A Summer Mortality Outbreak of Lactococcosis by Lactococcus garvieae in a Raceway System Affecting Farmed Rainbow Trout (Oncorhynchus mykiss) and Brook Trout (Salvelinus fontinalis)
Simple Summary
Abstract
1. Introduction
2. Material and Methods
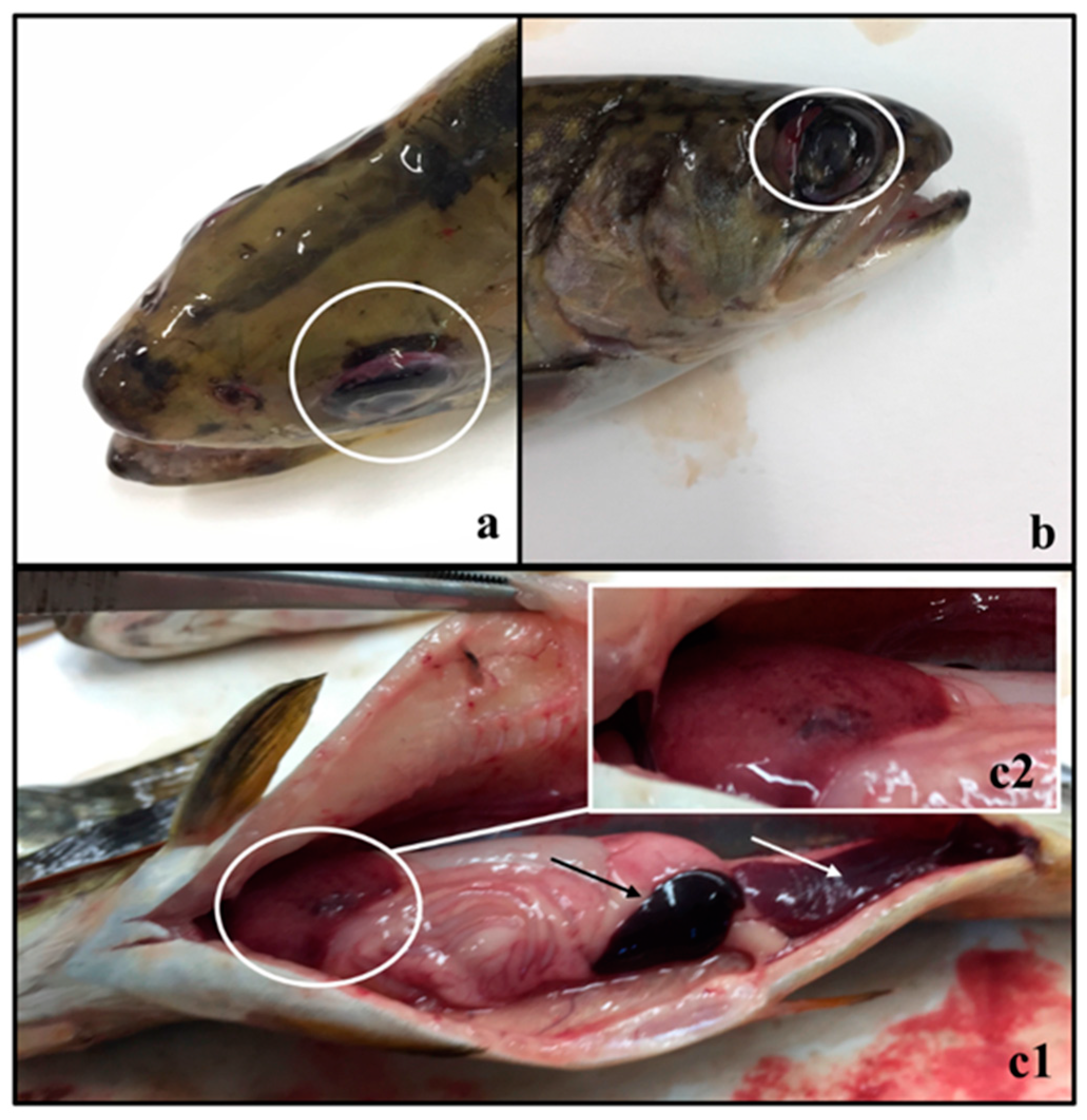
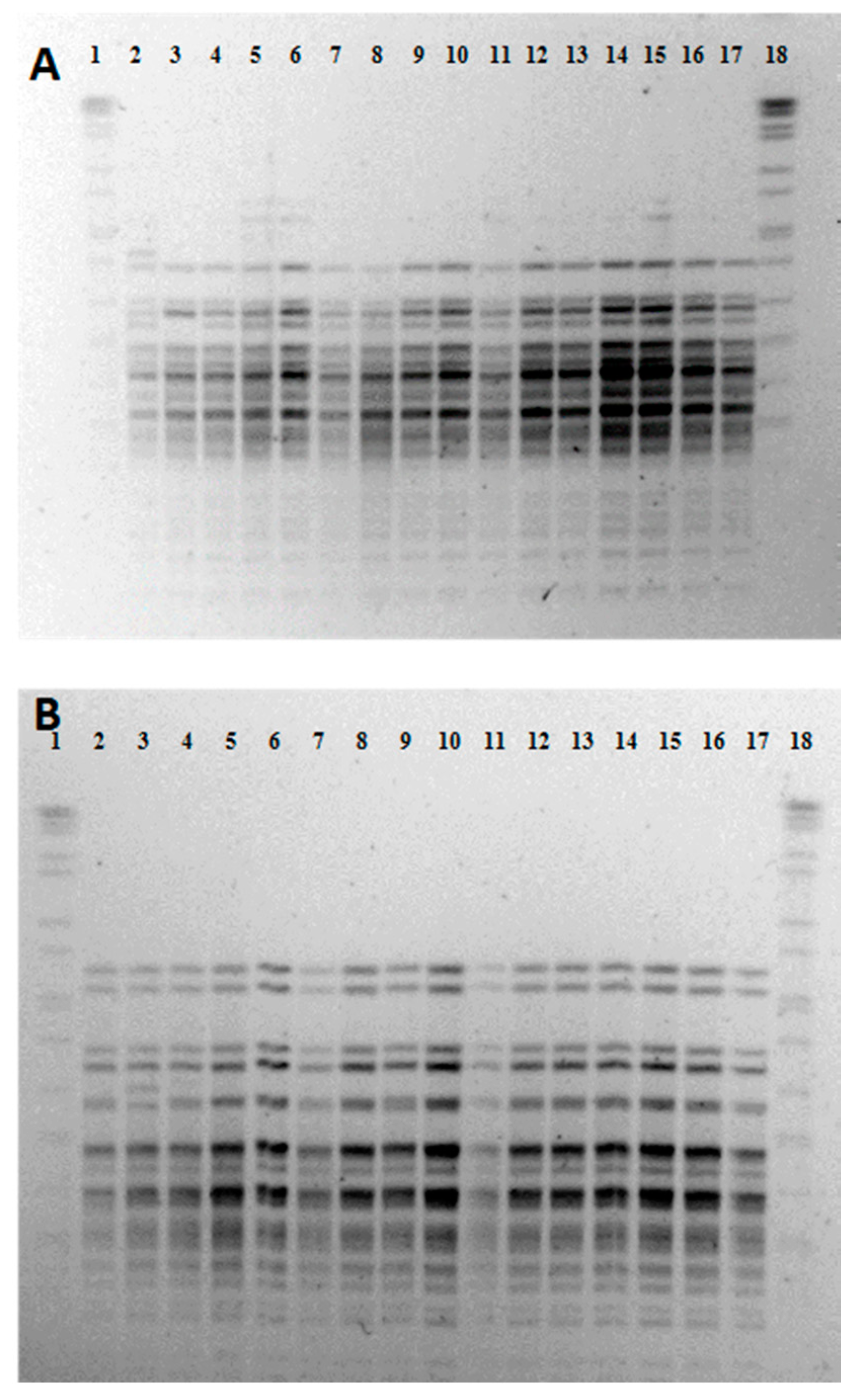
3. Results and Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Osman, K.M.; Al-Maary, K.S.; Mubarak, A.S.; Dawoud, T.M.; Moussa, I.M.I.; Ibrahim, M.D.S.; Hessain, A.M.; Orabi, A.; Fawzy, N.M. Characterization and susceptibility of streptococci and enterococci isolated from Nile tilapia (Oreochromis niloticus) showing septicaemia in aquaculture and wild sites in Egypt. BMC Vet. Res. 2017, 13, 357. [Google Scholar] [CrossRef]
- Eldar, A.; Ghittino, C. Lactococcus garvieae and Streptococcus iniae infection in rainbow trout (Oncorhynchus mykiss): Similar but different diseases. Dis. Aquat. Org. 1999, 36, 227–231. [Google Scholar] [CrossRef]
- Wallbanks, S.; Martinez-Murcia, A.J.; Fryer, J.L.; Phillips, B.A.; Collins, M.D. 16s rRNA Sequence Determination for Carnobacterium and Related Lactic Acid of Vagococcus salmoninarum sp. nov. Members of the Genus Bacteria and Description. Int. J. Syst. Evol. 1990, 40, 224–230. [Google Scholar] [CrossRef]
- Michel, C.; Nougayrede, P.; Eldar, A.; Sochon, E.; de Kinkelin, P. Vagococcus salmoninarum, a bacterium of pathological significance in rainbow trout (Oncorhynchus mykiss) farming. Dis. Aquat. Organ. 1997, 30, 189–208. [Google Scholar] [CrossRef]
- Vendrell, D.; Balcázar, J.L.; Ruiz-Zarzuela, I.; de Blas, I.; Gironés, O.; Múzquiz, J.L. Lactococcus garvieae in fish: A review. Comp. Immunol. Microbiol. Infect. Dis. 2006, 29, 177–198. [Google Scholar] [CrossRef] [PubMed]
- Ghittino, C.; Prearo, M. Report of Streptococcosis in rainbow trout (Oncorhynchus mykiss) in Italy: Preliminary note. Boll. Soc. Ital. Patol. Ittica 1992, 8, 4–11. [Google Scholar]
