Genomic Analysis Reveals Specific Patterns of Homozygosity and Heterozygosity in Inbred Pigs
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods and Materials
2.1. Ethics Statement
2.2. Animals
2.3. DNA Extraction and Genotyping
2.4. Data Management and Quality Control
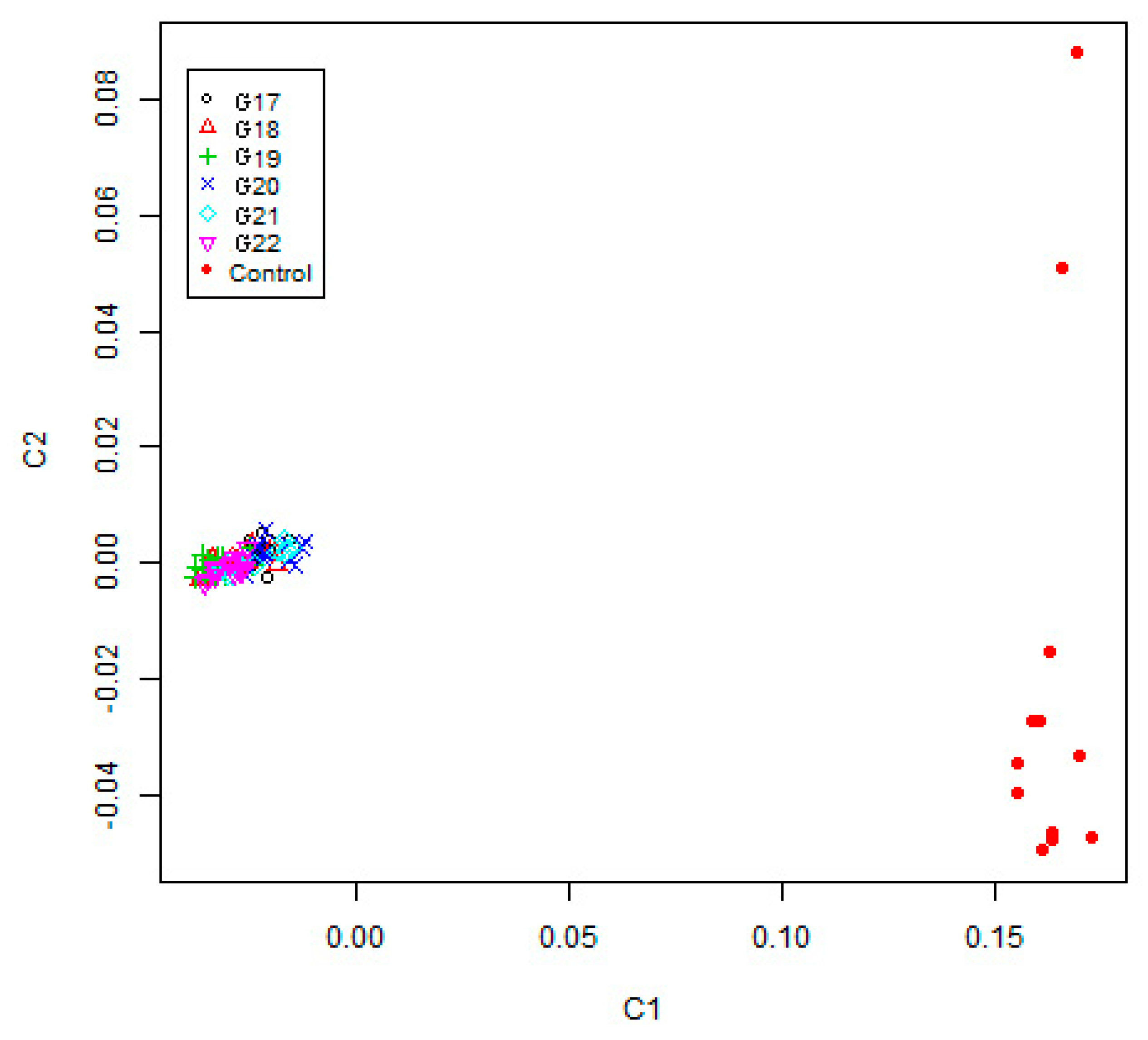
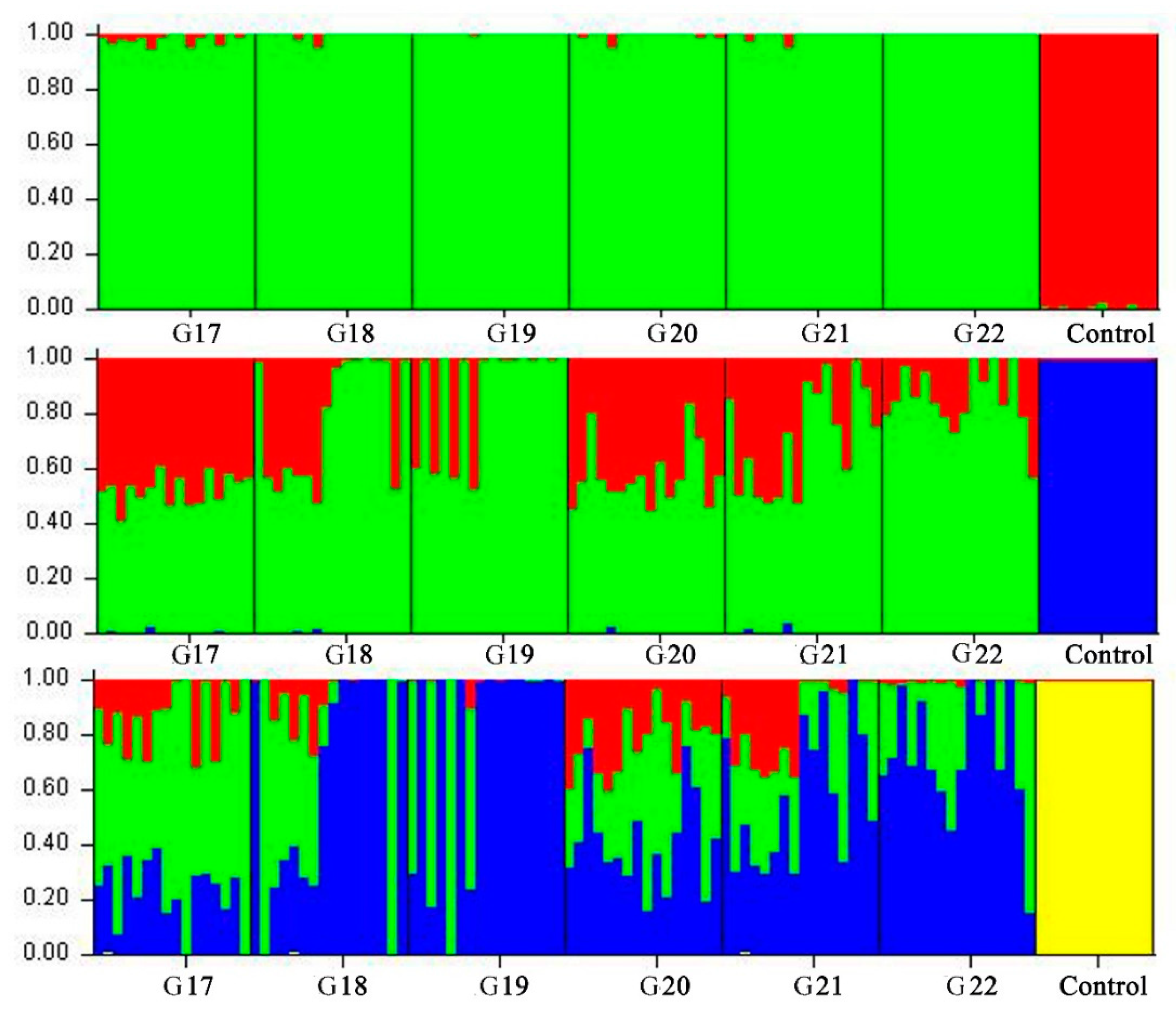
2.5. Genetic Diversity, Multidimensional Scaling and Admixture Analyses
2.6. Runs of Homozygosity Analysis and the Changes of Heterozygosity
2.7. Gene Ontology and Pathway Analysis of Genes with He=1 SNPs
3. Results
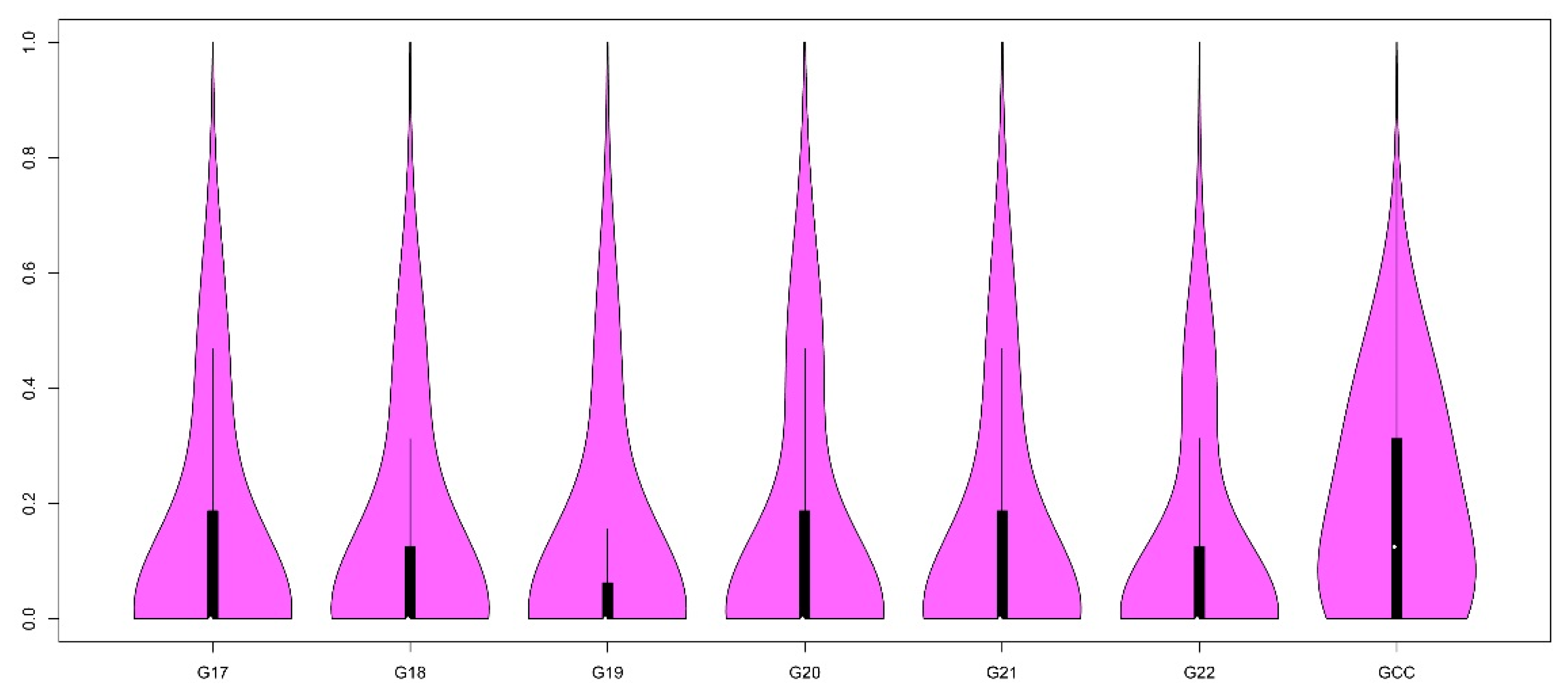
3.1. Estimation of Heterozygosity and Inbreeding Coefficient
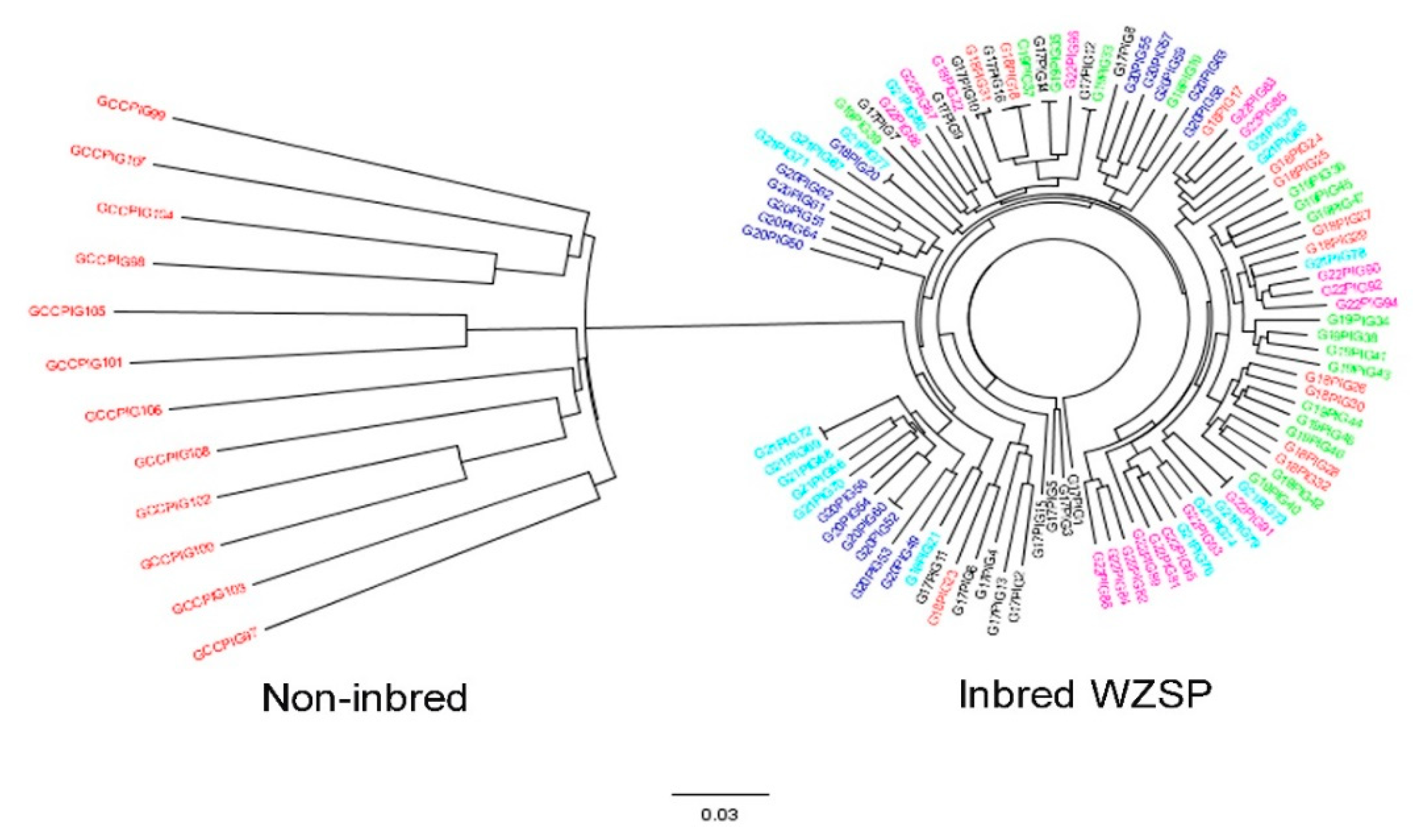
3.2. Genetic Structure Estimation for the Inbred WZSP
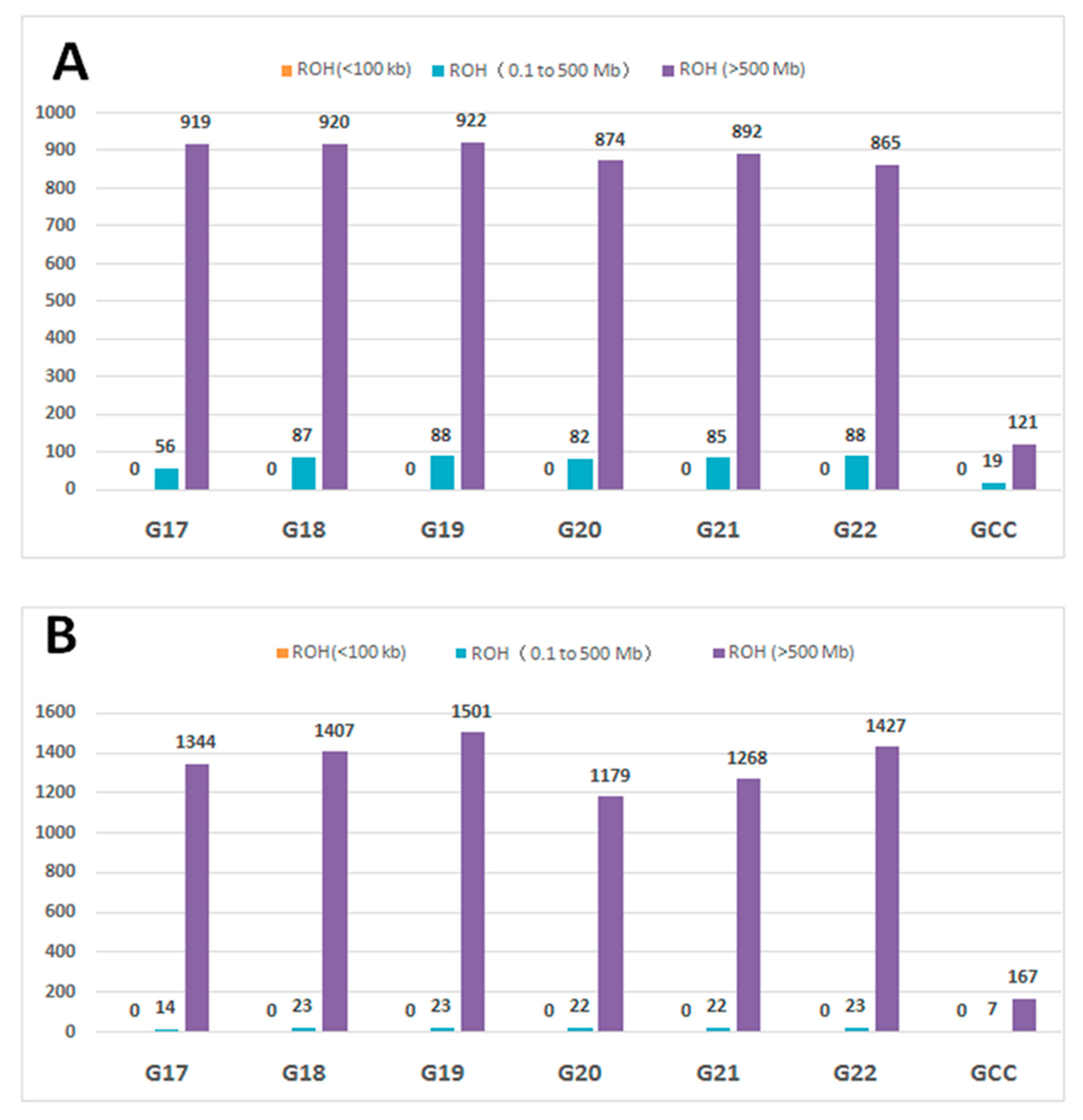
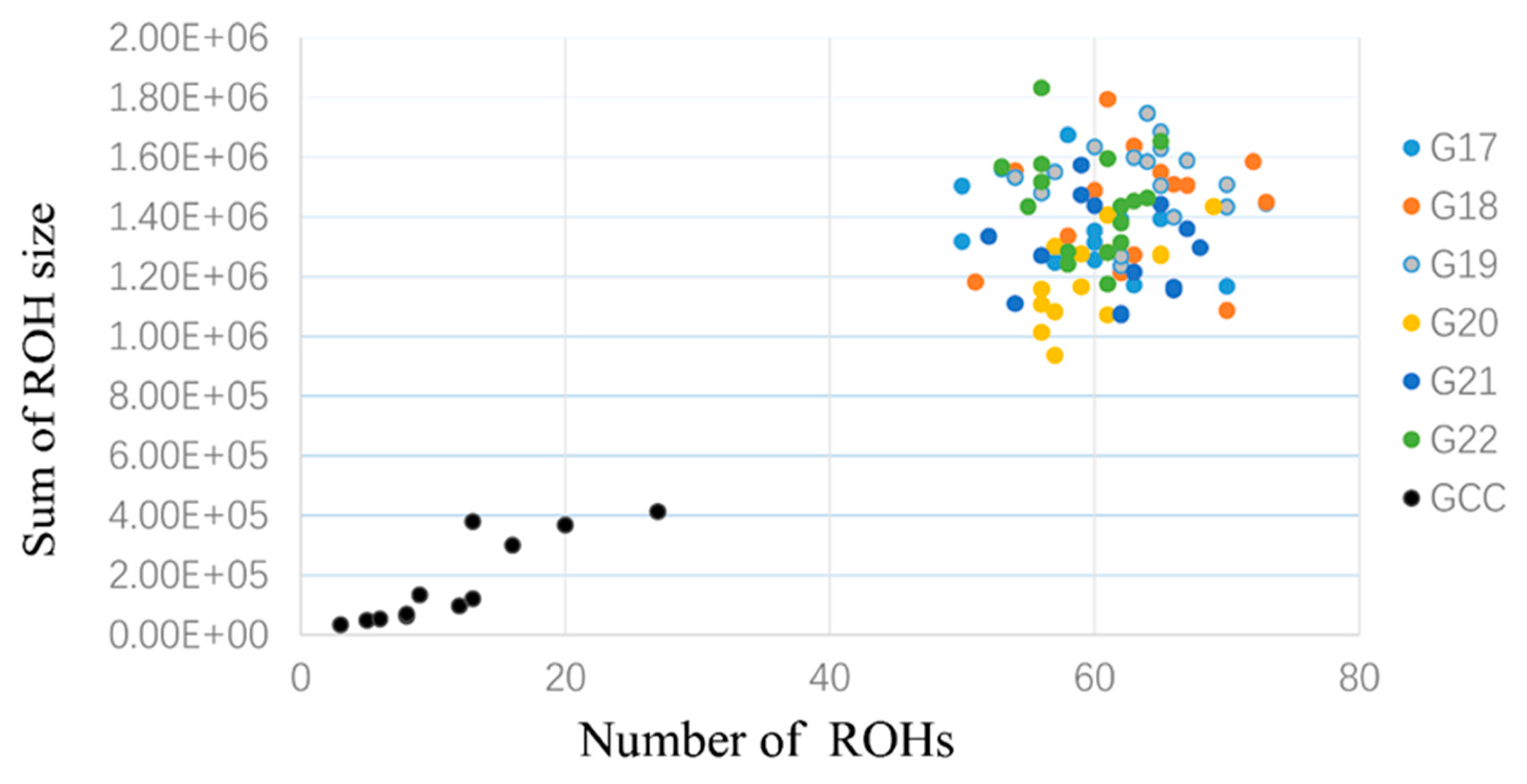
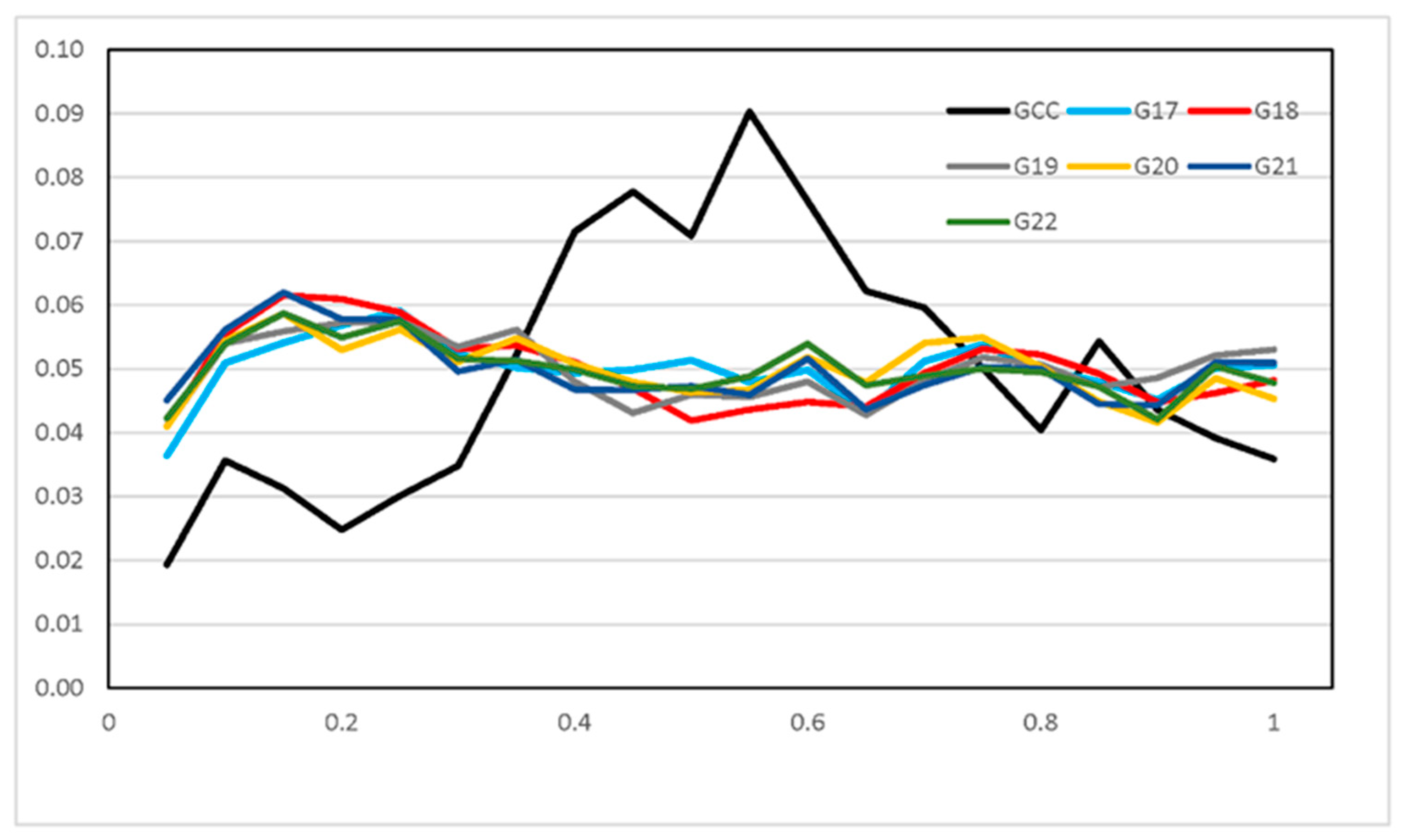
3.3. Runs of Homozygosity and ROH Regions across Generations
3.4. ROH Number, Length, Distribution, and Their Implications
3.5. Functional Gene related to the Change of Heterozygosity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Charlesworth, D.; Willis, J.H. The genetics of inbreeding depression. Nat. Rev. Microbiol. 2009, 10, 783–796. [Google Scholar] [CrossRef] [PubMed]
- Leroy, G. Inbreeding depression in livestock species: Review and meta-analysis. Anim. Genet. 2014, 45, 618–628. [Google Scholar] [CrossRef] [PubMed]
- Howrigan, D.P.; Simonson, M.A.; Keller, M.C. Detecting autozygosity through runs of homozygosity: A comparison of three autozygosity detection algorithms. BMC Genom. 2011, 12, 460. [Google Scholar] [CrossRef]
- Gibson, J.; Morton, N.E.; Collins, A. Extended tracts of homozygosity in outbred human populations. Hum. Mol. Genet. 2006, 15, 789–795. [Google Scholar] [CrossRef]
