Effect of Dietary Supplementation of Moringa Oleifera on the Production Performance and Fecal Methanogenic Community of Lactating Dairy Cows
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Sample Collection and Measurements
2.3. DNA Extraction and High-Throughput Sequencing using McrA Gene
2.4. Bioinformatics Analysis of the Sequence Data
2.5. Statistical Analyses
3. Results
3.1. Feed Intake and Milk Yield and Composition
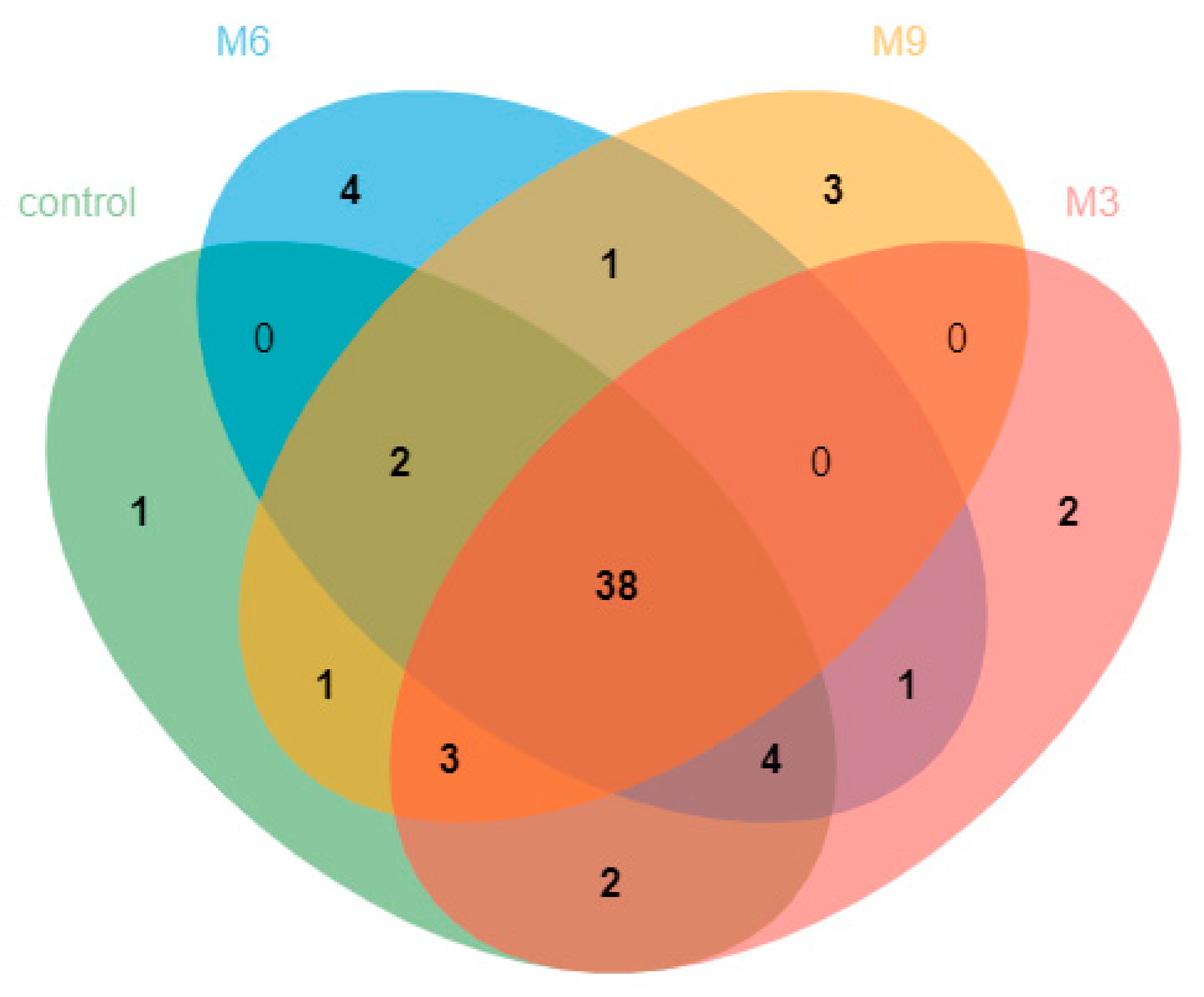
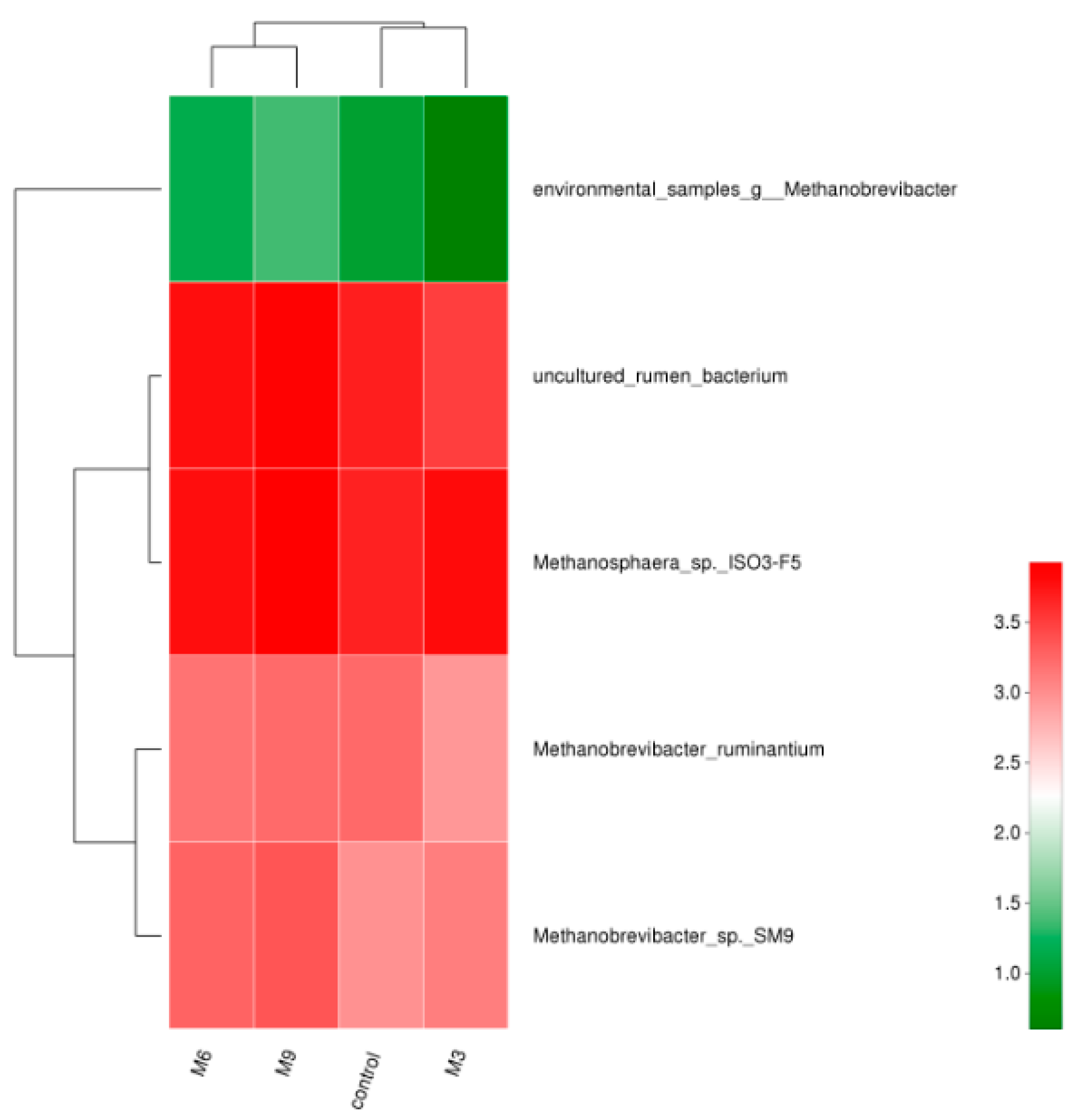
3.2. Composition and Dynamics of Fecal Methanogen Community
4. Discussion
4.1. Feed Intake, Milk Yield and Composition
4.2. Fecal Methanogenic Composition and Dynamics
4.3. Effect of Moringa Oleifera Supplementation on Fecal Methanogenic Archaea
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kholif, A.E.; Gouda, G.A.; Anele, U.Y.; Galyean, M.L. Extract of Moringa oleifera leaves improves feed utilization of lactating Nubian goats. Small Rumin. Res. 2018, 158, 69–75. [Google Scholar] [CrossRef]
- Zhang, T.T.; Si, B.W.; Deng, K.D.; Tu, Y.; Zhou, C.L.; Diao, Q.Y. Effects of feeding a Moringa oleifera rachis and twig preparation to dairy cows on their milk production and fatty acid composition, and plasma antioxidants. J. Sci. Food Agric. 2018, 98, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Azzaz, H.H.; Farahat, E.S.A.; Morsy, T.A.; Aziz, H.A.; Hadhoud, F.I.; Abd-Alla, M.S. Moringa oleifera and Echinacea purpurae as supplements for Rhamani lactating ewe’s diets and their effect on rumen characteristics, nutrients digestibility, blood parameters, milk production, composition and its fatty acid profile. Asian J. Anim. Vet. Adv. 2016, 11, 684–692. [Google Scholar] [CrossRef][Green Version]
- Mendieta-Araica, B.; Sporndly, R.; Reyes-Sanchez, N.; Sporndly, E. Moringa (Moringa oleifera) leaf meal as a source of protein in locally produced concentrates for dairy cows fed low protein diets in tropical areas. Livest. Sci. 2011, 137, 10–17. [Google Scholar] [CrossRef]