- Colorni, A.; Ravelo, C.; Romalde, J.L.; Toranzo, A.E.; Diamant, A. Lactococcus garvieae in wild Red Sea wrasse Coris aygula (Labridae). Dis. Aquat. Org. 2003, 56, 275–278. [Google Scholar] [CrossRef]
- Evans, J.J.; Klesius, P.H.; Gilbert, P.M.; Shoemaker, C.A.; Al Sarawi, M.A.; Landsberg, J.; Duremdez, R.; Al Marzouk, A.; Al Zenki, S. Characterization of β—Haemolytic Group B Streptococcus agalactiae in cultured seabream, Sparus auratus L., and wild mullet, Liza klunzingeri (Day), in Kuwait. J. Fish Dis. 2002, 25, 505–513. [Google Scholar] [CrossRef]
- Doménech, A.; Fernández-Garayzábal, J.F.; Pascual, C.; Garcia, J.A.; Cutuli, M.T.; Moreno, M.A.; Collins, M.D.; Domínguez, L. Streptococcosis in cultured turbot, Scophthalmus maximus (L.), associated with Streptococcus parauberis. J. Fish Dis. 1996, 19, 33–38. [Google Scholar] [CrossRef]
- Collins, M.D.; Farrow, J.A.; Phillips, B.A.; Kandler, O. Streptococcus garvieae sp. nov. and Streptococcus plantarum sp. nov. J. Gen. Microbiol. 1983, 129, 3427–3431. [Google Scholar] [CrossRef]
- Eldar, A.; Ghittino, C.; Asanta, L.; Bozzetta, E.; Goria, M.; Prearo, M.; Bercovier, H. Enterococcus seriolicida is a junior synonym of Lactococcus garvieae, a causative agent of septicemia and meningoencephalitis in fish. Curr. Microbiol. 1996, 32, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Meyburgh, C.M.; Bragg, R.R.; Boucher, C.E. Lactococcus garvieae: An emerging bacterial pathogen of fish. Dis. Aquat. Org. 2017, 123, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, L.M.; Merquior, V.L.C.; Vianni, M.C.E.; Carvalho, M.G.S.; Fracalanzza, S.E.L.; Steigerwalt, A.G.; Brenner, D.J.; Facklam, R.R. Phenotypic and genotypic characterization of atypical Lactococcus garvieae strains isolated from water buffalos with subclinical mastitis and confirmation of L. garvieae as a senior subjective synonym of Enterococcus seriolicida. Int. J. Syst. Bacteriol. 1996, 46, 664–668. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.F.W.; Woo, P.C.Y.; Teng, J.L.L.; Lau, S.K.P.; Leung, S.S.M.; Tam, F.C.C.; Yuen, K.Y. Primary infective spondylodiscitis caused by Lactococcus garvieae and a review of human L. garvieae infections. Infection 2011, 39, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Ceschia, G.; Giorgetti, G.; Giavenni, R.; Sarti, M. A new problem for Italian trout farms: Streptococcosis in rainbow trout (Oncorhynchus mykiss). Bull. Eur. Assoc. Fish Pathol. 1992, 12, 2–72. [Google Scholar]
- Hoshina, T.; Sano, T.; Morimoto, Y. A Streptococcus pathogenic to fish. J. Tokyo Univ. Fish. 1958, 44, 57–58. [Google Scholar]
- Prieta, J.; Domènech, A.M.; Fernández-Garayzábal, J.F.; Collins, M.D.; Rodrígues, U.M.; Jones, D. Lactococcosis de la trucha arco iris (Oncorhynchus mykiss). Med. Vet. 1993, 10, 367–673. [Google Scholar]
- Eyngor, M.; Zlotkin, A.; Ghittino, C.; Prearo, M.; Douet, D.G.; Chilmonczyk, S.; Eldar, A. Clonality and Diversity of the Fish Pathogen Lactococcus garvieae in Mediterranean Countries. Appl. Environ. Microbiol. 2004, 70, 5132–5137. [Google Scholar] [CrossRef]
- Bercovier, H.C.; Ghittino, C.; Eldar, A. Immunization with bacterial antigens: Infections with streptococci and related organisms. Dev. Biol. Stand. 1997, 90, 153–160. [Google Scholar]
- Perera, R.P.; Johnson, S.K.; Collins, M.D.; Lewis, D.H. Streptococcus iniae associated with mortality of Tilapia nilotica X T. aurea hybrids. J. Aquat. Anim. Health 1994, 6, 335–340. [Google Scholar] [CrossRef]
- Afonso, A.; Silva, J.; Gomes, S. Lactococcus garvieae trout infections in Portugal: A new challenge on fish vaccinology. IBMC News 2003, 7, 4–6. [Google Scholar]
- Algöet, M.; Bayley, A.E.; Roberts, E.G.; Feist, S.W.; Wheeler, R.W.; Verner-Jeffreys, D.W. Susceptibility of selected freshwater fish species to a UK Lactococcus garvieae isolate. J. Fish Dis. 2009, 32, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Zlotkin, A.; Eldar, A.; Ghittino, C.; Bercovier, H. Identification of Lactococcus garvieae by PCR. J. Clin. Microbiol. 1998, 36, 983–998. [Google Scholar] [PubMed]