- Kim, E.-S.; Sonstegard, T.S.; Van Tassell, C.P.; Wiggans, G.; Rothschild, M.F. The Relationship between Runs of Homozygosity and Inbreeding in Jersey Cattle under Selection. PLoS ONE 2015, 10, e0129967. [Google Scholar] [CrossRef] [PubMed]
- Bosse, M.; Megens, H.-J.; Madsen, O.; Paudel, Y.; Frantz, L.A.F.; Schook, L.B.; Crooijmans, R.P.M.A.; Groenen, M.A.M. Regions of Homozygosity in the Porcine Genome: Consequence of Demography and the Recombination Landscape. PLoS Genet. 2012, 8, e1003100. [Google Scholar] [CrossRef]
- Metzger, J.; Karwath, M.; Tonda, R.; Beltran, S.; Agueda, L.; Gut, M.; Gut, I.G.; Distl, O. Runs of homozygosity reveal signatures of positive selection for reproduction traits in breed and non-breed horses. BMC Genom. 2015, 16, 764. [Google Scholar] [CrossRef]
- Zhang, Q.; Guldbrandtsen, B.; Bosse, M.; Lund, M.S.; Sahana, G. Runs of homozygosity and distribution of functional variants in the cattle genome. BMC Genom. 2015, 16, 1313. [Google Scholar] [CrossRef]
- Herrero-Medrano, J.M.; Megens, H.-J.; Groenen, M.A.; Bosse, M.; Perez-Enciso, M.; Crooijmans, R.P. Whole-genome sequence analysis reveals differences in population management and selection of European low-input pig breeds. BMC Genom. 2014, 15, 601. [Google Scholar] [CrossRef]
- Keller, M.C.; Visscher, P.M.; Goddard, M.E.; Rosenberg, N.A. Quantification of Inbreeding Due to Distant Ancestors and Its Detection Using Dense Single Nucleotide Polymorphism Data. Genetics 2011, 189, 237–249. [Google Scholar] [CrossRef]
