Influence of Maternal Factors (Weight, Body Condition, Parity, and Pregnancy Rank) on Plasma Metabolites of Dairy Ewes and Their Lambs
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
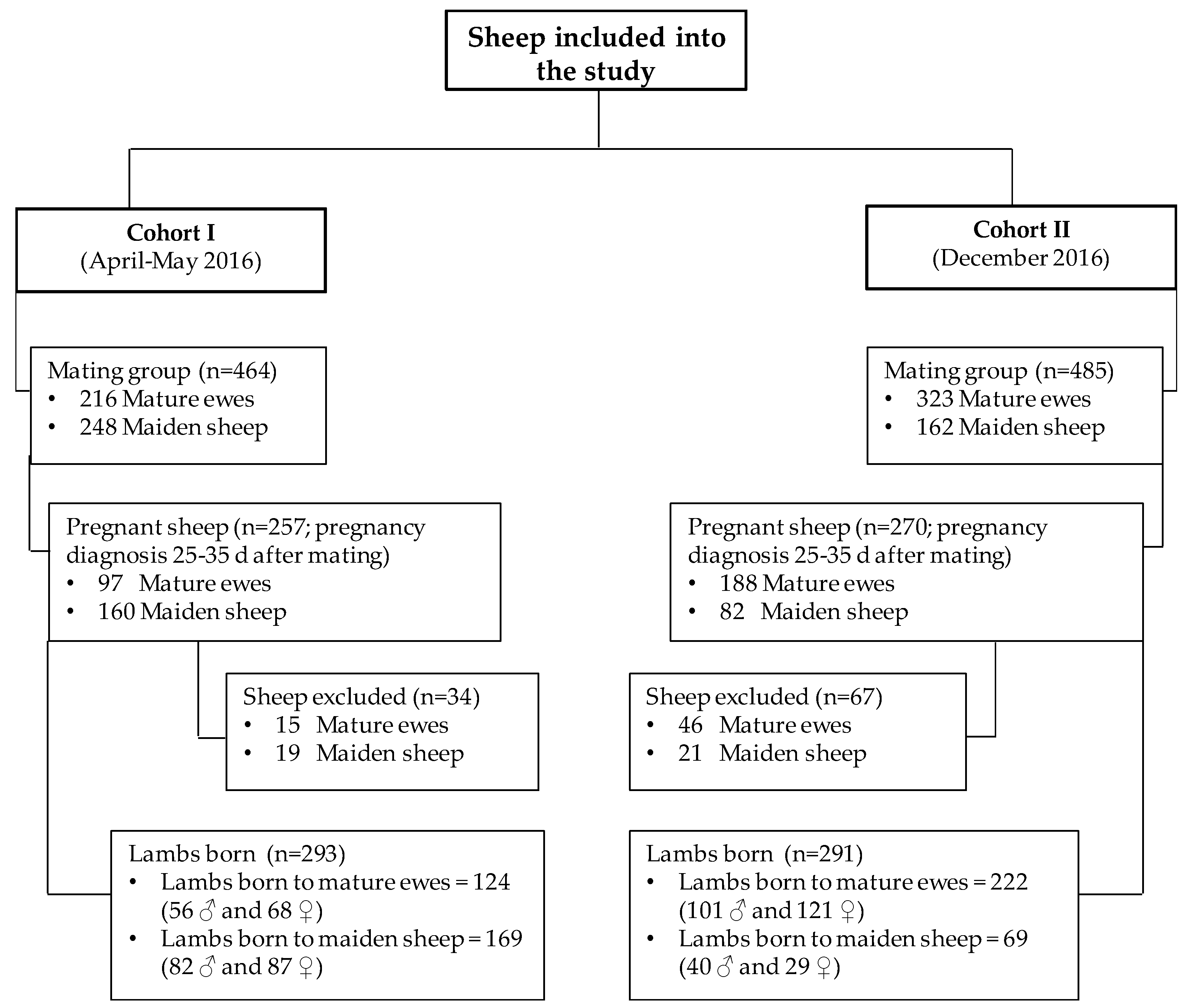
2.1. Animals and Handling
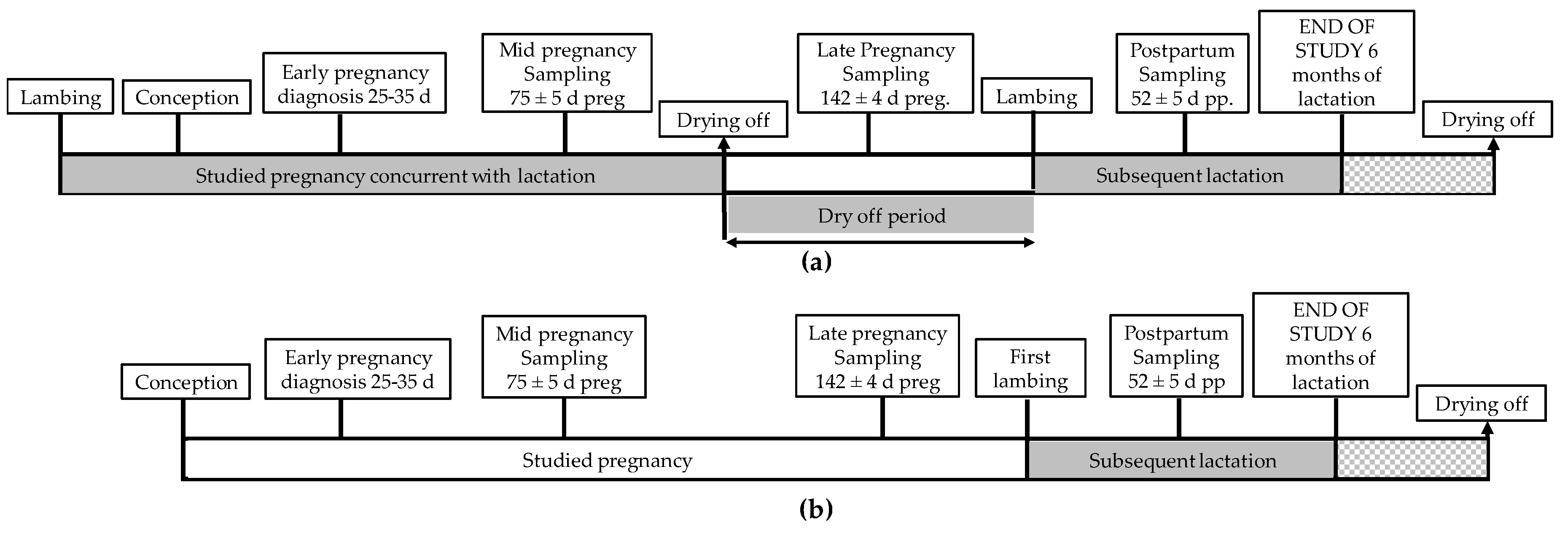
2.2. Study Design and Measured Variables
Parameters recorded and used to categorize and compare sheep and lambs
- Pregnancy rank: single vs. multiple (diagnosed by ultrasonography and verified after lambing)
- Parity: maiden sheep vs. adult sheep
- Body condition score (BCS), as assessed by two trained observers using the 5-point scale (1 = emaciated, 5 = obese) [23]. BCS was determined three times: in mid-pregnancy (BCS-1; 75 ± 5 days of pregnancy), in late pregnancy (BCS-2; 142 ± 4 days of pregnancy), and postpartum (BCS-3; 52 ± 5 days after lambing). On the basis of BCS, sheep were divided into three groups: thin (BCS ≤ 2), average (2 < BCS < 3), or fat (BCS ≥ 3).