- Zeng, B.; Sun, J.J.; Chen, T.; Sun, B.L.; He, Q.; Chen, X.Y.; Zhang, Y.L.; Xi, Q.Y. Effects of Moringa oleifera silage on milk yield, nutrient digestibility and serum biochemical indexes of lactating dairy cows. J. Anim. Physiol. Anim. Nutr. 2018, 1, 75–81. [Google Scholar] [CrossRef]
- Sarkar, S.; Mohini, M.; Nampoothiri, V.M.; Mondal, G.; Pandita, S.; Mahesh, M.S. Effect of supplementation of Moringa oleifera leaves on in vitro methane emissions and rumen fermentation on roughage based ration. In Proceedings of the XVI Biennial Animal Nutrition Conference on Innovative Approaches for Animal Feeding and Nutritional Research, Karnal, India, 6–8 February 2016. [Google Scholar]
- Shaani, Y.; Eliyahu, D.; Mizrahi, I.; Yosef, E.; Ben-Meir, Y.; Nikbachat, M.; Solomon, R.; Mabjeesh, S.J.; Miron, J. Effect of feeding ensiled mixture of pomegranate pulp and drier feeds on digestibility and milk performance in dairy cows. J. Dairy Res. 2016, 1, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Daquiado, A.R.; Cho, K.M.; Kim, T.Y.; Kim, S.C.; Chang, H.H.; Lee, Y.B. Methanogenic archaea diversity in Hanwoo (Bos taurus coreanae) rumen fluid, rectal dung, and barn floor manure using a culture-independent method based on mcrA gene sequence. Anaerobe 2014, 27, 77–81. [Google Scholar] [CrossRef]
- Jin, D.X.; Kang, K.; Wang, H.Z.; Wang, Z.S.; Xue, B.; Wang, L.Z.; Xu, F.; Peng, Q.H. Effects of dietary supplementation of active dried yeast on fecal methanogenic archaea diversity in dairy cows. Anaerobe 2017, 44, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Mohammadzadeh, H.; Yáñez-Ruiz, D.R.; Martínez-Fernandez, G. Molecular comparative assessment of the microbial ecosystem in rumen and faeces of goats fed alfalfa hay alone or combined with oats. Anaerobe 2014, 29, 52–58. [Google Scholar] [CrossRef]
- AOAC International. Official Methods of Analysis, 20nd ed.; AOAC International: Rockville, MD, USA, 2016. [Google Scholar]
- Luton, P.E.; Wayne, J.M.; Sharp, R.J.; Riley, P.W. The mcrA gene as an alternative to 16S rRNA in the phylogenetic analysis of methanogen populations in landfill. Microbiol. 2002, 148, 3521–3530. [Google Scholar] [CrossRef]
