Age-Dependent Utilization of Shelters and Habitat in Two Reptile Species with Contrasting Intraspecific Interactions
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
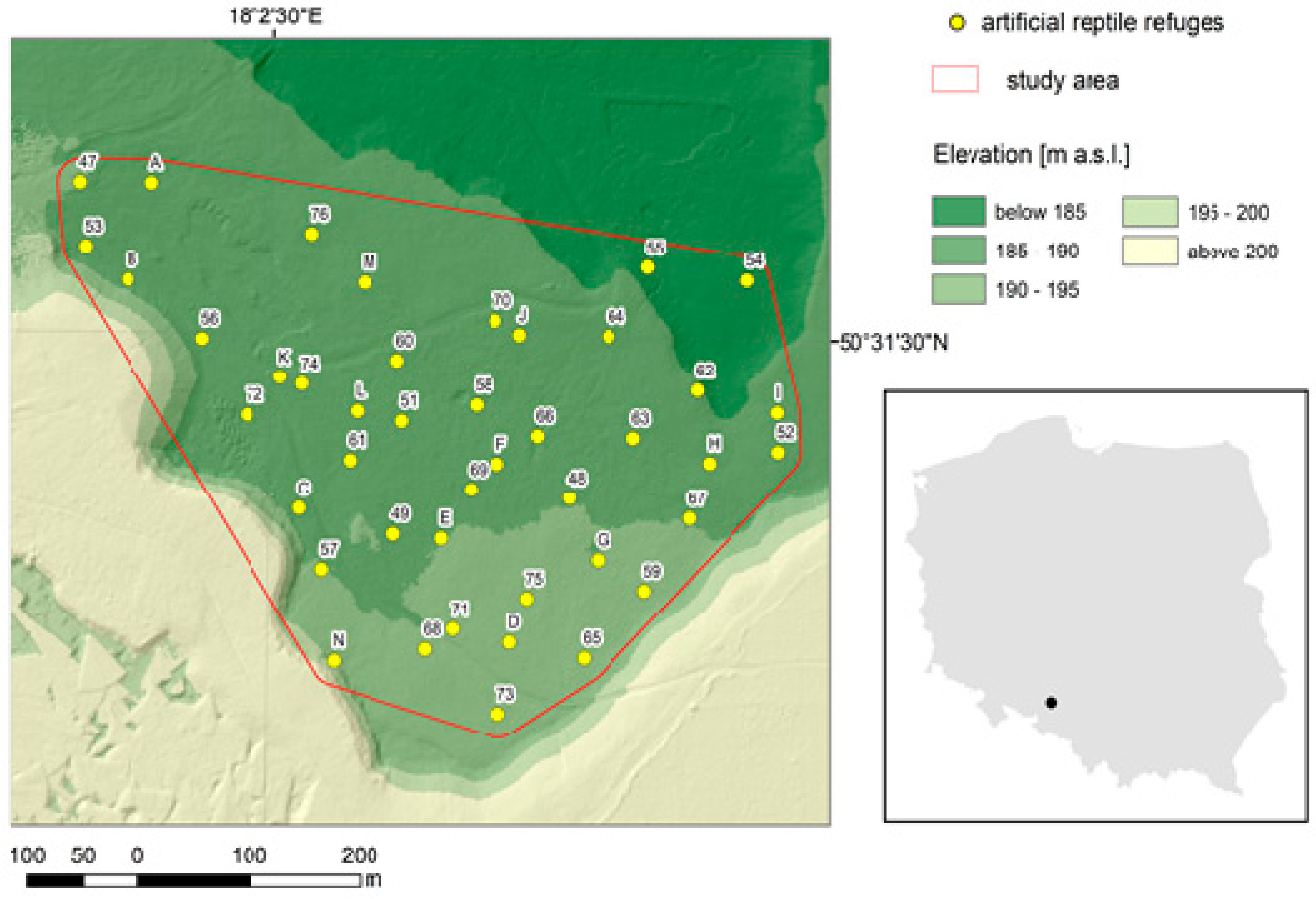
2.1. Study Area
2.2. Experimental Design and Sampling Data