- Lamb parameters:
- ○
- Sex of the lamb: Male vs. female
- ○
- Sex of sibling(s) (in multiple lambings)
- ▪
- Male and female siblings
- ▪
- Only male sibling(s) (same as the lamb)
- ▪
- Only female sibling(s) (same as the lamb)
- ○
- Lamb birth weight
2.3. Metabolic Parameters of Sheep
2.4. Assessment of Early Postnatal Development and Metabolic Features of Lambs
2.5. Statistical Analyses
3. Results
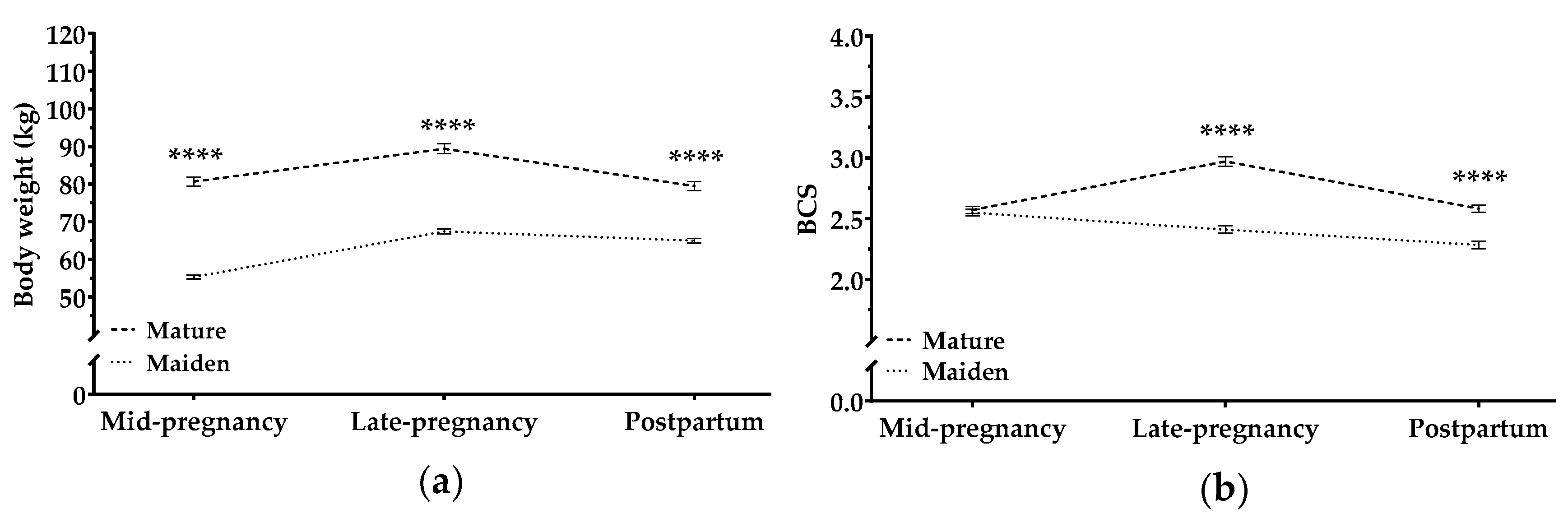
3.1. Changes in Maternal BW and BCS Throughout Pregnancy
3.2. Changes in Maternal Metabolic Indices Throughout Pregnancy
3.2.1. Glucose
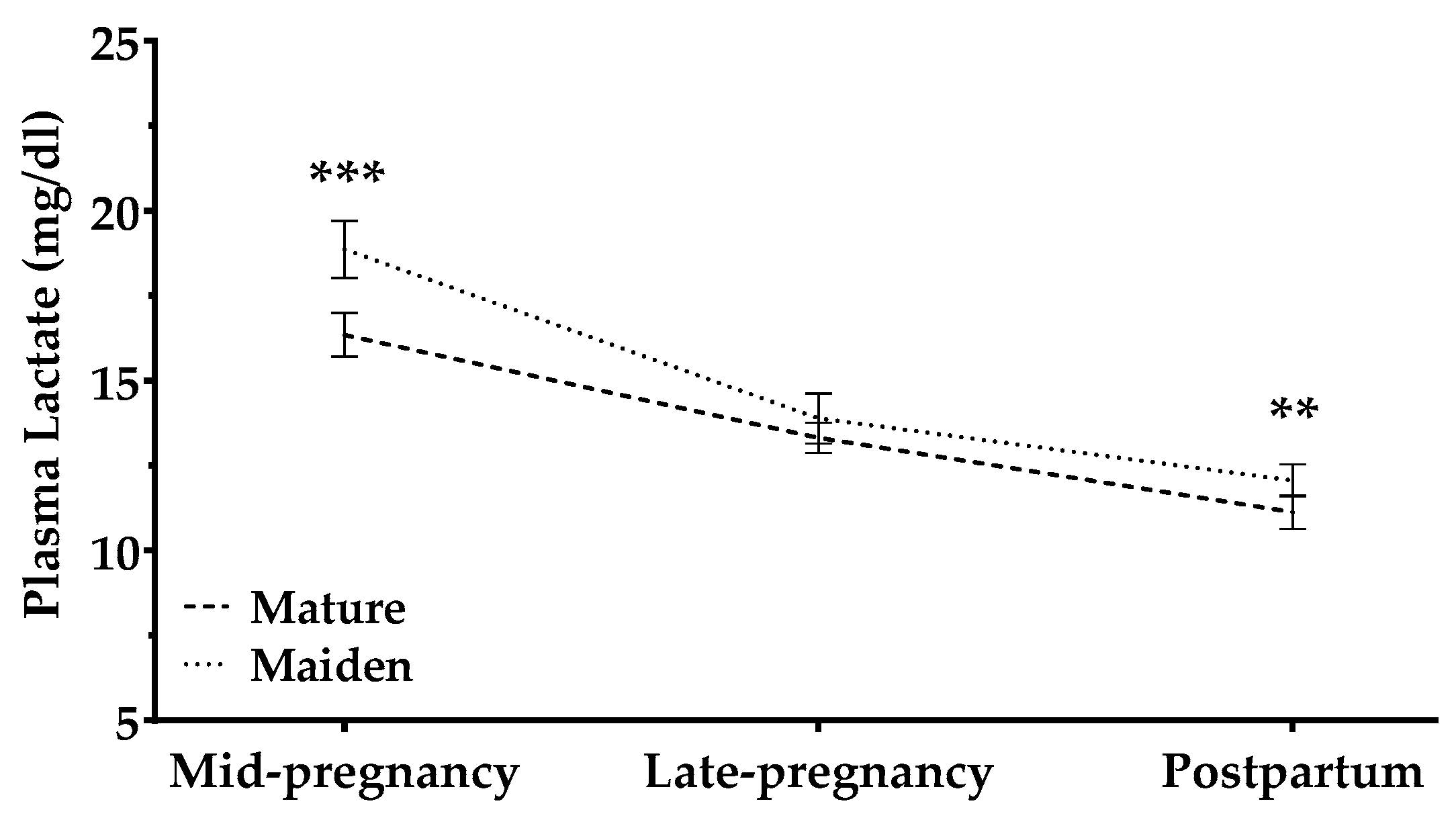
3.2.2. Lactate
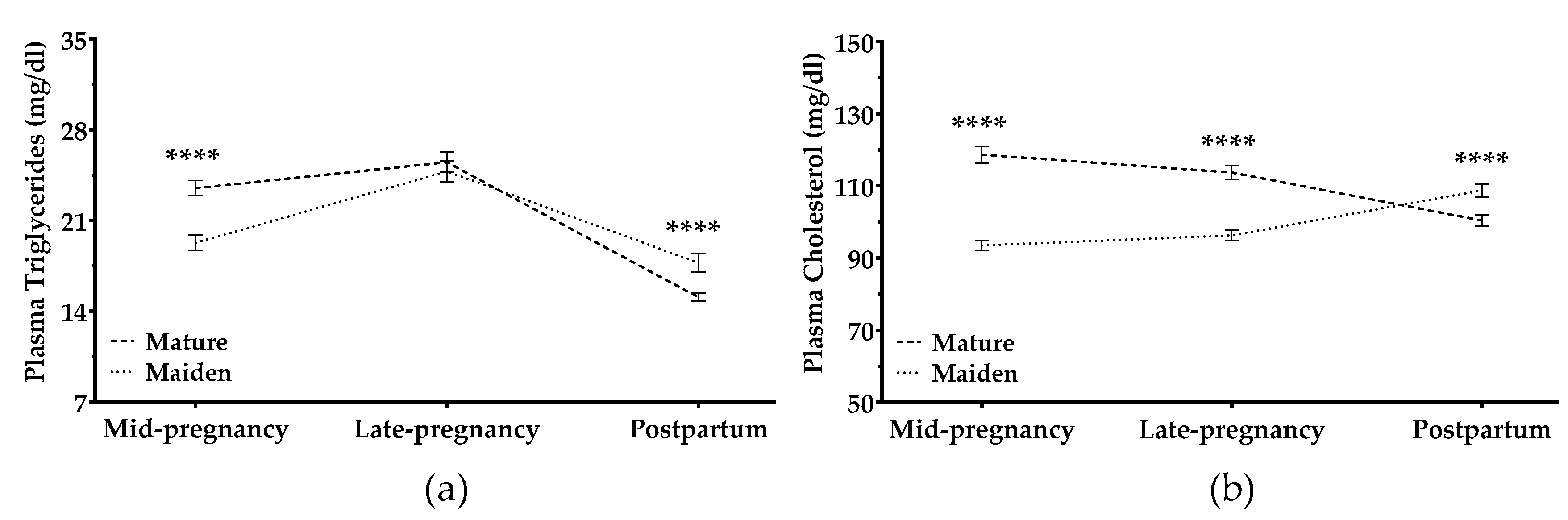
3.2.3. Cholesterol and Triglycerides
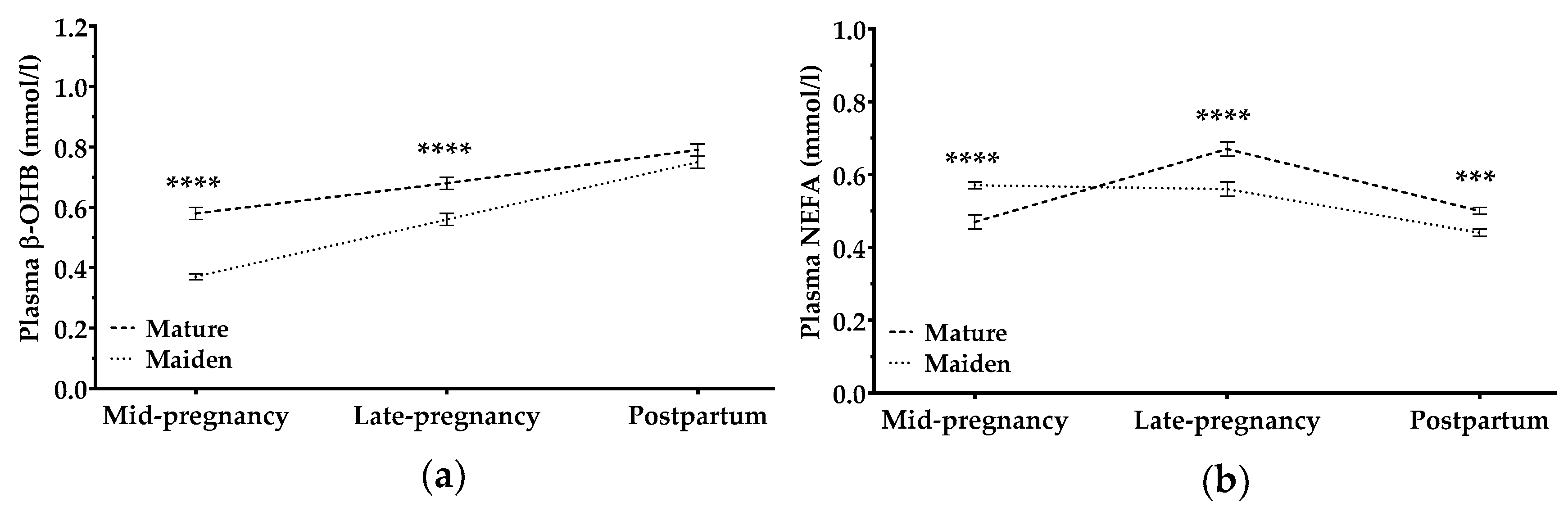
3.2.4. β-Hydroxybutyrate and Non-Esterified Fatty Acids
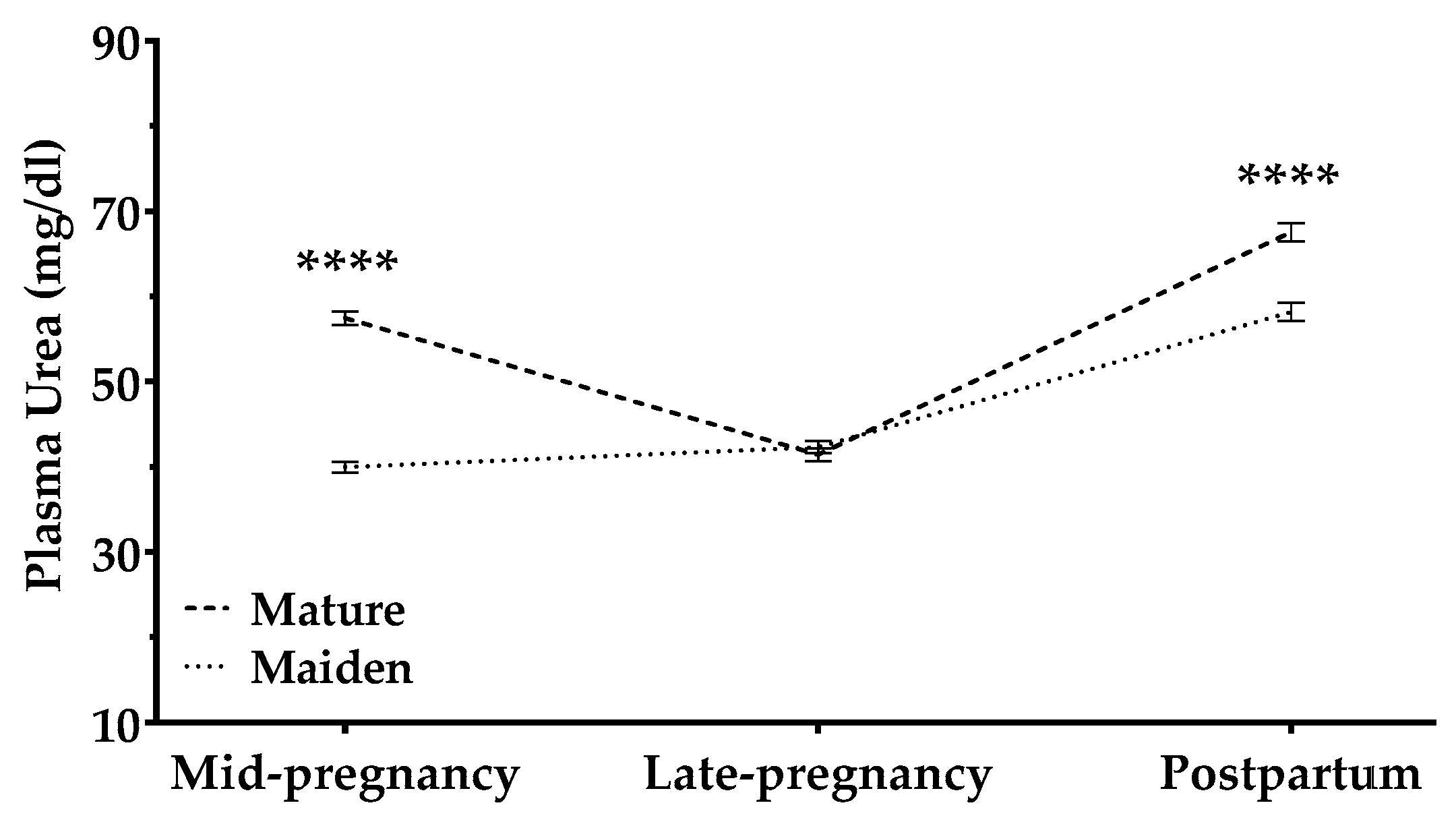
3.2.5. Urea
3.3. BW, Body Size, and Metabolic Phenotype of Lambs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Swali, A.; Wathes, D.C. Influence of Primiparity on Size at Birth, Growth, the Somatotrophic Axis and Fertility in Dairy Heifers. Anim. Reprod. Sci. 2007, 102, 122–136. [Google Scholar] [CrossRef]
- Piccione, G.; Caola, G.; Giannetto, C.; Grasso, F.; Calanni Runzo, S.; Zumbo, A.; Pennisi, P. Selected Biochemical Serum Parameters in Ewes during Pregnancy, Post-Parturition, Lactation and Dry Period. Anim. Sci. Pap. Rep. 2009, 27, 321–330. [Google Scholar]
- Mohammadi, V.; Anassori, E.; Jafari, S. Measure of Energy Related Biochemical Metabolites Changes during Peri-Partum Period in Makouei Breed Sheep. Vet. Res. Forum Int. Q. J. 2016, 7, 35–39. [Google Scholar]
- Bell, A.W. Regulation of Organic Nutrient Metabolism during Transition from Late Pregnancy to Early Lactation. J. Anim. Sci. 1995, 73, 2804–2819. [Google Scholar] [CrossRef]
- Joy, M.; Ripoll-Bosch, R.; Sanz, A.; Molino, F.; Blasco, I.; Álvarez-Rodríguez, J. Effects of Concentrate Supplementation on Forage Intake, Metabolic Profile and Milk Fatty Acid Composition of Unselected Ewes Raising Lambs. Anim. Feed Sci. Technol. 2014, 187, 19–29. [Google Scholar] [CrossRef]
- Hu, G.; Mccutcheon, S.N.; Parker, W.J.; Walsh, P.A. Blood Metabolite Levels in Late Pregnant Ewes as Indicators of Their Nutritional Status. N. Z. J. Agric. Res. 1990, 33, 63–68. [Google Scholar] [CrossRef]
- Russel, A.J.F. Means of Assessing the Adequacy of Nutrition of Pregnant Ewes. Livest. Prod. Sci. 1984, 11, 429–436. [Google Scholar] [CrossRef]
- Sanson, D.W.; West, T.R.; Tatman, W.R.; Riley, M.L.; Judkins, M.B.; Moss, G.E. Relationship of Body Composition of Mature Ewes with Condition Score and Body Weight. J. Anim. Sci. 1993, 71, 1112–1116. [Google Scholar] [CrossRef]
- Bell, A.W.; Bauman, D.E. Adaptations of Glucose Metabolism during Pregnancy and Lactation. J. Mammary Gland Biol. Neoplasia 1997, 2, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Chilliard, Y.; Ferlay, A.; Faulconnier, Y.; Bonnet, M.; Rouel, J.; Bocquier, F. Adipose Tissue Metabolism and Its Role in Adaptations to Undernutrition in Ruminants. Proc. Nutr. Soc. 2000, 59, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Vernon, G.R.; Clegg, A.R.; Flint, J.D. Metabolism of Sheep Adipose Tissue during Pregnancy and Lactation. Adaptation and Regulation. Biochem. J. 1981, 200, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Holtenius, P.; Holtenius, K. New Aspects of Ketone Bodies in Energy Metabolism of Dairy Cows: A Review. J. Vet. Med. Ser. A 1996, 43, 579–587. [Google Scholar] [CrossRef]