- Do, D.N.; Strathe, A.B.; Ostersen, T.; Jensen, J.; Mark, T.; Kadarmideen, H.N. Genome-Wide Association Study Reveals Genetic Architecture of Eating Behavior in Pigs and Its Implications for Humans Obesity by Comparative Mapping. PLoS ONE 2013, 8, e71509. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Yang, J.; Zhou, L.; Zhang, Z.; Ma, H.; Xie, X.; Zhang, F.; Xiong, X.; Cui, L.; Yang, H.; et al. Genome-wide association study of meat quality traits in a white durocxerhualian f2 intercross and chinese sutai pigs. PLoS ONE 2013, 8, e64047. [Google Scholar]
- Sanchez, M.-P.; Tribout, T.; Iannuccelli, N.; Bouffaud, M.; Servin, B.; Tenghe, A.; Déhais, P.; Muller, N.; Del Schneider, M.P.; Mercat, M.-J.; et al. A genome-wide association study of production traits in a commercial population of Large White pigs: Evidence of haplotypes affecting meat quality. Genet. Sel. Evol. 2014, 46, 12. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Chen, S.; Cheng, D.; Wang, L.; Li, Y.; Ma, X.; Song, X.; Liu, X.; Li, W.; Liang, J.; et al. Genome-wide association study of porcine hematological parameters in a large white x minzhu f2 resource population. Int. J. Biol. Sci. 2012, 8, 870–881. [Google Scholar] [CrossRef] [PubMed]
- Qiao, R.; Gao, J.; Zhang, Z.; Li, L.; Xie, X.; Fan, Y.; Cui, L.; Ma, J.; Ai, H.; Ren, J.; et al. Genome-wide association analyses reveal significant loci and strong candidate genes for growth and fatness traits in two pig populations. Genet. Sel. Evol. 2015, 47, 17. [Google Scholar] [CrossRef] [PubMed]
- Veroneze, R.; Bastiaansen, J.W.; Knol, E.F.; Guimarães, S.E.; Silva, F.F.; Harlizius, B.; Lopes, M.S.; Lopes, P.S. Linkage disequilibrium patterns and persistence of phase in purebred and crossbred pig (Sus scrofa) populations. BMC Genet. 2014, 15, 126. [Google Scholar] [CrossRef] [PubMed]
