Effects of the Forage Type and Chop Length of Ramie Silage on the Composition of Ruminal Microbiota in Black Goats
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Ethics
2.2. Preparation of Silages
2.3. Animal and Experimental Design
2.4. Sampling and Measurements
2.5. DNA Extraction, Polymerase Chain Reaction (PCR), and Amplicon Sequencing
2.6. PCR Amplication and 16s rDNA Sequencing
2.7. Sequence Analysis
2.8. Statistical Analysis
3. Results
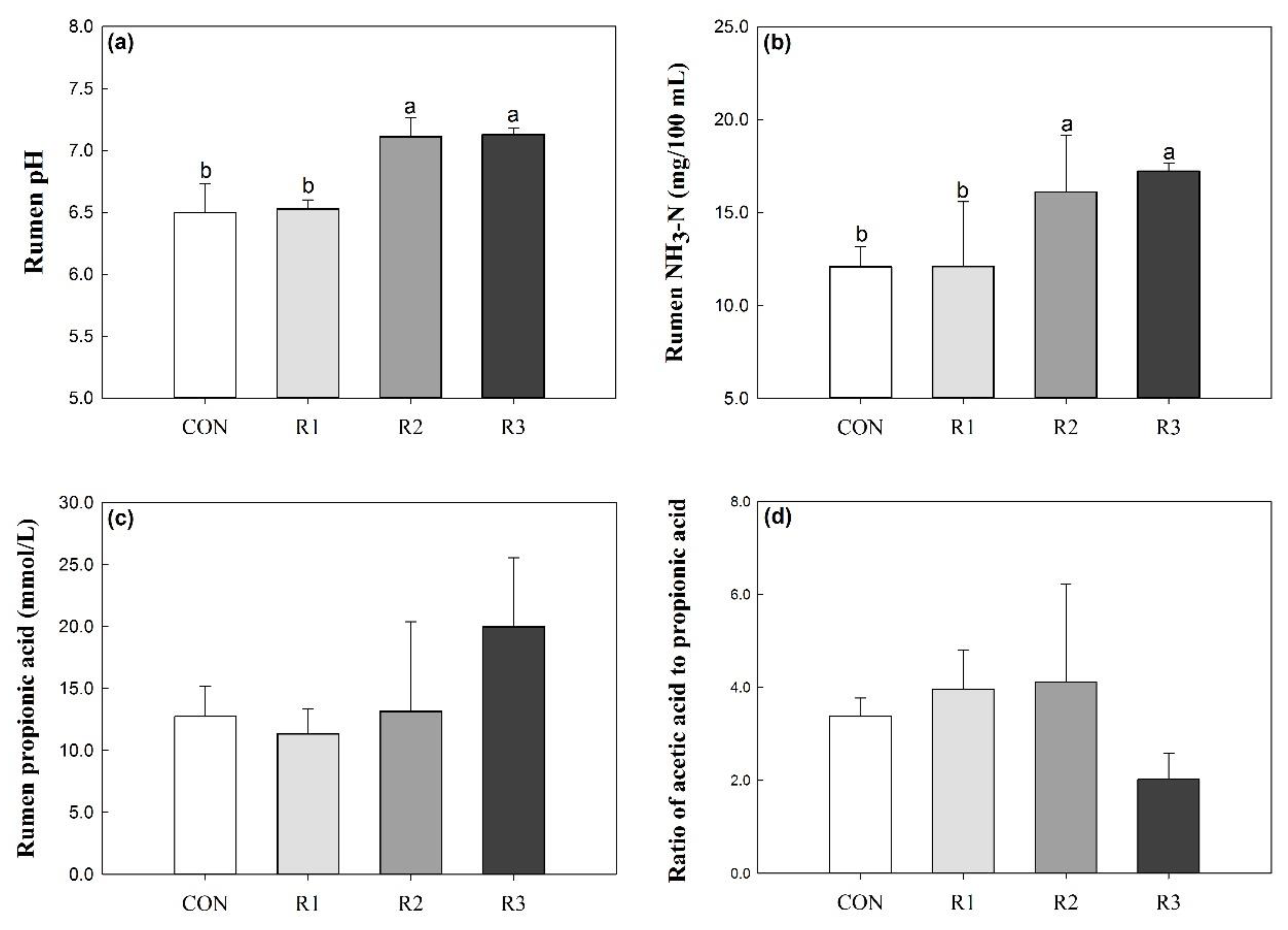
3.1. Rumen Fermentation
3.2. Alpha Diversity of Ruminal Microbiota
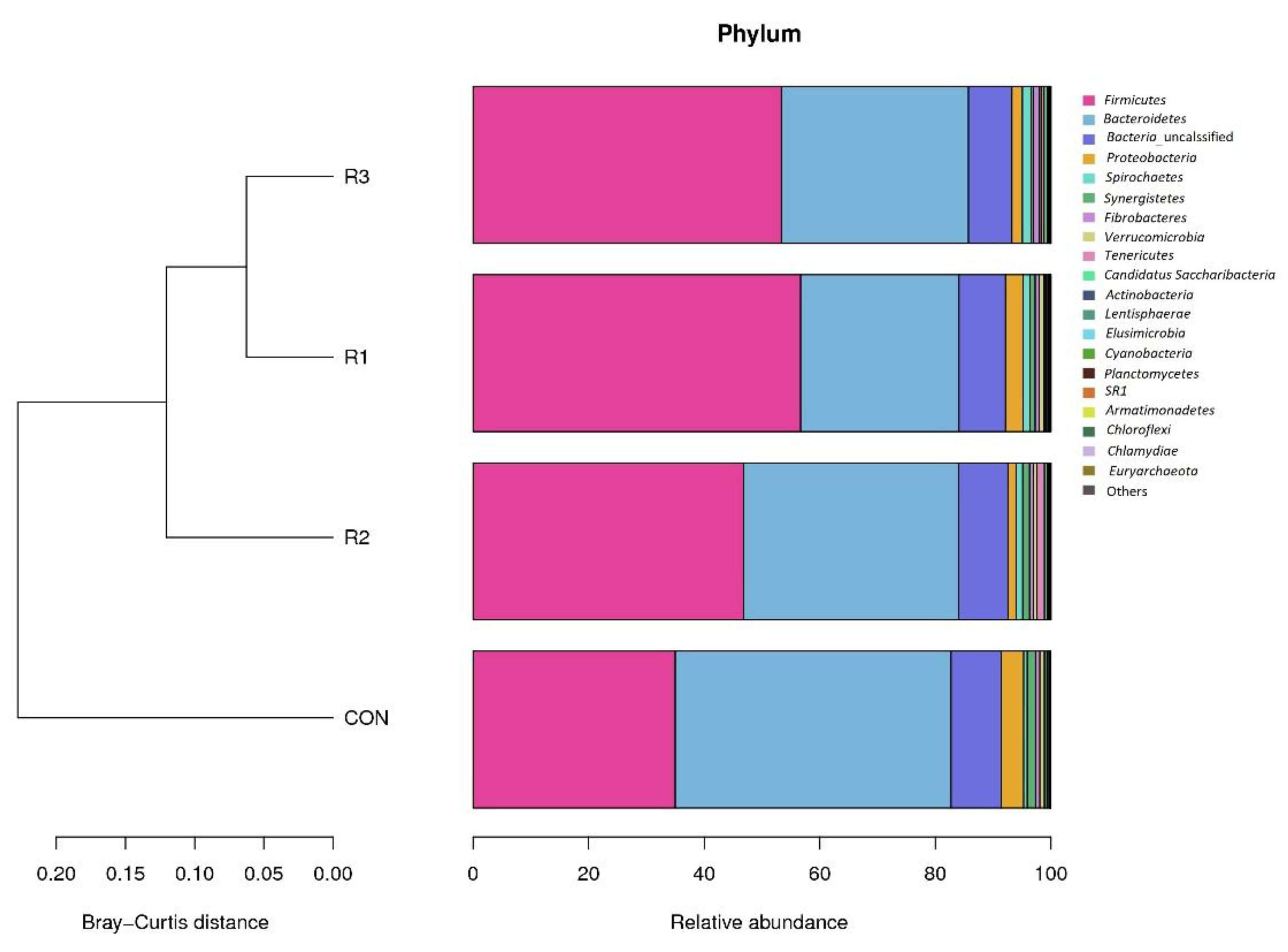
3.3. Rumen Microbial Composition at the Phylum Level
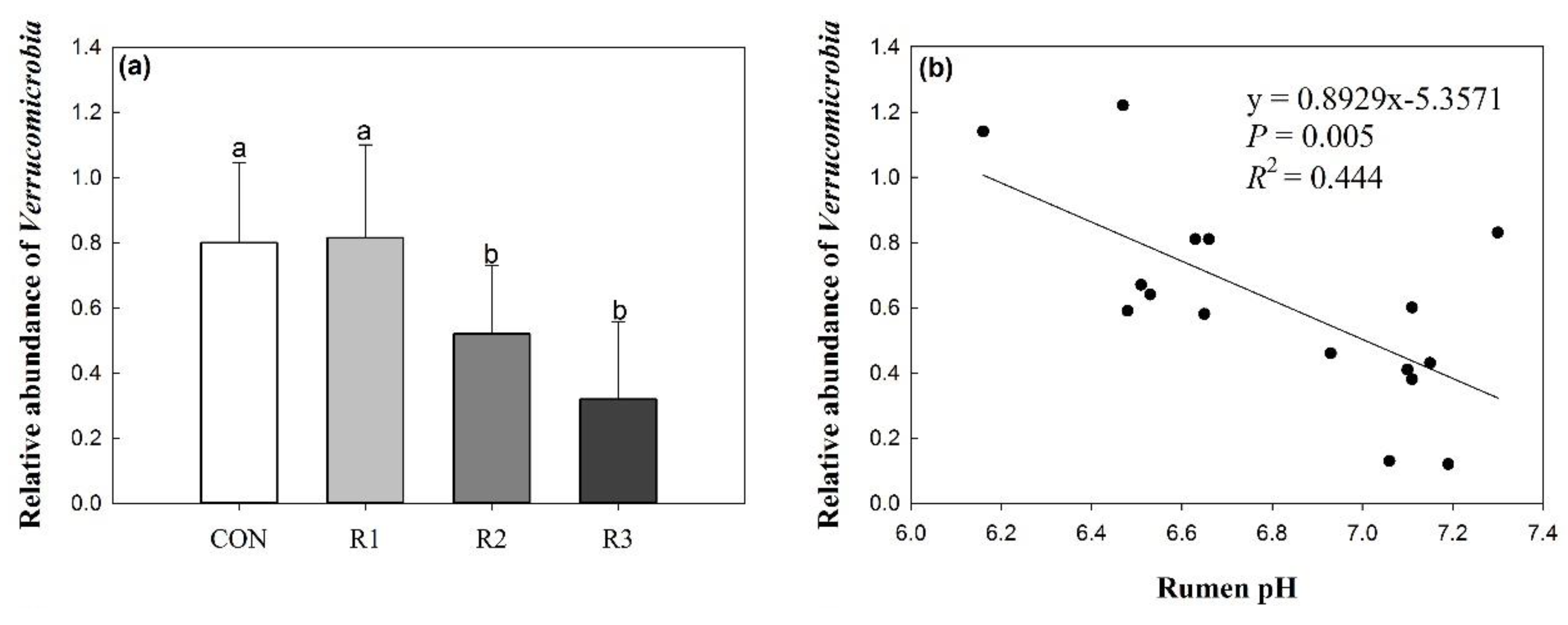
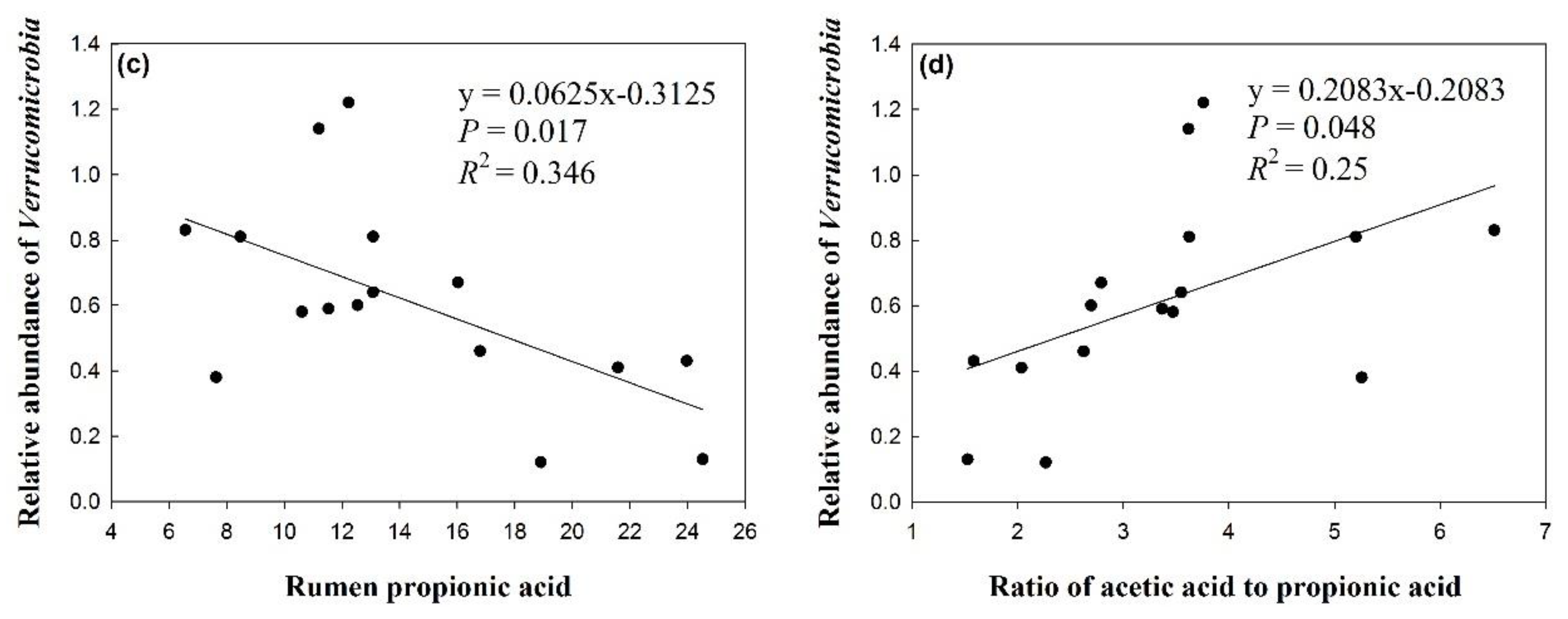
3.4. Effects of Silage Type and Chop Length on Verrucomicrobia
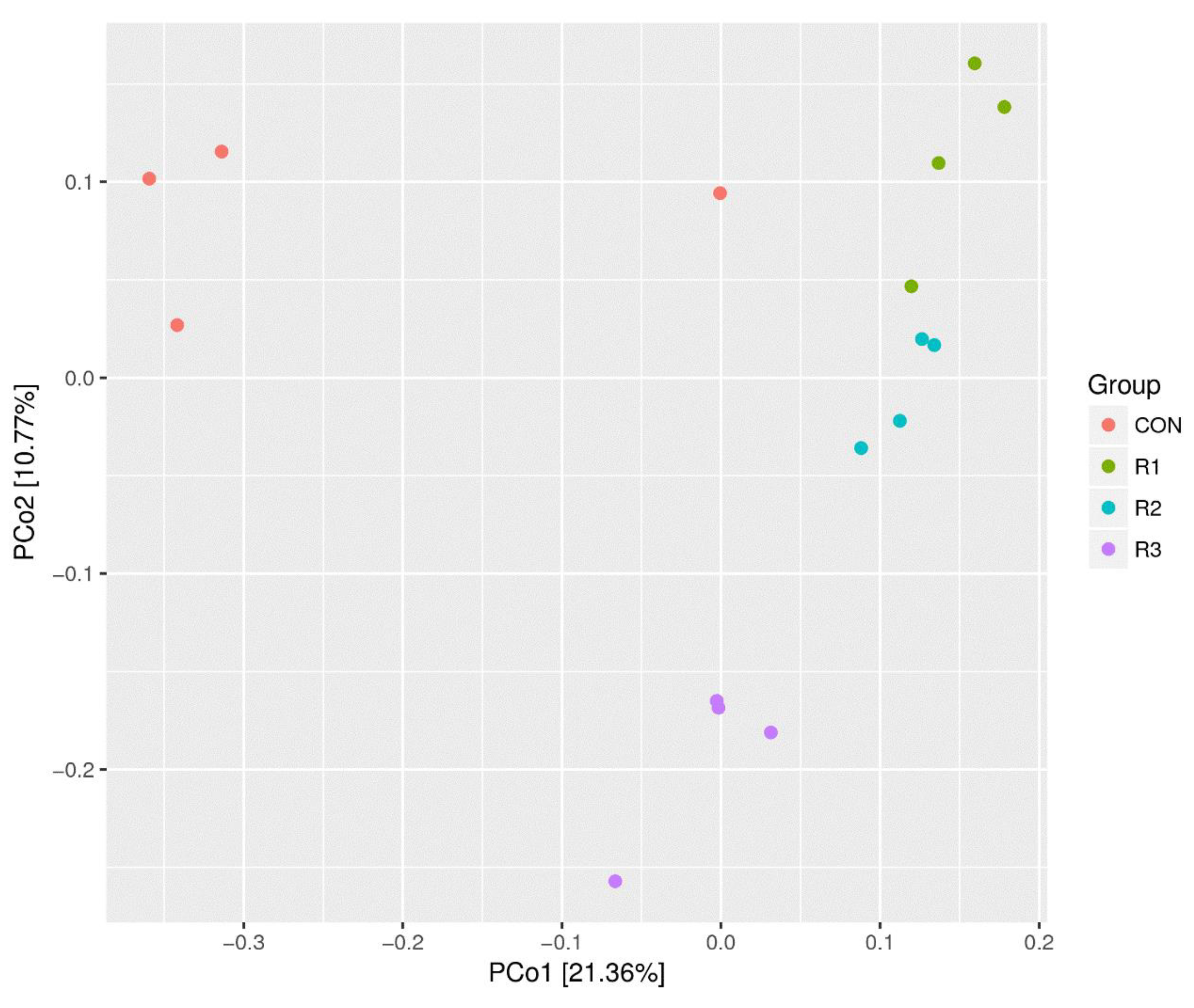
3.5. Analysis of Weighted UniFrac Distance
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chaucheyras-Durand, F.; Ameilbonne, A.; Bichat, A.; Mosoni, P.; Ossa, F.; Forano, E. Live yeasts enhance fibre degradation in the cow rumen through an increase in plant substrate colonization by fibrolytic bacteria and fungi. J. Appl. Microbiol. 2016, 120, 560–570. [Google Scholar] [CrossRef] [PubMed]
- Lengowski, M.B.; Witzig, M.; MÖhring, J.; Seyfang, G.M.; Rodehutscord, M. Effects of corn silage and grass silage in ruminant rations on diurnal changes of microbial populations in the rumen of dairy cows. Anaerobe 2016, 42, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Staerfl, S.M.; Zeitz, J.O.; Kreuzer, M.; Soliva, C.R. Methane conversion rate of bulls fattened on grass or maize silage as compared with the IPCC default values, and the long-term methane mitigation efficiency of adding acacia tannin, garlix, maca and lupine. Agric. Ecosyst. Environ. 2012, 148, 111–120. [Google Scholar] [CrossRef]
- Angelini, L.G.; Tavarini, S. Ramie [Boehmeria nivea (L.) Gaud.] as a potential new fibre crop for the Mediterranean region: Growth, crop yield and fibre quality in a long-term field experiment in Central Italy. Ind. Crop Prod. 2013, 51, 138–144. [Google Scholar] [CrossRef]
- Squibb, R.L.; Mendez, J.; Guzman, M.A.; Scrimshaw, N.S. Ramie—A high protein forage crop for tropical areas. Grass Forage Sci. 2010, 9, 313–322. [Google Scholar] [CrossRef]
- Kipriotis, E.; Heping, X.; Vafeiadakis, T.; Kiprioti, M.; Alexopoulou, E. Ramie and kenaf as feed crops. Ind. Crop Prod. 2015, 68, 126–130. [Google Scholar] [CrossRef]
- Wu, D.Q.; Tang, S.X.; He, Z.X.; Odongo, E.N.; Tan, Z.L.; Han, X.F.; Zhou, C.S.; Kang, J.H.; Wang, M. Oleic and linoleic acids alter fermentation characteristics, methane and fatty acid isomers production during in vitro incubation with mixed ruminal microbes. J. Food Agric. Environ. 2013, 11, 464–469. [Google Scholar]
- Fadrosh, D.W.; Ma, B.; Gajer, P.; Sengamalay, N.; Ott, S.; Brotman, R.M.; Ravel, J. An improved dual-indexing approach for multiplexed 16S rRNA gene sequencing on the Illumina MiSeq platform. Microbiome 2014, 2, 6. [Google Scholar] [CrossRef]