- Caldeira, R.M.; Belo, A.T.; Santos, C.C.; Vazques, M.I.; Portugal, A.V. The Effect of Body Condition Score on Blood Metabolites and Hormonal Profiles in Ewes. Small Rumin. Res. 2007, 68, 233–241. [Google Scholar] [CrossRef]
- Bauman, D.E.; Bruce Currie, W. Partitioning of Nutrients during Pregnancy and Lactation: A Review of Mechanisms Involving Homeostasis and Homeorhesis. J. Dairy Sci. 1980, 63, 1514–1529. [Google Scholar] [CrossRef]
- Karen, A.M.; Kovács, P.; Beckers, J.F.; De Sousa, N.M.; Szenci, O. Plasma Urea Nitrogen in Relation to Pregnancy Rate in Dairy Sheep. Anim. Reprod. Sci. 2011, 124, 69–72. [Google Scholar] [CrossRef]
- González-Recio, O.; Ugarte, E.; Bach, A. Trans-Generational Effect of Maternal Lactation during Pregnancy: A Holstein Cow Model. PLoS ONE 2012, 7, e51816. [Google Scholar] [CrossRef]
- Johnston, L.B.; Clark, A.J.L.; Savage, M.O. Genetic Factors Contributing to Birth Weight. Arch. Dis. Child. Fetal Neonatal Ed. 2002, 86, 2F–3F. [Google Scholar] [CrossRef]
- Robinson, J.J.; Rooke, J.; McEvoy, T.G. Nutrition for Conception and Pregnancy. In Sheep Nutrition; Free, M., Dove, H., Eds.; CSIRO: Collingwood, ON, Canada, 2002; pp. 189–211. [Google Scholar]
- Caton, J.S.; Hess, B.W. Maternal Plane of Nutrition: Impacts on Fetal Outcomes and Postnatal Offspring Responses. In Proceedings of the 4th Grazing Livestock Nutrition Conference, Estes Park, CO, Canada, 9–10 July 2010; Hess, B.W., DelCurto, T., Bowman, J.G.P., Waterman, R.C., Eds.; pp. 104–122. [Google Scholar]
- Barker, D.J.P. The Developmental Origins of Well Being. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2004, 359, 1359–1366. [Google Scholar] [CrossRef] [PubMed]
- Gluckman, P.D.; Hanson, M.A. Maternal Constraint of Fetal Growth and Its Consequences. Semin. Fetal Neonatal Med. 2004, 9, 419–425. [Google Scholar] [CrossRef]
- Gardener, D.S.; Buttery, P.L.; Daniel, Z.; Symonds, M.E. Factors Affecting Birth Weight in Sheep: Maternal Environment. Reproduction 2007, 133, 297–307. [Google Scholar] [CrossRef]
- Russel, A.J.F.; Doney, J.M.; Gunn, R.G. Subjective Assessment of Body Fat in Live Sheep. J. Agric. Sci. 1969, 72, 451–454. [Google Scholar] [CrossRef]
- Blomberg, L.A.; Schreier, L.L.; David Guthrie, H.; Sample, G.L.; Vallet, J.; Caperna, T.; Ramsay, T. The Effect of Intrauterine Growth Retardation on the Expression of Developmental Factors in Porcine Placenta Subsequent to the Initiation of Placentation. Placenta 2010, 31, 549–552. [Google Scholar] [CrossRef]
- Vázquez-Gómez, M.; García-Contreras, C.; Torres-Rovira, L.; Astiz, S.; Óvilo, C.; González-Bulnes, A.; Isabel, B. Maternal Undernutrition and Offspring Sex Determine Birth-Weight, Postnatal Development and Meat Characteristics in Traditional Swine Breeds. J. Anim. Sci. Biotechnol. 2018, 9, 27. [Google Scholar] [CrossRef]
- Goodman, E.; Hinden, B.R.; Khandelwal, S. Accuracy of Teen and Parental Reports of Obesity and Body Mass Index. Pediatrics 2000, 106 Pt 1, 52–58. [Google Scholar] [CrossRef]
- Luther, J.; Aitken, R.; Milne, J.; Matsuzaki, M.; Reynolds, L.; Redmer, D.; Wallace, J. Maternal and Fetal Growth, Body Composition, Endocrinology, and Metabolic Status in Undernourished Adolescent Sheep. Biol. Reprod. 2007, 77, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Remond, B.; Cisse, M.; Ollier, A.; Chilliard, Y. Slow Release Somatotropin in Dairy Heifers and Cows Fed Two Levels of Energy Concentrate. 1. Performance and Body Condition. J. Dairy Sci. 1991, 74, 1370–1381. [Google Scholar] [CrossRef]