- Ai, H.; Huang, L.; Ren, J. Genetic Diversity, Linkage Disequilibrium and Selection Signatures in Chinese and Western Pigs Revealed by Genome-Wide SNP Markers. PLoS ONE 2013, 8, e56001. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Onsins, S.E.; Burgos-Paz, W.; Manunza, A.; Amills, M. Mining the pig genome to investigate the domestication process. Heredity 2014, 113, 471–484. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.; Kim, T.-H.; Lee, K.-T.; Kwak, W.; Lee, T.; Lee, S.-W.; Kim, M.-J.; Cho, K.; Kim, N.; Chung, W.-H.; et al. A genome-wide scan for signatures of directional selection in domesticated pigs. BMC Genom. 2015, 16, 130. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wei, J.; Zhang, Q.; Chen, L.; Wang, J.; Liu, J.; Ding, X. A Genome Scan for Selection Signatures in Pigs. PLoS ONE 2015, 10, e0116850. [Google Scholar] [CrossRef] [PubMed]
- Saura, M.; Fernández, A.; Varona, L.; Fernández, A.I.; De Cara, M.; Ángeles, R.; Barragán, C.; Villanueva, B. Detecting inbreeding depression for reproductive traits in Iberian pigs using genome-wide data. Genet. Sel. Evol. 2015, 47, 1. [Google Scholar] [CrossRef]
- Zanella, R.; Peixoto, J.O.; Cardoso, F.F.; Cardoso, L.L.; Biegelmeyer, P.; Cantão, M.E.; Otaviano, A.; Freitas, M.S.; Caetano, A.R.; Ledur, M.C. Genetic diversity analysis of two commercial breeds of pigs using genomic and pedigree data. Genet. Sel. Evol. 2016, 48, 24. [Google Scholar] [CrossRef] [PubMed]
- Silio, L.; Rodriguez, M.C.; Fernandez, A.; Barragan, C.; Benitez, R.; Ovilo, C.; Fernandez, A.I. Measuring inbreeding and inbreeding depression on pig growth from pedigree or snp-derived metrics. J. Anim. Breed. Genet. 2012, 130, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Lopes, M.S.; Silva, F.F.; Harlizius, B.; Duijvesteijn, N.; Lopes, P.S.; Guimarães, S.E.F.; Knol, E.F. Improved estimation of inbreeding and kinship in pigs using optimized snp panels. BMC Genet. 2013, 14, 1. [Google Scholar] [CrossRef]