- Amato, K.R.; Yeoman, C.J.; Kent, A.; Righini, N.; Carbonero, F.; Estrada, A.; Gaskins, H.R.; Stumpf, R.M.; Yildirim, S.; Torralba, M.; et al. Habitat degradation impacts black howler monkey (Alouatta pigra) gastrointestinal microbiomes. ISME J. 2013, 7, 1344–1353. [Google Scholar] [CrossRef] [PubMed]
- Oliveros, J.C. VENNY. An Interactive tool for Comparing Lists with Venn Diagrams; BioinfoGP of CNB-CSIC: Madrid, Spain, 2007. [Google Scholar]
- Cohen-Zinder, M.; Leibovich, H.; Vaknin, Y.; Sagi, G.; Shabtay, A.; Ben-MEIR, Y.; Nikbachat, M.; Protnik, Y.; Yishay, M.; Miron, J. Effect of feeding lactating cows with ensiled mixture of Moringa oleifera, wheat hay and molasses, on digestibility and efficiency of milk production. Anim. Feed Sci. Technol. 2016, 211, 75–83. [Google Scholar] [CrossRef]
- Verma, A.R.; Vijayakumar, M.; Mathela, C.S.; Rao, C.V. In vitro and in vivo antioxidant properties of different fractions of Moringa oleifera leaves. Food Chem. Toxicol. 2009, 47, 2110–2196. [Google Scholar] [CrossRef]
- Aerts, R.J.; Barry, T.N.; McNabbm, W.C. Polyphenols and agriculture: Beneficial effects of proanthocyanidins in forages. Agric. Ecosyst. Environ. 1999, 75, 1–12. [Google Scholar] [CrossRef]
- Faulkner, M.J.; Wenner, B.A.; Solden, L.M.; Weiss, W.P. Source of supplemental dietary copper, zinc, and manganese affects fecal microbial relative abundance in lactating dairy cows. J. Dairy Sci. 2017, 100, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhu, Z.P.; Liu, Y.F.; Guo, T.J.; Dong, H.M. Diversity and abundance of the rumen and fecal methanogens in Altay sheep native to Xinjiang and the influence of diversity on methane emissions. Arch. Microbiol. 2012, 194, 353–361. [Google Scholar] [CrossRef]
- Zhou, M.; Chen, Y.H.; Griebel, P.J.; Guan, L.L. Methanogen prevalence throughout the gastrointestinal tract of pre-weaned dairy calves. Gut Microbes 2014, 5, 628–638. [Google Scholar] [CrossRef]
- Min, B.R.; Solaiman, S.; Shange, R.; Eun, J.S. Gastrointestinal bacterial and methanogenic archaea diversity dynamics associated with condensed tannin-Containing pine bark diet in goats using 16S rDNA amplicon pyrosequencing. Int. J. Microbiol. 2014, 4, 1–11. [Google Scholar] [CrossRef]