- Castromontoya, J.M.; Hps, M.; Becker, K. Chemical composition of rumen microbial fraction and fermentation parameters as affected by tannins and saponins using an in vitro rumen fermentation system. Can. J. Anim. Sci. 2001, 91, 433–448. [Google Scholar] [CrossRef]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef]
- Sun, S.N.; Lin, Q.; Xiang, H.; Hou, Z.P.; Zheng, R.Z.; Dai, Q.Z.; Wu, D.Q. Effects of Different lengths of feeding-ramie silage on rumen fermentation parameters and cellulase activities of black goats. Chin. J. Anim. Sci. 2019, 31, 477–484. [Google Scholar]
- Hoover, W. Effect of solid and liquid flows on fermentation in continuous cultures. IV. pH and dilution rate. J. Anim. Sci. 1984, 58, 1133–1139. [Google Scholar] [CrossRef]
- Xie, X.; Wang, J.K.; Guan, L.L.; Liu, J.X. Effect of changing forage on the dynamic variation in rumen fermentation in sheep. Anim. Sci. J. 2018, 89, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Soita, H.W.; Christensen, D.A.; Mckinnon, J.J. Effects of barley silage particle size and concentrate level on rumen kinetic parameters and fermentation patterns in steers. Can. J. Anim. Sci. 2003, 83, 533–539. [Google Scholar] [CrossRef]
- Schwab, E.C.; Shaver, R.D.; Shinners, K.J.; Lauer, J.G.; Coors, J.G. Processing and chop length effects in Brown-Midrib corn silage on intake, digestion, and milk production by dairy cows. J. Dairy Sci. 2002, 85, 613–623. [Google Scholar] [CrossRef]