- Saura, M.; Fernández, A.; Rodríguez, M.C.; Toro, M.A.; Barragan, C.; Fernandez, A.I.; Villanueva, B. Genome-Wide Estimates of Coancestry and Inbreeding in a Closed Herd of Ancient Iberian Pigs. PLoS ONE 2013, 8, e78314. [Google Scholar] [CrossRef]
- Fernández, A.; Rodrigáñez, J.; Toro, M.A.; Rodríguez, M.C.; Silió, L. Inbreeding effects on the parameters of the growth function in three strains of Iberian pigs. J. Sci. 2002, 80, 2267. [Google Scholar] [CrossRef]
- Fabuel, E.; Barragán, C.; Silió, L.; Rodriguez, M.C.; Toro, M.A. Analysis of genetic diversity and conservation priorities in Iberian pigs based on microsatellite markers. Heredity 2004, 93, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Toro, M.A.; Rodrigañez, J.; Silio, L.; Rodriguez, C. Genealogical analysis of a closed herd of black hairless iberian pigs. Conserv. Biol. 2000, 14, 1843–1851. [Google Scholar] [CrossRef]
- Fernández, J.; Villanueva, B.; Pong-Wong, R.; Toro, M. Ángel Efficiency of the Use of Pedigree and Molecular Marker Information in Conservation Programs. Genetics 2005, 170, 1313–1321. [Google Scholar] [CrossRef] [PubMed]
- Esteve-Codina, A.; Paudel, Y.; Ferretti, L.; Raineri, E.; Megens, H.-J.; Silió, L.; Rodríguez, M.C.; Groenen, M.A.M.; Ramos-Onsins, S.E.; Pérez-Enciso, M.; et al. Dissecting structural and nucleotide genome-wide variation in inbred Iberian pigs. BMC Genom. 2013, 14, 148. [Google Scholar] [CrossRef] [PubMed]
- Fernández, A.I.; Barragán, C.; Rodriguez, M.C.; Villanueva, B. Copy number variants in a highly inbred Iberian porcine strain. Anim. Genet. 2014, 45, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Herrero-Medrano, J.M.; Megens, H.