- Guzman, C.E.; Bereza-Malcolm, L.T.; Groef, B.D.; Franks, A.E. Presence of selected methanogens, fibrolytic bacteria, and proteobacteria in the gastrointestinal tract of neonatal dairy calves from birth to 72 hours. PLoS ONE 2015, 7, e0133048. [Google Scholar] [CrossRef]
- Dowd, S.E.; Callaway, T.R.; Wolcott, R.D.; Sun, Y.; McKeehan, T.; Hagevoort, R.G.; Edrington, T.S. Evaluation of the bacterial diversity in the feces of cattle using 16S rDNA bacterial tag-encoded FLX amplicon pyrosequencing (bTEFAP). BMC Microbiol. 2008, 8, 125. [Google Scholar] [CrossRef]
- Shanks, O.C.; Kelty, C.A.; Archibeque, S.; Jenkins, M.; Newton, R.J.; McLellan, S.L.; Huse, S.M.; Sogin, M.L. Community structures of fecal bacteria in cattle from different animal feeding operations. Appl. Environ. Microbiol. 2011, 77, 2992–3001. [Google Scholar] [CrossRef]
- Durso, L.M.; Wells, J.E.; Harhay, G.P.; Rice, W.C.; Kuehn, L.; Bono, J.L.; Shackelford, S.; Wheeler, T.; Smith, T.P.L. Comparison of bacterial communities in faeces of beef cattle fed diets containing corn and wet distillers’ grain with solubles. Lett. Appl. Microbiol. 2012, 55, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Wallace, R.J.; McEwan, N.R.; McIntosh, F.M.; Teferedegne, B.; Newbold, C.J. Natural products as manipulators of rumen fermentation. Asian Austral. J. Anim. Sci. 2002, 15, 1458–1468. [Google Scholar] [CrossRef]
- Elghandour, M.M.Y.; Vallejo, L.H.; Salem, A.Z.M.; Mellado, M.; Camacho, L.M.; Cipriano, M.; Olafadehan, O.A.; Olivares, J.; Rogas, S. Moringa oleifera leaf meal as an environmental friendly protein source for ruminants: Biomethane and carbon dioxide production, and fermentation characteristics. J. Clean. Prod. 2017, 165, 1229–1238. [Google Scholar] [CrossRef]
- Ferreira, P.M.P.; Farias, D.F.; de Abreu Oliveira, J.T.; de Fátima Urano Carvalho, A. Moringa oleifera: Bioactive compounds and nutritional potential. Revista de Nutrição 2008, 21, 431–437. [Google Scholar] [CrossRef]
- Teixeira, E.M.B.; Carvalho, M.R.B.; Neves, V.A.; Silva, M.A.; Arantes-Pereira, L. Chemical characteristics and fractionation of proteins from Moringa oleifera Lam. leaves. Food Chem. 2014, 147, 51–54. [Google Scholar] [CrossRef]