- Elvira, L.; Hernandez, F.; Cuesta, P.; Cano, S.; Gonzalez-Martin, J.V.; Astiz, S. Factors Affecting the Lactation Curves of Intensively Managed Sheep Based on a Clustering Approach. J. Dairy Res. 2013, 80, 439–447. [Google Scholar] [CrossRef]
- Harmeyer, J.; Schlumbohm, C. Pregnancy Impairs Ketone Body Disposal in Late Gestating Ewes: Implications for Onset of Pregnancy Toxaemia. Res. Vet. Sci. 2006, 81, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Kappel, L.C.; Ingraham, R.H.; Morgan, E.B.; Zeringue, L.; Wilson, D.; Babcock, D.K. Relationship between Fertility and Blood Glucose and Cholesterol Concentrations in Holstein Cows. Am. J. Vet. Res. 1984, 45, 2607–2612. [Google Scholar]
- Vizcarra, J.A.; Wettemann, R.P.; Spitzer, J.C.; Morrison, D.G. Body Condition at Parturition and Postpartum Weight Gain Influence Luteal Activity and Concentrations of Glucose, Insulin, and Nonesterified Fatty Acids in Plasma of Primiparous Beef Cows 1,2. J. Anim. Sci. 1998, 76, 927–936. [Google Scholar] [CrossRef]
- Taylor, V.J.; Beever, D.E.; Bryant, M.J.; Wathes, D.C. Metabolic Profiles and Progesterone Cycles in First Lactation Dairy Cows. Theriogenology 2003, 59, 1661–1677. [Google Scholar] [CrossRef]
- Kaufman, C.F.; Bergman, E.N. Renal Ketone Body Metabolism and Gluconeogenesis in Normal and Hypoglycemic Sheep. Am. J. Physiol. 1974, 226, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Antunovic, Z.; Novoselec, J.; Speranda, M.; Steiner, Z.; Cavar, S. Monitoring of Blood Metabolic Profile and Milk Quality of Ewes during Lactation in Organic Farming. Mljekarstvo J. Dairy Prod. Process. Improv. 2017, 67, 243–252. [Google Scholar] [CrossRef]
- Alonso, A.; Teresa, R.D.E.; Garcia, M.; Gonzalez, R.; Vallejo, M. The Effects of Age and Reproductive Status on Serum and Blood Parameters in Merino Breed Sheep. J. Vet. Med. 1997, 44, 223–231. [Google Scholar] [CrossRef]
- Kassim, W.Y.; Al-Hellou, M.F. Effect of Geographic Location and Age on Levels of Some Biochemical Parameters of Ewes in Southern of Iraq. J. Biosci. Med. 2018, 6, 21–29. [Google Scholar] [CrossRef]
- Seidel, H.; Novotny, J.; Kovac, G. Selected Biochemical Indices in Sheep during Pregnancy and after Parturition. Bull. Vet. Inst. Pulawy 2006, 50, 167–170. [Google Scholar]
- Moallem, U.; Rozov, A.; Gootwine, E.; Honig, H. Plasma Concentrations of Key Metabolites and Insulin in Late-Pregnant Ewes Carrying 1 to 5 Fetuses. J. Anim. Sci. 2015, 90, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Pesantez-Pacheco, J.L.; Torres-Rovira, L.; Hernandez, F.; Sanz-Fernandez, M.V.; Villalobos, N.P.; Heras-Molina, A.; Garcia-Contreras, C.; Vazquez Gomez, M.; Martinez-Ros, P.; Gonzalez-Martin, J.V.; et al. Efficiency and Demographics of a High-Yield Dairy Ewe Farm with Two Managing Systems Involving Five or 10 Lambings per Year. Animal 2018, 12, 2181–2190. [Google Scholar] [CrossRef]
- Abecia, J.A.; Palacios, C. Ewes Giving Birth to Female Lambs Produce More Milk than Ewes Giving Birth to Male Lambs. Ital. J. Anim. Sci. 2018, 17, 736–739. [Google Scholar] [CrossRef]
