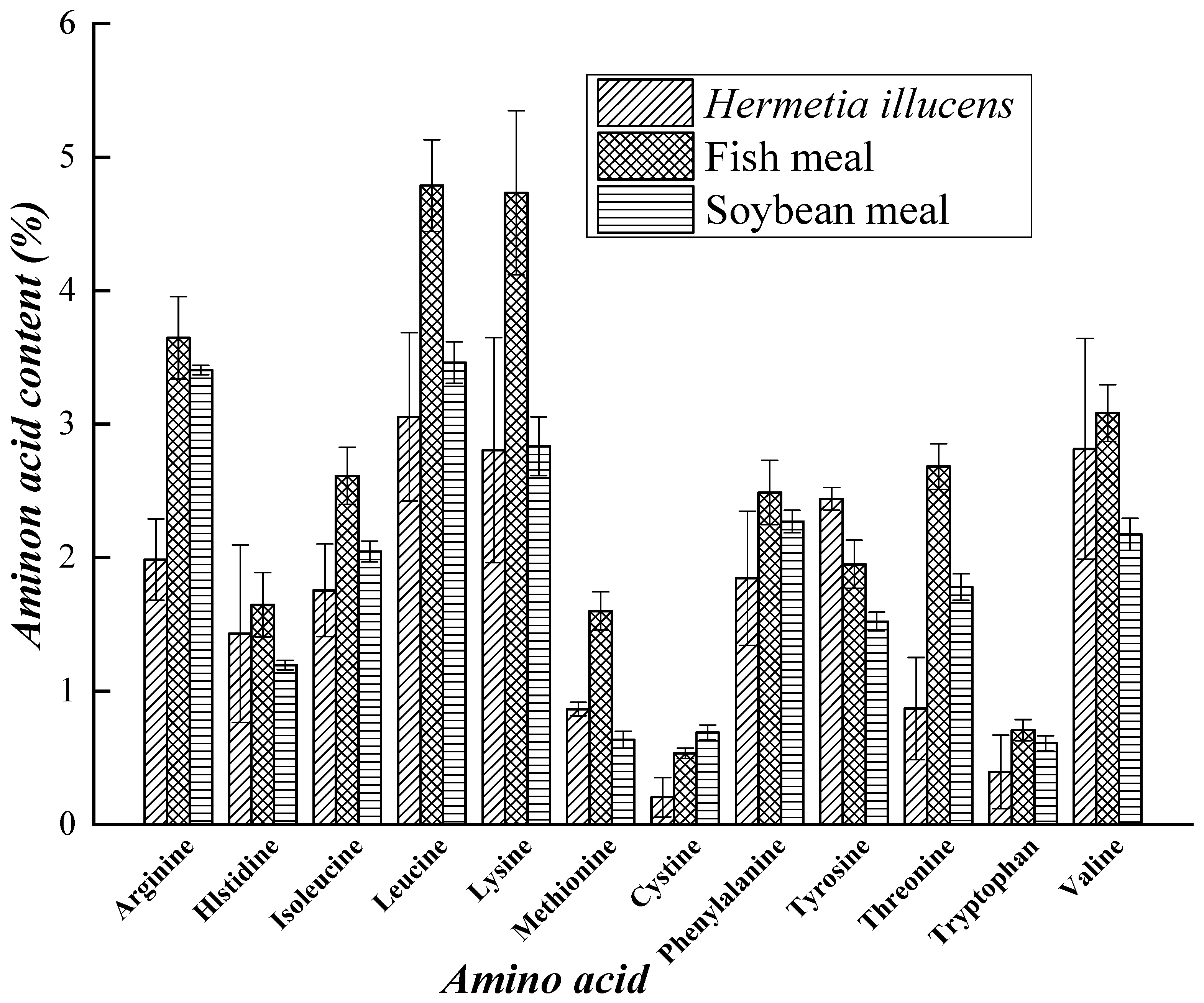
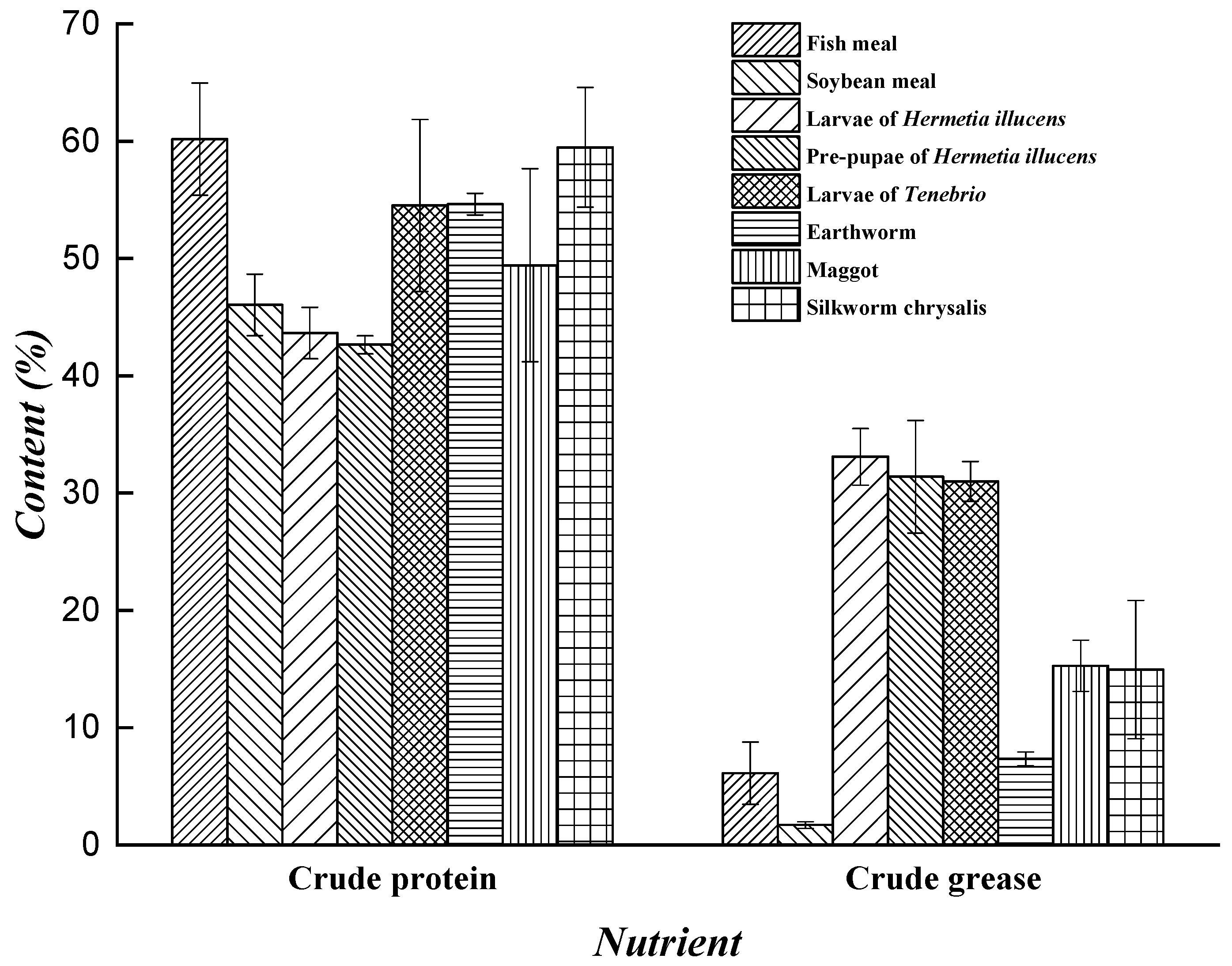
| Substrate | Conversion Rate of Substrate (%) | Output (%) | Survival Rate (%) | Larvae Composition (%) | References |
|---|---|---|---|---|---|
| Poultry feed | 23–70 | 1.4–16 | 40–93 | P: ~40, G: ~35 | [12,13,14] |
| Kitchen waste | ~60 | NA | NA | P: ~40, G: ~35 | [15,16] |
| Livestock waste | 25–53 | 8–22 | 71–99 | P: ~40, G: ~30 | [17,18] |
| Domestic or municipal organic wastes | 39–79 | ~14.5 | NA | NA | [9,19,20] |
| Agro-industry by-products | 6.3–27 | 4–6 | NA | P: ~30, G: ~28 | [21,22,23,24] |
| Crop straw | 9–68 | 5–10 | 51–98 | NA | [25] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Wang, C.; Yao, H. Comprehensive Resource Utilization of Waste Using the Black Soldier Fly (Hermetia illucens (L.)) (Diptera: Stratiomyidae). Animals 2019, 9, 349. https://doi.org/10.3390/ani9060349
Liu C, Wang C, Yao H. Comprehensive Resource Utilization of Waste Using the Black Soldier Fly (Hermetia illucens (L.)) (Diptera: Stratiomyidae). Animals. 2019; 9(6):349. https://doi.org/10.3390/ani9060349
Chicago/Turabian StyleLiu, Cuncheng, Cunwen Wang, and Huaiying Yao. 2019. "Comprehensive Resource Utilization of Waste Using the Black Soldier Fly (Hermetia illucens (L.)) (Diptera: Stratiomyidae)" Animals 9, no. 6: 349. https://doi.org/10.3390/ani9060349
APA StyleLiu, C., Wang, C., & Yao, H. (2019). Comprehensive Resource Utilization of Waste Using the Black Soldier Fly (Hermetia illucens (L.)) (Diptera: Stratiomyidae). Animals, 9(6), 349. https://doi.org/10.3390/ani9060349