- Ramírez-Restrepoa, C.A.; Tan, C.; O’Neill, C.J.; López-Villalobos, N.; Padmanabha, J.; Wang, J.K.; McSweeney, C.S. Methane production, fermentation characteristics, and microbial profiles in the rumen of tropical cattle fed tea seed saponin supplementation. Anim. Feed. Sci. Technol. 2016, 216, 58–67. [Google Scholar] [CrossRef]
- Holtshausen, L.; Chaves, A.V.; Beauchemin, K.A.; McGinn, S.M.; McAllister, T.A.; Odongo, N.E.; Cheeke, P.R.; Benchaar, C. Feeding saponin-containing Yucca schidigera and Quillaja saponaria to decrease enteric methane production in dairy cows. J. Dairy Sci. 2009, 92, 2809–2821. [Google Scholar] [CrossRef] [PubMed]
- Finlay, B.J.; Esteban, G.; Clarke, K.J.; Williams, A.G.; Embley, T.M.; Hirt, R.P. Some rumen ciliates have endosymbiotic methanogens. FEMS Microbiol. Lett. 1994, 117, 157–162. [Google Scholar] [CrossRef]
- Morgavi, D.P.; Martin, C.; Jouany, J.P.; Ranilla, M.J. Rumen protozoa and methanogenesis: Not a simple cause-effect relationship. Br. J. Nutr. 2012, 107, 388–397. [Google Scholar] [CrossRef]
- Soliva, C.R.; Kreuzer, M.; Foidl, N.; Foidl, G.; Machnüller, A.; Hess, H.D. Feeding value of whole and extracted Moringa oleifera leaves for ruminants and their effects on ruminal fermentation in vitro. Anim. Feed Sci. Technol. 2005, 118, 47–62. [Google Scholar] [CrossRef]
- Zhou, M.; Hernandez-Sanabria, E.; Guan, L.L. Assessment of the microbial ecology of ruminal methanogens in cattle with different feed efficiency. Appl. Environ. Microbiol. 2009, 75, 6524–6533. [Google Scholar] [CrossRef] [PubMed]
- Henderson, G.; Cox, F.; Ganesh, S.; Jonker, A.; Young, W.; Janssen, P.H. Rumen microbial community composition varies with diet and host, but a core microbiome is found across a wide geographical range. Sci. Rep. 2015, 5, 14567. [Google Scholar] [CrossRef] [PubMed]
| Items | Dietary Moringa Oleifera Content | |||
|---|---|---|---|---|
| 0 | 3% | 6% | 9% | |
| Ingredients, % of DM | ||||
| Ground corn | 21.2 | 21.7 | 22.1 | 22.7 |
| Soybean meal | 10.5 | 11.6 | 12.7 | 13.8 |
| DDGS | 8.4 | 8.4 | 8.4 | 8.4 |
| Cottonseed meal | 7.5 | 7.5 | 7.5 | 7.5 |
| Palm fat | 7.5 | 7.5 | 7.5 | 7.5 |