2.3. Data Analysis
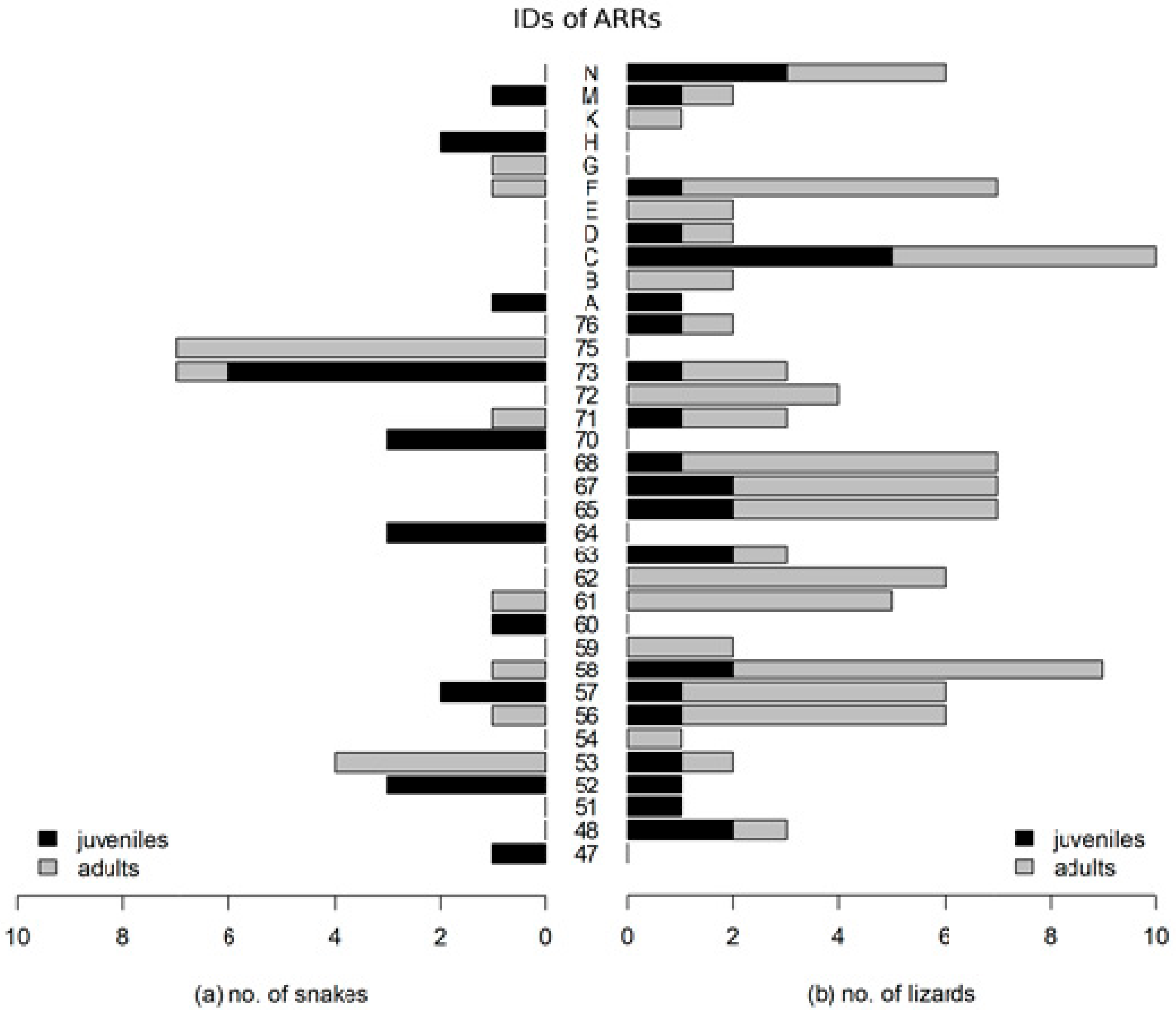
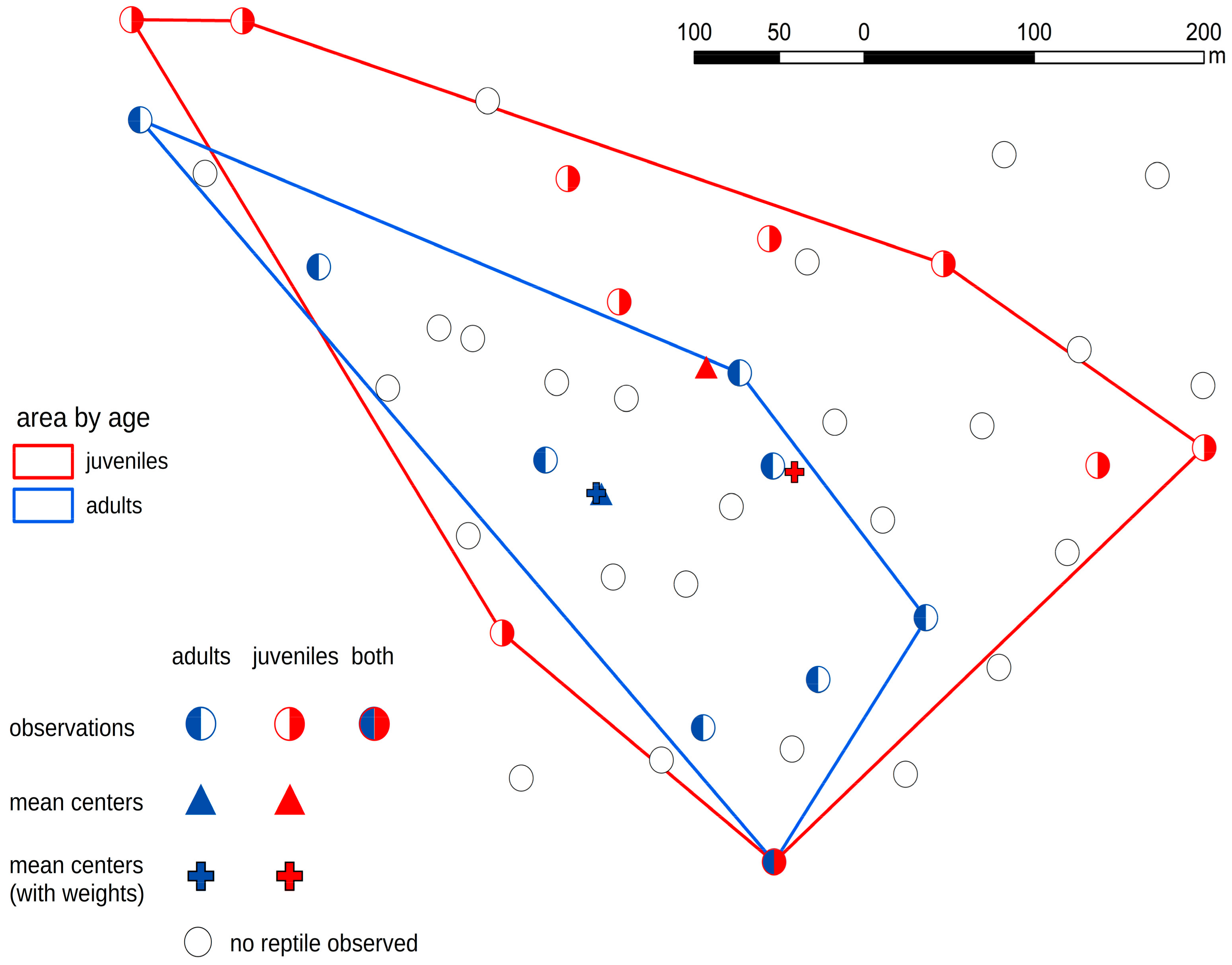
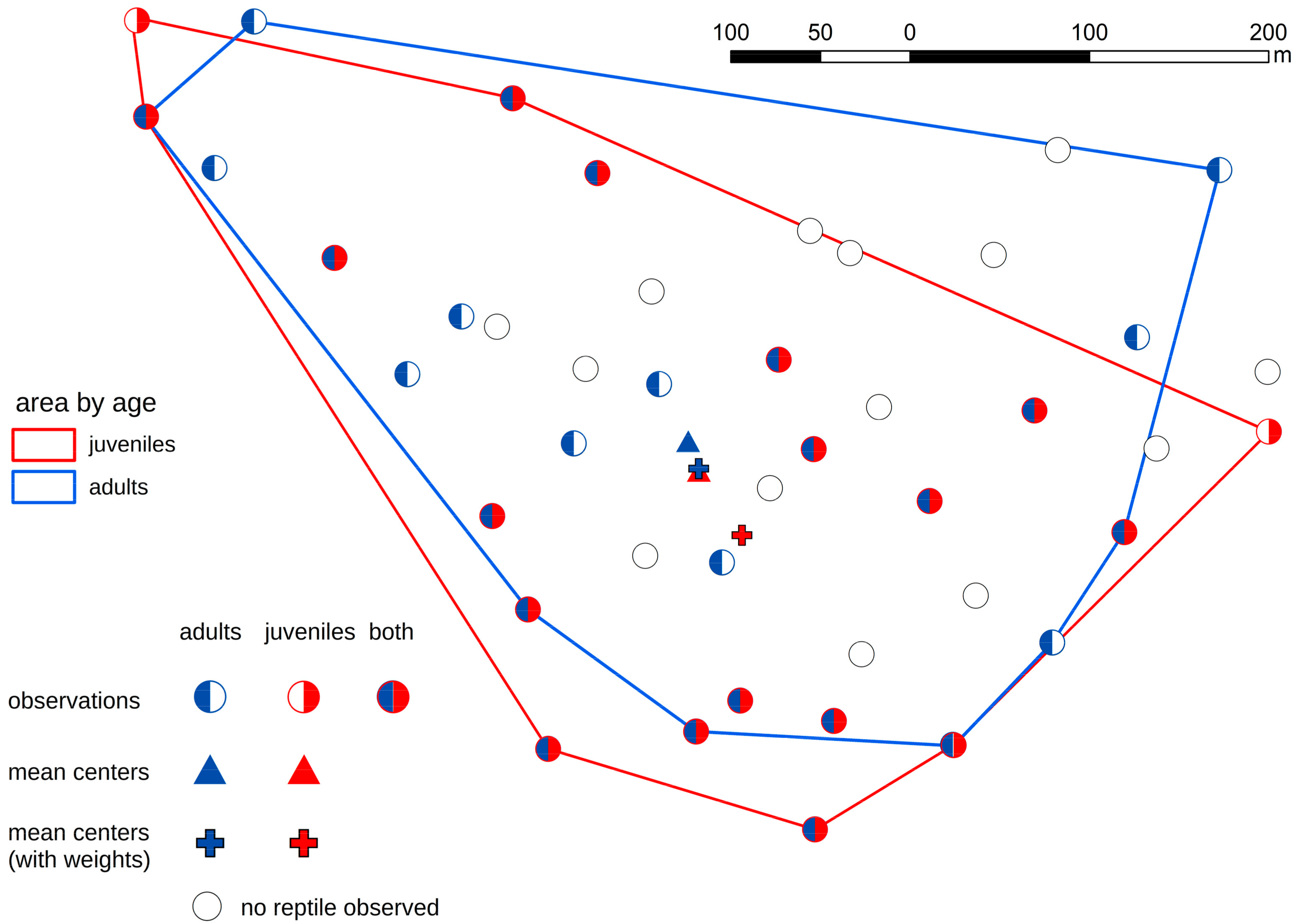
3. Results
4. Discussion
5. Conclusions
- Smooth snakes and slow worms showed different age-dependent spatial distribution.
- In the case of the smooth snake, we discovered that juvenile specimens occupied different artificial shelters from adults. In the case of lizards, there were no such dependencies.
- Juvenile snakes chose sites with suboptimal habitat conditions probably because sites with better habitat condition are occupied by adult snakes.
- Taken together, our results provide strong support for the role of age and behavioral cues in shaping the spatial ecology of terrestrial squamates.