- Einarson, M.S.; Plaizier, J.C.; Wittenberg, K.M. Effects of barley silage chop length on productivity and rumen conditions of lactating dairy cows fed a total mixed ration. J. Dairy Sci. 2004, 87, 2987–2996. [Google Scholar] [CrossRef]
- Thomson, A.L.; Humphries, D.J.; Kliem, K.E.; Dittmann, M.T.; Reynolds, C.K. Effects of replacing maize silage with Lucerne silage and Lucerne silage chop length on rumen function and milk fatty acid composition. J. Dairy Sci. 2017, 100, 7127–7138. [Google Scholar] [CrossRef] [PubMed]
- Krause, K.M.; Combs, D.K.; Beauchemin, K.A. Effects of forage particle size and grain fermentability in midlactation cows. I. Milk production and diet digestibility. J. Dairy Sci. 2002, 85, 1936–1946. [Google Scholar] [CrossRef]
- Beauchemin, K.A.; Yang, W.Z.; Rode, L.M. Effects of particle size of alfalfa based dairy cow diets on chewing activity, ruminal fermentation, and milk production. J. Dairy Sci. 2003, 86, 630–643. [Google Scholar] [CrossRef]
- Yang, W.Z.; Beauchemin, K.A.; Rode, L.M. Effects of grain processing, forage to concentrate ratio, and forage particle size on rumen pH and digestion in dairy cows. J. Dairy. Sci. 2001, 84, 2203–2216. [Google Scholar] [CrossRef]
- Jin, D.; Zhao, S.G.; Zhang, Y.D.; Sun, P.; Bu, D.P.; Beckers, Y.; Wang, J.Q. Diversity shifts of rumen bacteria induced by dietary forages in dairy cows and quantification of the changed bacteria using a new primer design strategy. J. Integr. Agric. 2016, 15, 2596–2603. [Google Scholar] [CrossRef]