-J.; Groenen, M.A.; Ramis, G.; Bosse, M.; Perez-Enciso, M.; Crooijmans, R.P. Conservation genomic analysis of domestic and wild pig populations from the Iberian Peninsula. BMC Genet. 2013, 14, 106. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Xu, H.; Yu, Z.; Gao, P.; Liu, S. Inbred chinese wuzhishan (wzs) minipig model for soybean glycinin and beta-conglycinin allergy. J. Agric. Food Chem. 2010, 58, 5194–5198. [Google Scholar] [CrossRef] [PubMed]
- Gong, P.; Li, C.S.; Hua, R.; Zhao, H.; Tang, Z.R.; Mei, X.; Zhang, M.Y.; Cui, J. Mild hypothermia attenuates mitochondrial oxidative stress by protecting respiratory enzymes and upregulating mnsod in a pig model of cardiac arrest. PLoS ONE 2012, 7, e35313. [Google Scholar] [CrossRef]
- Mu, Y.-L.L.L.; Feng, S.-T.; Wu, T.-W.; Li, K.; Li, J.-Y.; He, W.; Gao, Q.; Zhou, W.-F.; Wei, J.-L.; Tang, F.; et al. Identification of the miniature pig inbred line by skin allograft. J. Integr. Agric. 2015, 14, 1376–1382. [Google Scholar] [CrossRef]
- Huang, H.; Deng, H.; Yang, Y.; Tang, Z.; Yang, S.; Mu, Y.; Cui, W.; Yuan, J.; Wu, Z.; Li, K. Molecular characterization and association analysis of porcine pane1 gene. Mol. Biol. Rep. 2010, 37, 2571–2577. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Mu, Y.; Zhang, L.; Yang, S.; Li, K.; Feng, S. New mutations in growth hormone and receptor genes from Chinese Wuzhishan miniature pig. Acta Agric. Scand. Sect. A 2007, 57, 97–100. [Google Scholar] [CrossRef]
- Sun, J.-L.; Mu, Y.-L.; Liu, X.-L.; Feng, S.-T.; Wang, S.-R. Study of Swine Leukocyte Antigen Class I-3 (SLA-3) Gene for Inbreeding Wuzhishan Pig. Agric. Sci. China 2007, 6, 1502–1510. [Google Scholar] [CrossRef]
- Fang, X.; Mou, Y.; Huang, Z.; Li, Y.; Han, L.; Zhang, Y.; Feng, Y.; Chen, Y.; Jiang, X.; Zhao, W.; et al. The sequence and analysis of a Chinese pig genome. GigaScience 2012, 1, 16. [Google Scholar] [CrossRef]