| Beet pulp | 4.7 | 4.7 | 4.7 | 4.7 |
| Alfalfa hay | 20.5 | 17.1 | 13.7 | 10.2 |
| Corn silage | 16.8 | 15.7 | 14.5 | 13.4 |
| Moringa oleifera | 0.0 | 3.0 | 6.0 | 9.0 |
| Premix 1 | 2.0 | 2.0 | 2.0 | 2.0 |
| Sodium hydrogen carbonate | 0.6 | 0.6 | 0.6 | 0.6 |
| Calcium hydrogen phosphate | 0.2 | 0.2 | 0.2 | 0.2 |
| Sodium chloride | 0.3 | 0.3 | 0.3 | 0.3 |
| Nutrient composition 2 | ||||
| CP | 178.70 | 179.00 | 179.30 | 179.60 |
| EE | 44.80 | 46.90 | 48.80 | 50.90 |
| Ash | 76.80 | 84.60 | 73.50 | 79.20 |
| NDF | 437.00 | 434.30 | 431.70 | 428.60 |
| ADF | 211.30 | 214.30 | 217.20 | 219.30 |
| NEL, MJ/kg | 6.60 | 6.61 | 6.62 | 6.63 |
| Item 1 | Treatment 2 | SEM 3 | p-Value | |||
|---|---|---|---|---|---|---|
| Control | M3 | M6 | M9 | |||
| DM intake, kg/day | 20.6 | 20.9 | 20.7 | 19.3 | 0.53 | 0.09 |
| Milk yield, kg/day | 29.6 | 29.7 | 31.0 | 28.6 | 0.46 | 0.46 |
| ECM kg/day | 31.7 | 32.2 | 34.5 | 31.4 | 0.57 | 0.69 |
| Milk composition, % | ||||||
| Fat | 3.54 b | 3.62 b | 3.82 a | 3.68 ab | 0.07 | 0.04 |
| Protein | 3.65 | 3.70 | 3.66 | 3.70 | 0.03 | 0.49 |
| Lactose | 5.10 | 5.08 | 5.08 | 5.05 | 0.03 | 0.14 |
| Total solid | 13.0 | 13.1 | 13.2 | 13.1 | 0.08 | 0.13 |
| Item 1 | Treatment 2 | SEM 3 | p-Value | |||
|---|---|---|---|---|---|---|
| Control | M3 | M6 | M9 | |||
| Ace | 21.05 | 19.78 | 15.30 | 14.35 | 1.788 | 0.510 |
| Chao | 20.63 | 19.25 | 19.75 | 18.25 | 0.569 | 0.561 |
| Shannon | 1.653 b | 1.438 b | 1.628 b | 1.783 a | 0.0570 | 0.019 |
| Simpson | 0.310 a | 0.393 a | 0.328 a | 0.265 b | 0.0199 | 0.014 |
| Item | Treatment | SEM 2 | p-Value | |||
|---|---|---|---|---|---|---|
| Control | M3 | M6 | M9 | |||
| Phylum | ||||||
| Euryarchaeota | 0.734 | 0.743 | 0.757 | 0.705 | 0.022 | 0.883 |
| Uncultured rumen archaea | 0.216 | 0.150 | 0.213 | 0.272 | 0.016 | 0.456 |
| Unclassified | 0.051ab | 0.108a | 0.03ab | 0.023b | 0.015 | 0.172 |
| Order | ||||||
| Methanobacteriales | 0.582 | 0.677 | 0.615 | 0.606 | 0.023 | 0.561 |
| Methanomicrobiales | 0.150 | 0.065 | 0.142 | 0.099 | 0.026 | 0.676 |
| Uncultured rumen archaea | 0.216 | 0.150 | 0.213 | 0.272 | 0.016 | 0.456 |