- Abecia, L.; Molina-Alacaide, E.; Cantalapiedra-Híjar, G.; Soto, E.C.; Carro, M.D. Effects of forage type on diversity in bacterial pellets isolated from liquid and solid phases of the rumen content in sheep and goats. Options Méditerranéennes Sér. A Mediterr. Semin. 2013, 107, 9–16. [Google Scholar]
- Abecia, L.; Molina-Alcaide, E.; Cantalapiedra-Hijar, G.; Soto, E.C.; Carro, M.D. Effects of forage type on diversity in bacterial pellets isolated from liquid and solid phases of the rumen content in sheep and goats. In Feeding and Management Strategies to Improve Livestock Productivity, Welfare and Product Quality under Climate Change; Ben Salem, H., López-Fran cos, A., Eds.; CIHEAM/INRAT/OEP/IRESA/FAO: Zaragoza, Spain, 2013; pp. 9–16. [Google Scholar]
- Brulc, J.M.; Antonopoulos, D.A.; Miller, M.E.; Wilson, M.K.; Yannarell, A.C.; Dinsdale, E.A.; Edwards, R.E.; Frank, E.D.; Emerson, J.B.; Wacklin, P.; et al. Gene-centric metagenomics of the fiber-adherent bovine rumen microbiome reveals forage specific glycoside hydrolases. Proc. Natl. Acad. Sci. USA 2009, 106, 1948–1953. [Google Scholar] [CrossRef]
- Sadet-Bourgeteau, S.; Martin, C.; Morgavi, D.P. Bacterial diversity dynamics in rumen epithelium of wethers fed forage and mixed concentrate forage diets. Vet. Microbiol. 2010, 146, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.H.; Teather, R.; Forster, R. Composition, spatial distribution, and diversity of the bacterial communities in the rumen of cows fed different forages. FEMS Microbiol. Ecol. 2010, 74, 612–622. [Google Scholar] [CrossRef] [PubMed]