- Our findings may also indicate that habitat-based reptile conservation needs to account for the social mode of a given species and associated spatial structuring of the population.
6. Licenses
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brooks, T.M.; Mittermeier, R.A.; Mittermeier, C.G.; Fonseca, G.A.B.D.; Rylands, A.B.; Konstant, W.R.; Flick, P.; Pilgrim, J.; Oldfield, S.; Magin, G.; et al. Habitat Loss and Extinction in the Hotspots of Biodiversity. Conserv. Biol. 2002, 16, 909–923. [Google Scholar] [CrossRef]
- Law, B.S.; Dickman, C.R. The use of habitat mosaics by terrestrial vertebrate fauna: Implications for conservation and management. Biodivers. Conserv. 1998, 7, 323–333. [Google Scholar] [CrossRef]
- Goulart, F.V.B.; Cáceres, N.C.; Graipel, M.E.; Tortato, M.A.; Ghizoni, I.R.; Oliveira-Santos, L.G.R. Habitat selection by large mammals in a southern Brazilian Atlantic Forest. Mamm. Biol. 2009, 74, 182–190. [Google Scholar] [CrossRef]
- Touihri, M.; Séguy, M.; Imbeau, L.; Mazerolle, M.J.; Bird, D.M. Effects of agricultural lands on habitat selection and breeding success of American kestrels in a boreal context. Agric. Ecosyst. Environ. 2019, 272, 146–154. [Google Scholar] [CrossRef]
- Regosin, J.V.; Windmiller, B.S.; Homan, R.N.; Reed, J.M. Variation in terrestrial habitat use by four pool-breeding amphibian species. J. Wildl. Manag. 2005, 69, 1481–1493. [Google Scholar] [CrossRef]
- Kurek, K.; Król, W.; Najberek, K.; Ćmiel, A.M.; Solarz, W.; Bury, S.; Baś, G.; Najbar, B.; Okarma, H. Habitat use of the Aesculapian snake at different spatial scales. J. Wildl. Manag. 2018, 82, 1746–1755. [Google Scholar] [CrossRef]
- Shine, R.; Wall, M. Why is intraspecific niche partitioning more common in snakes than in lizards? In Lizard Ecology; Reilly, S.M., McBrayer, L.B., Miles, D.B., Eds.; Cambridge University Press: Cambridge, UK, 2007; pp. 173–208. [Google Scholar] [CrossRef]
- Peterson, C.R. Snake thermal ecology: The causes and consequences of body-temperature variation. In Snakes: Ecology and Behavior; McGraw-Hill: New York, NY, USA, 1993; pp. 241–314. [Google Scholar]
- Huang, W.-S.; Greene, H.W.; Chang, T.-J.; Shine, R. Territorial behavior in Taiwanese kukrisnakes (Oligodon formosanus). Proc. Natl. Acad. Sci. USA 2011, 108, 7455–7459. [Google Scholar] [CrossRef]
- Schuett, G.W.; Clark, R.W.; Repp, R.A.; Amarello, M.; Smith, C.F.; Greene, H.W. Social behavior of rattlesnakes: A shifting paradigm. Ratt. Ariz. Conserv. Behav. Venom Evol. 2016, 2, 161–244. [Google Scholar]
- Shine, R.; Harlow, P.S.; Keogh, J.S. The influence of sex and body size on food habits of a giant tropical snake, Python reticulatus. Funct. Ecol. 1998, 12, 248–258. [Google Scholar] [CrossRef]
- Camilleri, C.; Shine, R. Sexual Dimorphism and Dietary Divergence: Differences in Trophic Morphology between Male and Female Snakes. Copeia 1990, 1990, 649–658. [Google Scholar] [CrossRef]
- Luiselli, L.; Angelici, F.M. Sexual size dimorphism and natural history traits are correlated with intersexual dietary divergence in royal pythons (Python regius) from the rainforests of southeastern Nigeria. Ital. J. Zool. 1998, 65, 183–185. [Google Scholar] [CrossRef]