- Barnett, R.; Larson, G. A phenol-chloroform protocol for extracting DNA from ancient samples. Methods Mol Biol. 2012, 840, 13–19. [Google Scholar]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.R.; Bender, D.; Maller, J.; Sklar, P.; De Bakker, P.I.W.; Daly, M.J.; et al. PLINK: A Tool Set for Whole-Genome Association and Population-Based Linkage Analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 2000, 155, 945–959. [Google Scholar]
- Meyer, L.R.; Zweig, A.S.; Hinrichs, A.S.; Karolchik, D.; Kuhn, R.M.; Wong, M.; Sloan, C.A.; Rosenbloom, K.R.; Roe, G.; Rhead, B.; et al. The ucsc genome browser database: Extensions and updates 2013. Nucleic Acids Res. 2013, 41, D64–D69. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.I.; Simpson, F.; David, P.; Rijks, J.M.; Kuiken, T.; Thorne, M.A.S.; Lacy, R.C.; DasMahapatra, K.K. High-throughput sequencing reveals inbreeding depression in a natural population. Proc. Natl. Acad. Sci. USA 2014, 111, 3775–3780. [Google Scholar] [CrossRef] [PubMed]
- Rijks, J.M.; Hoffman, J.I.; Kuiken, T.; Osterhaus, A.D.M.E.; Amos, W. Heterozygosity and lungworm burden in harbour seals (Phoca vitulina). Heredity 2008, 100, 587–593. [Google Scholar] [CrossRef] [PubMed]
| Chr | Pos 1 | Gene | Gene Accession Number | Gene Involved GO Terms and KEGG Pathways | Terms/Pathway ID |
|---|---|---|---|---|---|
| 1 | 16767735 | ESR1 | 397435 | Gene expression and signaling by G protein-coupled receptors (GPCR). | GO:0007200 |
| 4 | 51508361 | RIPK2 | 100518835 | Signaling by GPCR and immune system. | ssc04621, ssc04722 |
| 7 | 37570312 | FGD2 | 100524814 | Signaling by GPCR and signaling by Rho GTPases. | GO:0035023, GO:0007200 |