- Schmitt, E.; Maffi, A.S.; Raimondo, R.F.S.; Lima, M.E.; Hoffmann, D.A.C.; Farofa, T.S.; Montagner, P.; Rincon, J.A.A.; Del Pino, F.A.B.; Correa, M.N. Energetic Metabolic Profile of Ewes Presenting Low Body Condition Score Induced to Subclinical Hypocalcemia in Early Postpartum. Austral J. Vet. Sci. 2018, 50, 15–20. [Google Scholar] [CrossRef]
- Antunovi, Z.; Sencic, D.; Šperanda, M.; Liker, B. Influence of the Season and the Reproductive Status of Ewes on Blood Parameters. Small Rumin. Res. 2002, 45, 39–44. [Google Scholar] [CrossRef]
- Schlumbohm, C.; Harmeyer, J. Twin-Pregnancy Increases Susceptibility of Ewes to Hypoglycaemic Stress and Pregnancy Toxaemia. Res. Vet. Sci. 2008, 84, 286–299. [Google Scholar] [CrossRef]
- Haffaf, S.; Benallou, B. Changes in Energetic Profile of Pregnant Ewes in Relation with the Composition of the Fetal Fluids. Asian Pac. J. Trop. Biomed. 2016, 6, 256–258. [Google Scholar] [CrossRef]
- Pinent, M.; Hackl, H.; Rainer Burkard, T.; Prokesch, A.; Papak, C.; Scheideler, M.; Hammerle, G.; Zechner, R.; Trajanoski, Z.; Gertrude Strauss, J. Differential Transcriptional Modulation of Biological Processes in Adipocyte Triglyceride Lipase and Hormone-Sensitive Lipase-Deficient Mice. Genomics 2008, 92, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Novoselec, J.; Speranda, M.; Klir, Z.; Mioc, B.; Steiner, Z.; Antunovic, Z. Blood Biochemical Indicators and Concentration of Thyroid Hormones in Heavily Pregnant and Lactating Ewes Depending on Selenium Supplementation. Acta Vet. Brno 2017, 86, 353–363. [Google Scholar] [CrossRef]
- Thomas, P.C.; Chamberlain, D.G.; Martin, P.A.; Robertson, S. Dietary Energy Intake and Milk Yield and Composition in Dairy Cows. In Proceedings of the 10th International Symposium on Energy Metabolism, Airlie, VA, USA, 12–17 September 1985; Moe, P.W., Tyrell, F.H., Reynolds, P.W., Eds.; pp. 17–20. [Google Scholar]
- Jalilian, M.T.; Moeini, M.M. The Effect of Body Condition Score and Body Weight of Sanjabi Ewes on Immune System, Productive and Reproductive Performance. Acta Argic. Slov. 2013, 102, 99–106. [Google Scholar] [CrossRef]
- Baird, G.D.; Van Der Walt, J.G.; Bergman, E.N. Whole Body Metabolism of Glucose and Lactate in Productive Sheep and Cows. Br. J. Nutr. 1983, 50, 249–265. [Google Scholar] [CrossRef]
- Canfield, R.W.; Sniffen, C.J.; Butler, W.R. Effects of Excess Degradable Protein on Postpartum Reproduction and Energy Balance in Dairy Cattle. J. Dairy Sci. 1990, 73, 2342–2349. [Google Scholar] [CrossRef]
- Karapehlivan, M.; Atakisi, E.; Atakisi, O.; Yucayurt, R.; Pancarci, S.M. Blood Biochemical Parameters during the Lactation and Dry Period in Tuj Ewes. Small Rumin. Res. 2007, 73, 267–271. [Google Scholar] [CrossRef]
- Kumar, H.; Nakao, T.; Suzuki, T.; Akita, M.; Higaki, T. Relationships between Body Condition Score, Body Weight, and Some Nutritional Parameters in Plasma and Resumption of Ovarian Cyclicity Postpartum during Pre-Service Period in High-Producing Dairy Cows in a Subtropical Region in Japan. Theriogenology 2005, 64, 855–866. [Google Scholar] [CrossRef]
- Holtenius, K.; Agena, S.; Delavaud, C.; Chilliard, Y. Effects of Feeding Intensity during the Dry Period. 2. Metabolic and Hormonal Responses. J. Anim. Sci. 2003, 86, 883–891. [Google Scholar] [CrossRef]
- Lacetera, N.; Bernabucci, U.; Ronchi, B.; Nardone, A. Effects of Subclinical Pregnancy Toxemia on Immune Responses in Sheep. Am. J. Vet. Res. 2001, 62, 1020–1024. [Google Scholar] [CrossRef] [PubMed]