- Vela, A.I.; Vazquez, J.; Gibello, A.; Blanco, M.M.; Moreno, M.A.; Liebana, P.; Albendea, C.; Alcala, B.; Mendez, A.; Dominguez, L.; et al. Phenotypic and genetic characterization of Lactococcus garvieae isolated in Spain from lactococcosis outbreaks and comparison with isolates of other countries and sources. J. Clin. Microbiol. 2000, 38, 3791–3795. [Google Scholar]
- Tejedor, J.L.; Vela, A.I.; Gibello, A.; Casamayor, A.; Domínguez, L.; Fernández-Garayzábal, J.F. A genetic comparison of pig, cow and trout isolates of Lactococcus garvieae by PFGE analysis. Lett. Appl. Microbiol. 2011, 53, 614–619. [Google Scholar] [CrossRef]
- Kusuda, K.; Kawai, K.; Salati, F.; Banner, C.R.; Fryer, J.L. Enterococcus seriolicida sp. nov., a fish pathogen. Int. J. Syst. Bacteriol. 1991, 41, 406–409. [Google Scholar] [CrossRef]
- Lee, D.C.; Lee, J.I.; Park, C.I.; Park, S.I. The study on the causal agent of Streptococcosis (Lactococcus garvieae), isolated from cultured marine fishes. J. Fish Pathol. 2001, 14, 71–80. [Google Scholar]
- Chen, S.C.; Lin, Y.D.; Liaw, L.L.; Wang, P.C. Lactococcus garvieae infection in the giant freshwater prawn Macrobranchium rosenbergii confirmed by polymerase chain reaction and 16S rDNA sequencing. Dis. Aquat. Org. 2001, 45, 45–52. [Google Scholar] [CrossRef]
- Chen, S.C.; Liaw, L.L.; Su, H.Y.; Ko, S.C.; Wu, C.Y.; Chang, H.C.; Tsai, Y.H.; Yang, K.L.; Chen, Y.C.; Chen, T.H.; et al. Lactococcus garvieae, a cause of disease in grey mullet, Mugil cephalus L., in Taiwan. J. Fish Dis. 2002, 25, 727–732. [Google Scholar] [CrossRef]
- Ravelo, C.; Magarinos, B.; López-Romalde, S.; Toranzo, A.E.; Romalde, J.L. Molecular fingerprinting of fish-pathogenic Lactococcus garvieae strains by random amplified polymorphic DNA analysis. J. Clin. Microbiol. 2003, 41, 751–756. [Google Scholar] [CrossRef]