- Fernando, S.C.; Purvis, H.; Najar, F.; Sukharnikov, L.; Krehbiel, C.; Nagaraja, T. Rumen microbial population dynamics during adaptation to a high-grain diet. Appl. Environ. Microbiol. 2010, 76, 7482–7490. [Google Scholar] [CrossRef]
- Grilli, D.J.; Fliegerov, K.; Kopecný, J.; Lama, S.P.; Egea, V.; Sohaefer, N.; Pereyra, C.; Ruiz, M.S.; Sosa, M.A.; Arenas, G.N.; et al. Analysis of the rumen bacterial diversity of goats during shift from forage to concentrate diet. Anaerobe 2016, 42, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Jalali, A.R.; Weisbjerg, M.R.; Nadeau, E.; Randby, Å.T.; Rustas, B.O.; Eknæs, M.; Nørgaard, P. Effects of forage type, animal characteristics and feed intake on fecal particle size in goat, sheep, llama and cattle. Anim. Feed Sci. Technol. 2015, 208, 53–65. [Google Scholar] [CrossRef]
- Khaing, K.T.; Loh, T.C.; Ghizan, S.; Jahromi, M.F.; Halim, R.A.; Samsudin, A.A. Profiling of rumen fermentation and microbial population changes in goats fed with Napier grass supplemented with whole corn plant silage. Asian J. Anim. Sci. 2016, 10, 1–14. [Google Scholar]
| Items | CON | R1 | R2 | R3 |
|---|---|---|---|---|
| Ingredient (%) | ||||
| Corn | 20.4 | 20.4 | 20.4 | 20.4 |
| Wheat bran | 10.72 | 12.72 | 12.72 | 12.72 |
| Soybean meal | 6.8 | 4.8 | 4.8 | 4.8 |
| NaCl | 0.48 | 0.48 | 0.48 | 0.48 |