| Family | ||||||
| Methanobacteriaceae | 0.582 | 0.677 | 0.615 | 0.606 | 0.023 | 0.561 |
| Methanocorpusculaceae | 0.150 | 0.065 | 0.142 | 0.099 | 0.026 | 0.676 |
| Uncultured rumen archaea | 0.216 | 0.150 | 0.213 | 0.272 | 0.016 | 0.456 |
| Genus | ||||||
| Methanobrevibacter | 0.391 | 0.380 | 0.400 | 0.315 | 0.026 | 0.698 |
| Methanosphaera | 0.191b | 0.297a | 0.215b | 0.291a | 0.016 | 0.016 |
| Methanocorpusculum | 0.150 | 0.065 | 0.142 | 0.099 | 0.026 | 0.676 |
| Uncultured rumen archaea | 0.216 | 0.150 | 0.213 | 0.272 | 0.016 | 0.456 |
| Species | ||||||
| Unclassified Methanobrevibacter | 0.274 | 0.279 | 0.281 | 0.172 | 0.031 | 0.569 |
| Methanosphaera sp. ISO3-F5 | 0.191b | 0.297a | 0.215b | 0.291a | 0.016 | 0.016 |
| Unclassified Methanocorpusculum | 0.150 | 0.065 | 0.142 | 0.099 | 0.026 | 0.676 |
| Uncultured rumen archaea | 0.216 | 0.150 | 0.213 | 0.272 | 0.026 | 0.456 |
| Methanobrevibacter sp. SM9 | 0.038 | 0.060 | 0.065 | 0.080 | 0.007 | 0.258 |
| Methanobrevibacter ruminantium | 0.078a | 0.041b | 0.054b | 0.062b | 0.014 | 0.035 |
| Unclassified | 0.051 | 0.108 | 0.030 | 0.023 | 0.015 | 0.172 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, L.; Zhang, T.; Diao, Q. Effect of Dietary Supplementation of Moringa Oleifera on the Production Performance and Fecal Methanogenic Community of Lactating Dairy Cows. Animals 2019, 9, 262. https://doi.org/10.3390/ani9050262
Dong L, Zhang T, Diao Q. Effect of Dietary Supplementation of Moringa Oleifera on the Production Performance and Fecal Methanogenic Community of Lactating Dairy Cows. Animals. 2019; 9(5):262. https://doi.org/10.3390/ani9050262
Chicago/Turabian StyleDong, Lifeng, Tingting Zhang, and Qiyu Diao. 2019. "Effect of Dietary Supplementation of Moringa Oleifera on the Production Performance and Fecal Methanogenic Community of Lactating Dairy Cows" Animals 9, no. 5: 262. https://doi.org/10.3390/ani9050262
APA StyleDong, L., Zhang, T., & Diao, Q. (2019). Effect of Dietary Supplementation of Moringa Oleifera on the Production Performance and Fecal Methanogenic Community of Lactating Dairy Cows. Animals, 9(5), 262. https://doi.org/10.3390/ani9050262