- González-García, E.; Tesniere, A.; Camous, S.; Bocquier, F.; Barillet, F.; Hassoun, P. The Effects of Parity, Litter Size, Physiological State, and Milking Frequency on the Metabolic Profile of Lacaune Dairy Ewes. Domest. Anim. Endocrinol. 2015, 50, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Douglas, G.N.; Overton, T.R.; Bateman, H.G.; Dann, H.M.; Drackley, J.K. Prepartal Plane of Nutrition, Regardless of Dietary Energy Source, Affects Periparturient Metabolism and Dry Matter Intake in Holstein Cows. J. Dairy Sci. 2006, 89, 2141–2157. [Google Scholar] [CrossRef]
- Butler, S.T.; Pelton, S.H.; Butler, W.R. Energy Balance, Metabolic Status, and the First Postpartum Ovarian Follicle Wave in Cows Administered Propylene Glycol. J. Dairy Sci. 2006, 89, 2938–2951. [Google Scholar] [CrossRef]
- Fırat, A.; Ozpınar, A. Metabolic Profile of Pre-Pregnancy, Pregnancy and Early Lactation in Multiple Lambing Sakiz Ewes. 2. Changes in Plasma Progesterone, Estradiol-17beta and Cholesterol Levels. Anim. Nutr. Metab. 2002, 46, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Cal-Pereyra, L.; Benech, A.; González-Montaña, J.R.; Acosta-Dibarrat, J.; Da Silva, S.; Martin, A. Changes in the Metabolic Profile of Pregnant Ewes to an Acute Feed Restriction in Late Gestation. N. Z. Vet. J. 2015, 63, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Durak, M.H.; Altiner, A. Effect of Energy Deficiency during Late Pregnancy in Chios Ewes on Free Fatty Acids, β-Hydroxybutyrate and Urea Metabolites. Turkish J. Vet. Anim. Sci. 2006, 30, 497–502. [Google Scholar]
- Barillet, M.C.; Such, X.; Bocquier, F.; Caja, G. Nutrition, Alimentation et Élevage Des Brebis Laitières. Maîtrise de Facteurs de Production Pour Réduire Les Coûts et Améliorer La Qualité Des Produits. In Options Méditerranéennes: Série B. Etudes et Recherches: Série B. Etudes et Recherches; CIHEAM: Zaragoza, Spain, 2002; pp. 57–71. [Google Scholar]
- Thomas, D.L.; Berger, Y.M.; McKusick, B.C.; Mikolayunas, C.M. Dairy Sheep Production Research at the University of Wisconsin-Madison, USA—A Review. J. Anim. Sci. Biotechnol. 2014, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Berry, M.J.; Jaquiery, A.L.; Oliver, M.H.; Harding, J.E.; Bloomfield, F.H. Neonatal Milk Supplementation in Lambs Has Persistent Effects on Growth and Metabolic Function That Differ by Sex and Gestational Age. Br. J. Nutr. 2016, 116, 1912–1925. [Google Scholar] [CrossRef]
- Ramos Nieves, J.M.; Bernal Santos, G.; Faciola, A.; Van Ambrugh, M.E.; Boisclair, Y.R. Effects of Birth Weight and Dietary Caloric Density on Growth, Voluntary Intake and Body Composition of Newborn Lambs. In Energy and Protein Metablism and Nutrition, Proceedings of the 3rd EAAP International Symposiumon Energy and Protein Metabolism and Nutrition, Parma, Italy, 6–10 September 2010; Crovetto Mateo, G., Ed.; Wageningen Academic: Parma, Italy, 2010; pp. 215–216. [Google Scholar]
- Greenwood, P.L.; Bell, A.W. Consequences of Intrauterine Growth Retardation for Postnatal Growth, Metabolism and Pathophysiology. Reprod. Suppl. 2003, 61, 195–206. [Google Scholar] [PubMed]