| 9 | 43255454 | FDX1 | 397133 | Related pathways are metabolism and infectious disease | IPR012675 |
| 13 | 29861003 | ABHD5 | 497624 | Lipoprotein metabolism and metabolism. | GO:0019915,GO:0051006 |
| 13 | 34681199 | PRKAR2A | 397493 | Platelet activation, signaling and aggregation and sertoli–sertoli cell junction dynamics | GO:1903538,GO:1904146 |
| 13 | 34775057 | PFKFB4 | 100158056 | Metabolism and glycosaminoglycan metabolism. | GO:0003873 |
| 13 | 35107691 | USP4 | 100512072 | Signaling by GPCR and Tumor necrosis factor (TNF) signaling | GO:0007200, GO:0006511 |
| 14 | 30401893 | NCOR2 | 733643 | gene expression and signaling by GPCR. | GO:0007200 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Mu, Y.; Xu, L.; Li, K.; Han, J.; Wu, T.; Liu, L.; Gao, Q.; Xia, Y.; Hou, G.; et al. Genomic Analysis Reveals Specific Patterns of Homozygosity and Heterozygosity in Inbred Pigs. Animals 2019, 9, 314. https://doi.org/10.3390/ani9060314
Wang L, Mu Y, Xu L, Li K, Han J, Wu T, Liu L, Gao Q, Xia Y, Hou G, et al. Genomic Analysis Reveals Specific Patterns of Homozygosity and Heterozygosity in Inbred Pigs. Animals. 2019; 9(6):314. https://doi.org/10.3390/ani9060314
Chicago/Turabian StyleWang, Ligang, Yulian Mu, Linyang Xu, Kui Li, Jianlin Han, Tianwen Wu, Lan Liu, Qian Gao, Ying Xia, Guanyu Hou, and et al. 2019. "Genomic Analysis Reveals Specific Patterns of Homozygosity and Heterozygosity in Inbred Pigs" Animals 9, no. 6: 314. https://doi.org/10.3390/ani9060314
APA StyleWang, L., Mu, Y., Xu, L., Li, K., Han, J., Wu, T., Liu, L., Gao, Q., Xia, Y., Hou, G., Yang, S., He, X., Liu, G. E., & Feng, S. (2019). Genomic Analysis Reveals Specific Patterns of Homozygosity and Heterozygosity in Inbred Pigs. Animals, 9(6), 314. https://doi.org/10.3390/ani9060314