| Premix | 1.6 | 1.6 | 1.6 | 1.6 |
| Ramie silage | 60.0 | 60.0 | 60.0 | |
| Corn silage | 60.0 | |||
| Total | 100 | 100.0 | 100.0 | 100.0 |
| Nutrient levels | ||||
| ME (MJ/Kg) | 8.71 | 8.72 | 8.72 | 8.72 |
| CP (%) | 15.0 | 14.9 | 15.2 | 14.9 |
| Starch (%) | 21.2 | 20.7 | 20.7 | 20.7 |
| NDF (%) | 37.0 | 37.5 | 36.7 | 37.3 |
| ADF (%) | 26.1 | 26.6 | 26.4 | 26.8 |
| Ca (%) | 2.85 | 2.87 | 2.86 | 2.88 |
| P (%) | 0.40 | 0.31 | 0.32 | 0.31 |
| CON | R1 | R2 | R3 | SEM | p-Value | |
|---|---|---|---|---|---|---|
| Shannon-Wiener | 8.73 | 9.25 | 9.26 | 9.18 | 0.36 | 0.10 |
| Chao 1 | 2632.79 | 3649.97 | 3956.94 | 3398.12 | 634.69 | <0.01 |
| Observed Species | 2050.00 | 2730.50 | 2826.00 | 2547.25 | 412.63 | 0.02 |
| Kingdom | Phylum | CON | R1 | R2 | R3 | SEM | p-Value |
|---|---|---|---|---|---|---|---|
| Bacteria | Firmicutes | 34.99 | 56.68 | 46.85 | 53.33 | 13.06 | 0.07 |
| Bacteroidetes | 47.73 | 27.41 | 37.16 | 32.39 | 11.50 | 0.06 | |
| Proteobacteria | 3.92 | 3.02 | 1.44 | 1.84 | 1.81 | 0.20 | |
| Spirochaetes | 0.62 | 1.23 | 1.15 | 1.49 | 0.68 | 0.35 | |
| Synergistetes | 1.42 | 0.90 | 1.18 | 0.45 | 0.72 | 0.27 | |
| Fibrobacteres | 0.78 | 0.71 | 0.69 | 1.02 | 0.70 | 0.92 | |
| Verrucomicrobia | 0.80 | 0.82 | 0.52 | 0.32 | 0.31 | 0.04 | |
| Tenericutes | 0.06 | 0.24 | 1.37 | 0.46 | 0.91 | 0.17 | |
| Candidatus Saccharibacteria | 0.38 | 0.19 | 0.47 | 0.56 | 0.42 | 0.69 | |
| Actinobacteria | 0.14 | 0.32 | 0.14 | 0.22 | 0.12 | 0.10 | |
| Lentisphaerae | 0.29 | 0.13 | 0.12 | 0.06 | 0.10 | <0.001 | |