- Thorn, S.R.; Rozance, P.J.; Brown, L.D.; Hay, W.W. The Intrauterine Growth Restriction Phenotype: Fetal Adaptations and Potential Implications for Later Life Insulin Resistance and Diabetes. Semin. Reprod. Med. 2011, 29, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Gama, L.T.; Dickerson, G.E.; Young, L.D.; Leymaster, K.A. Effects of Breed, Heterosis, Age of Dam, Litter Size, and Birth Weight on Lamb Mortality. J. Anim. Sci. 1991, 69, 2727–2743. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, P.L.; Hunt, A.S.; Hermanson, J.W.; Bell, A.W. Effects of Birth Weight and Postnatal Nutrition on Neonatal Sheep: I. Body Growth and Composition, and Some Aspects of Energetic Efficiency. J. Anim. Sci. 1998, 76, 2354–2367. [Google Scholar] [CrossRef]

| Parameters | 2016 | 2017 | 2018 |
|---|---|---|---|
| TMY (l) | 319 | 368 | 358 |
| YDIM (l/day) | 1.62 | 1.81 | 1.74 |
| LL (days) | 198 | 202 | 204 |
| ILI (days) | 262 | 266 | 269 |
| DPL (days) | 63 | 65 | 66 |
| Characteristic | Males (n = 255) | Females (n = 291) |
|---|---|---|
| Morphometric Characteristics | ||
| Birth body weight, kg | 4.14 ± 0.95 a | 3.79 ± 0.86 b |
| Body weight, 17 days *, kg | 8.12 ± 2.24 | 7.84 ± 2.02 |
| Average daily weight gain, kg | 0.238 ± 0.08 | 0.227 ± 0.06 |
| Birth trunk length, cm | 29.41 ± 2.37 e | 29.01 ± 2.44 f |
| Trunk length 17 days *, cm | 37.46 ± 4.17 | 37.49 ± 3.86 |
| Birth BMI-1, kg/m2 | 47.27 ± 6.33 a | 44.55 ± 6.89 b |
| BMI-2, 17 days *, kg/m2 | 57.28 ± 10.84 c | 55.20 ± 9.46 d |
| Thoracic girth, 17 days *, cm | 44.38 ± 4.45 | 44.32 ± 3.95 |
| Abdominal girth, 17 days *, cm | 44.55 ± 5.08 | 43.77 ± 4.66 |
| Metabolic Indices at 17 Days | ||
| Glucose, mg/dL | 123.1 ± 26.56 e | 120.3 ± 23.54 f |
| Lactate, mg/dL | 19.5 ± 5.40 | 18.9 ± 5.58 |
| Cholesterol, mg/dL | 92.9 ± 18.98 e | 96.5 ± 20.06 f |
| Triglycerides, mg/dL | 60.4 ± 36.33 e | 54.3 ± 30.41 f |
| β-OHB, mmol/L | 0.134 ± 0.07 | 0.134 ± 0.06 |
| NEFA, mmol/L | 0.533 ± 0.17 | 0.537 ± 0.16 |
| Urea, mg/dL | 31.8 ± 8.09 | 33.1 ± 9.72 |
| Parameters | Normal Birth Weight (n = 455) | Low Birth Weight (n = 122) | Males with Low Birth Weight (n = 50) | Females with Low Birth Weight (n = 72) |
|---|---|---|---|---|
| Morphometric Characteristics | ||||
| Birth body weight, kg | 4.27 ± 0.67 a | 2.62 ± 0.42 b | 2.66 ± 0.38 | 2.60 ± 0.46 |
| Body weight, 17 days *, kg | 8.39 ± 2.02 a | 6.24 ± 1.64 b | 6.21 ± 1.49 | 6.27 ± 1.74 |
| Average daily weight gain, kg | 0.237 ± 0.07 c | 0.213 ± 0.08 d | 0.225 ± 0.10 | 0.205 ± 0.06 |
| Birth trunk length, cm | 29.9 ± 1.73 a | 26 ± 2.04 b | 25.9 ± 1.81 | 26.1 ± 2.21 |
| Trunk length 17 days *, cm | 38.4 ± 3.49 a | 33.6 ± 3.67 b | 32.8 ± 3.08 | 34.2 ± 3.93 |
| Birth BMI-1, kg/m2 | 47.5 ± 5.81 a | 38.9 ± 5.77 b | 39.6 ± 4.62 | 38.4 ± 6.43 |
| BMI-2, 17 days *, kg/m2 | 56.5 ± 10.02 | 54.8 ± 10.72 | 57.6 ± 11.92 c | 53.0 ± 9.53 d |
| Thoracic girth, 17 days *, cm | 45.08 ± 3.93 a | 40.83 ± 3.59 b | 40.52 ± 3.56 | 41.03 ± 3.62 |
| Abdominal girth, 17 days *, cm | 45.01 ± 4.74 a | 40.78 ± 4.21 b | 40.91 ± 3.83 | 40.71 ± 4.47 |
| Metabolic Indices at 17 Days | ||||
| Glucose, mg/dL | 122.3 ± 25.93 | 118.4 ± 22.86 | 121.9 ± 20.00 | 116.2 ± 23.28 |
| Lactate, mg/dL | 19.2 ± 5.52 | 20.2 ± 5.17 | 19.6 ± 4.84 | 20.2 ± 5.40 |
| Cholesterol, mg/dL | 92.8 ± 17.99 c | 98.1 ± 22.11 d | 99.4 ± 21.56 | 97.3 ± 22.58 |
| Triglycerides, mg/dL | 56.6 ± 32.70 | 63.1 ± 37.78 | 65.7 ± 43.45 | 58.5 ± 33.79 |
| β-OHB, mmol/L | 0.135 ± 0.07 | 0.129 ± 0.06 | 0.134 ± 0.07 | 0.126 ± 0.06 |
| NEFA, mmol/L | 0.545 ± 0.17 c | 0.482 ± 0.14 d | 0.475 ± 0.14 | 0.486 ± 0.14 |
| Urea, mg/dL | 33.2 ± 8.76 e | 31.2 ± 10.27 f | 30.2 ± 5.98 | 31.9 ± 12.23 |