- Kang, S.H.; Shin, G.W.; Shin, Y.S.; Palaksha, K.J.; Kim, Y.R.; Yang, H.H.; Lee, E.Y.; Lee, E.G.; Huh, N.E.; Ju, O.M.; et al. Experimental evaluation of pathogenicity of Lactococcus garvieae in black rockfish (Sebastes schlegeli). J. Vet. Sci. 2004, 5, 387–390. [Google Scholar] [CrossRef] [PubMed]
- Kawanishi, M.; Kojima, A.; Ishihara, K.; Esaki, H.; Kijima, M.; Takahashi, T.; Suzuki, S.; Tamura, Y. Drug resistance and pulsed-field gel electrophoresis patterns of Lactococcus garvieae isolates from cultured Seriola (yellowtail, amberjack and kingfish) in Japan. Lett. Appl. Microbiol. 2005, 40, 322–328. [Google Scholar] [CrossRef]
- Ghittino, C.; Muzquiz, J.L. La estreptococosis de la trucha arco iris en Espana. Resumen de lo expuesto en la reunion de piscicultores de Zaragoza del 26 de enero de 1998. Rev. Aquat. 1998, 2, 1–3. [Google Scholar]
- McCormick, J.H.; Jones, B.R.; Hokanson, K.E.F. Effects of temperature on growth and survival of young brook trout, Salvelinus fontinalis. J. Fish. Res. Board Can. 1972, 29, 1107–1112. [Google Scholar] [CrossRef]
- Prearo, M. Igiene zootecnica: Pratiche gestionali per un’acquacoltura sostenibile. Ittiopatologia 2007, 4, 19–39. [Google Scholar]
- Soltani, M.; Nikbakht, G.; Ebrahimzadeh Moussavi, H.A.; Ahmadzadeh, N. Epizootic outbreak of lactococcosis caused by Lactococcus garvieae in farmed rainbow trout (Oncorhynchus mykiss) in Iran. Bull. Eur. Assoc. Fish. Pathol. 2008, 28, 95–106. [Google Scholar]
- Prearo, M. La vaccinazione in acquacoltura. Ittiopatologia 2009, 6, 171–186. [Google Scholar]
- Karsidani, S.H.; Soltani, M.; Nikbakhat-Brojeni, G.; Ghasemi, M.; Skall, H.F. Molecular epidemiology of zoonotic streptococcosis/lactococcosis in rainbow trout (Oncorhynchus mykiss) aquaculture in Iran. Iran. J. Microbiol. 2010, 2, 198–209. [Google Scholar]