| Elusimicrobia | 0.10 | 0.13 | 0.18 | 0.06 | 0.12 | 0.57 | |
| Cyanobacteria | 0.09 | 0.08 | 0.13 | 0.16 | 0.07 | 0.47 | |
| Planctomycetes | 0.01 | 0.04 | 0.01 | 0.05 | 0.04 | 0.34 | |
| SR1 | 0.00 | 0.01 | 0.02 | 0.05 | 0.05 | 0.56 | |
| Armatimonadetes | 0.01 | 0.02 | 0.01 | 0.02 | 0.01 | 0.82 | |
| Chloroflexi | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.75 | |
| Chlamydiae | 0.00 | 0.02 | 0.005 | 0.00 | 0.20 | 0.40 | |
| Archaea | Euryarchaeota | 0.00 | 0.01 | 0.005 | 0.01 | 0.01 | 0.36 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, S.; Hou, Z.; Dai, Q.; Wu, D. Effects of the Forage Type and Chop Length of Ramie Silage on the Composition of Ruminal Microbiota in Black Goats. Animals 2019, 9, 177. https://doi.org/10.3390/ani9040177
Sun S, Hou Z, Dai Q, Wu D. Effects of the Forage Type and Chop Length of Ramie Silage on the Composition of Ruminal Microbiota in Black Goats. Animals. 2019; 9(4):177. https://doi.org/10.3390/ani9040177
Chicago/Turabian StyleSun, Shengnan, Zhenping Hou, Qiuzhong Dai, and Duanqin Wu. 2019. "Effects of the Forage Type and Chop Length of Ramie Silage on the Composition of Ruminal Microbiota in Black Goats" Animals 9, no. 4: 177. https://doi.org/10.3390/ani9040177
APA StyleSun, S., Hou, Z., Dai, Q., & Wu, D. (2019). Effects of the Forage Type and Chop Length of Ramie Silage on the Composition of Ruminal Microbiota in Black Goats. Animals, 9(4), 177. https://doi.org/10.3390/ani9040177