| Parameters | Lambs Born to | Lambs Born as | ||
|---|---|---|---|---|
| Mature Ewes | Maiden Sheep | Single Pregnancy | Multiple Pregnancy | |
| Morphometric Characteristics | (n = 317) | (n = 224) | (n = 163) | (n = 378) |
| Birth body weight, kg | 4.25 ± 0.84 a | 3.53 ± 0.87 b | 4.28 ± 0.91 a | 3.81 ± 0.89 b |
| Body weight, 17 days *, kg | 8.80 ± 2.05 a | 6.80 ± 1.63 b | 8.06 ± 2.11 | 7.94 ± 2.14 |
| Average daily weight gain, kg | 0.244 ± 0.08 a | 0.215 ± 0.07 b | 0.229 ± 0.07 | 0.233 ± 0.07 |
| Birth trunk length, cm | 29.93 ± 2.04 a | 28.16 ± 2.54 b | 29.96 ± 2.14 a | 28.87 ± 2.46 b |
| Trunk length 17 days *, cm | 39.17 ± 3.62 a | 35.05 ± 3.19 b | 37.80 ± 3.91 | 37.32 ± 4.03 |
| Birth BMI-1, kg/m2 | 47.15 ± 6.62 a | 43.85 ± 6.04 b | 47.27 ± 7.03 a | 45.14 ± 6.55 b |
| BMI-2, 17 days *, kg/m2 | 56.95 ± 9.65 g | 55.20 ± 10.85 h | 56.00 ± 10.66 | 56.33 ± 10.00 |
| Thoracic girth, 17 days *, cm | 45.81 ± 3.96 a | 42.11 ± 3.61 b | 44.43 ± 4.23 | 44.22 ± 4.23 |
| Abdominal girth, 17 days *, cm | 45.87 ± 4.70 a | 41.83 ± 4.22 b | 44.71 ± 4.68 | 43.99 ± 5.01 |
| Metabolic Indices at 17 Days | ||||
| Glucose, mg/dL | 121.6 ± 24.83 | 121.6 ± 26.16 | 123.1 ± 29.43 | 120.9 ± 23.37 |
| Lactate, mg/dL | 18.8 ± 5.31 e | 20.0 ± 5.53 f | 19.2 ± 5.30 | 19.3 ± 5.49 |
| Cholesterol, mg/dL | 94.2 ± 18.04 | 93.4 ± 20.32 | 93.5 ± 18.42 | 94.0 ± 19.27 |
| Triglycerides, mg/dL | 54.7 ± 33.20 e | 61.6 ± 34.50 f | 54.4 ± 27.19 | 58.9 ± 36.38 |
| β-OHB, mmol/L | 0.132 ± 0.06 | 0.136 ± 0.07 | 0.131 ± 0.06 | 0.135 ± 0.07 |
| NEFA, mmol/L | 0.542 ± 0.17 | 0.518 ± 0.15 | 0.519 ± 0.15 | 0.538 ± 0.18 |
| Urea, mg/dL | 33.9 ± 9.97 c | 31.3 ± 7.19 d | 32.8 ± 7.70 | 32.9 ± 9.55 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pesántez-Pacheco, J.L.; Heras-Molina, A.; Torres-Rovira, L.; Sanz-Fernández, M.V.; García-Contreras, C.; Vázquez-Gómez, M.; Feyjoo, P.; Cáceres, E.; Frías-Mateo, M.; Hernández, F.; et al. Influence of Maternal Factors (Weight, Body Condition, Parity, and Pregnancy Rank) on Plasma Metabolites of Dairy Ewes and Their Lambs. Animals 2019, 9, 122. https://doi.org/10.3390/ani9040122
Pesántez-Pacheco JL, Heras-Molina A, Torres-Rovira L, Sanz-Fernández MV, García-Contreras C, Vázquez-Gómez M, Feyjoo P, Cáceres E, Frías-Mateo M, Hernández F, et al. Influence of Maternal Factors (Weight, Body Condition, Parity, and Pregnancy Rank) on Plasma Metabolites of Dairy Ewes and Their Lambs. Animals. 2019; 9(4):122. https://doi.org/10.3390/ani9040122
Chicago/Turabian StylePesántez-Pacheco, Jose Luis, Ana Heras-Molina, Laura Torres-Rovira, María Victoria Sanz-Fernández, Consolación García-Contreras, Marta Vázquez-Gómez, Pablo Feyjoo, Elisa Cáceres, Millán Frías-Mateo, Fernando Hernández, and et al. 2019. "Influence of Maternal Factors (Weight, Body Condition, Parity, and Pregnancy Rank) on Plasma Metabolites of Dairy Ewes and Their Lambs" Animals 9, no. 4: 122. https://doi.org/10.3390/ani9040122
APA StylePesántez-Pacheco, J. L., Heras-Molina, A., Torres-Rovira, L., Sanz-Fernández, M. V., García-Contreras, C., Vázquez-Gómez, M., Feyjoo, P., Cáceres, E., Frías-Mateo, M., Hernández, F., Martínez-Ros, P., González-Martin, J. V., González-Bulnes, A., & Astiz, S. (2019). Influence of Maternal Factors (Weight, Body Condition, Parity, and Pregnancy Rank) on Plasma Metabolites of Dairy Ewes and Their Lambs. Animals, 9(4), 122. https://doi.org/10.3390/ani9040122