- Múzquiz, J.L.; Royo, F.M.; Ortega, C.; de Blas, I.; Ruiz, I.; Alonso, J.L. Pathogenicity of streptococcosis in rainbow trout (Oncorhynchus mykiss): Dependence on age and diseased fish. Bull. Eur. Assoc. Fish Pathol. 1999, 19, 114–119. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pastorino, P.; Vela Alonso, A.I.; Colussi, S.; Cavazza, G.; Menconi, V.; Mugetti, D.; Righetti, M.; Barbero, R.; Zuccaro, G.; Fernández-Garayzábal, J.F.; et al. A Summer Mortality Outbreak of Lactococcosis by Lactococcus garvieae in a Raceway System Affecting Farmed Rainbow Trout (Oncorhynchus mykiss) and Brook Trout (Salvelinus fontinalis). Animals 2019, 9, 1043. https://doi.org/10.3390/ani9121043
Pastorino P, Vela Alonso AI, Colussi S, Cavazza G, Menconi V, Mugetti D, Righetti M, Barbero R, Zuccaro G, Fernández-Garayzábal JF, et al. A Summer Mortality Outbreak of Lactococcosis by Lactococcus garvieae in a Raceway System Affecting Farmed Rainbow Trout (Oncorhynchus mykiss) and Brook Trout (Salvelinus fontinalis). Animals. 2019; 9(12):1043. https://doi.org/10.3390/ani9121043
Chicago/Turabian StylePastorino, Paolo, Ana Isabel Vela Alonso, Silvia Colussi, Giulia Cavazza, Vasco Menconi, Davide Mugetti, Marzia Righetti, Raffaella Barbero, Gaetano Zuccaro, José Francisco Fernández-Garayzábal, and et al. 2019. "A Summer Mortality Outbreak of Lactococcosis by Lactococcus garvieae in a Raceway System Affecting Farmed Rainbow Trout (Oncorhynchus mykiss) and Brook Trout (Salvelinus fontinalis)" Animals 9, no. 12: 1043. https://doi.org/10.3390/ani9121043
APA StylePastorino, P., Vela Alonso, A. I., Colussi, S., Cavazza, G., Menconi, V., Mugetti, D., Righetti, M., Barbero, R., Zuccaro, G., Fernández-Garayzábal, J. F., Dondo, A., Acutis, P. L., & Prearo, M. (2019). A Summer Mortality Outbreak of Lactococcosis by Lactococcus garvieae in a Raceway System Affecting Farmed Rainbow Trout (Oncorhynchus mykiss) and Brook Trout (Salvelinus fontinalis). Animals, 9(12), 1043. https://doi.org/10.3390/ani9121043