- Shine, R.; Shine, T.; Shine, B. Intraspecific habitat partitioning by the sea snake Emydocephalus annulatus (Serpentes, Hydrophiidae): The effects of sex, body size, and colour pattern. Biol. J. Linn. Soc. 2003, 80, 1–10. [Google Scholar] [CrossRef]
- Brown, W.S.; MacLean, F.M. Conspecific Scent-Trailing by Newborn Timber Rattlesnakes, Crotalus horridus. Herpetologica 1983, 39, 430–436. [Google Scholar]
- Keren-Rotem, T.; Bouskila, A.; Geffen, E. Ontogenetic habitat shift and risk of cannibalism in the common chameleon (Chamaeleo chamaeleon). Behav. Ecol. Sociobiol. 2006, 59, 723–731. [Google Scholar] [CrossRef]
- Drobenkov, S.M. Distribution, ecological traits and conservation of the smooth snake (Coronella austriaca) in Belarus. Acta Biol. Univ. Daugavp. 2014, 14, 21–27. [Google Scholar]
- Pernetta, A.P.; Reading, C.J.; Allen, J.A. Chemoreception and kin discrimination by neonate smooth snakes, Coronella austriaca. Anim. Behav. 2009, 77, 363–368. [Google Scholar] [CrossRef]
- Gonzalo, A.; Cabido, C.; Martín, J.; López, P. Detection and Discrimination of Conspecific Scents by the Anguid Slow-Worm Anguis fragilis. J. Chem. Ecol. 2004, 30, 1565–1573. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.; Lee, J. Statistical Analysis of Geographic Information; John Wiley & Sons: Hoboken, NJ, USA, 2005. [Google Scholar]
- Santos, X.; Brito, J.C.; Sillero, N.; Pleguezuelos, J.M.; Llorente, G.A.; Fahd, S.; Parellada, X. Inferring habitat-suitability areas with ecological modelling techniques and GIS: A contribution to assess the conservation status of Vipera latastei. Biol. Conserv. 2006, 130, 416–425. [Google Scholar] [CrossRef]
- Franklin, J.; Wejnert, K.E.; Hathaway, S.A.; Rochester, C.J.; Fisher, R.N. Effect of species rarity on the accuracy of species distribution models for reptiles and amphibians in Southern California. Divers. Distrib. 2009, 15, 167–177. [Google Scholar] [CrossRef]
- Santos, X.; Brito, J.C.; Caro, J.; Abril, A.J.; Lorenzo, M.; Sillero, N.; Pleguezuelos, J.M. Habitat suitability, threats and conservation of isolated populations of the smooth snake (Coronella austriaca) in the southern Iberian Peninsula. Biol. Conserv. 2009, 142, 344–352. [Google Scholar] [CrossRef]
- Sillero, N.; Brito, J.C.; Skidmore, A.K.; Toxopeus, A.G. Biogeographical patterns derived from remote sensing variables: The amphibians and reptiles of the Iberian Peninsula. Amphib. Reptil. 2009, 30, 185–206. [Google Scholar] [CrossRef]
- Spellerberg, I.F.; Phelps, T.E. Biology, general ecology and behaviour of the snake, Coronella austriaca Laurenti. Biol. J. Linn. Soc. 1977, 9, 133–164. [Google Scholar] [CrossRef]
- Gent, A.H. Movement and Dispersion of the Smooth Snake Coronella austriaca Laurenti in Relation to Habitat. Ph.D. Thesis, University of Southampton, Southampton, UK, 1990. [Google Scholar]
- Meteorological Service with Climatic Data. Available online: http://pogodynka.pl/ (accessed on 2 December 2018).
- Juszczyk, W. Płazy i Gady Krajowe. Wyd. II; Polskie Wydawnictwo Naukowe: Warszawa, Poland, 1987. [Google Scholar]
- Brown, W.S.; Parker, W.S. A Ventral Scale Clipping System for Permanently Marking Snakes (Reptilia, Serpentes). J. Herpetol. 1976, 10, 247–249. [Google Scholar] [CrossRef]
- Ekner, A.; Sajkowska, Z.; Dudek, K.; Tryjanowski, P. Medical cautery units as a permanent and non-invasive method of marking lizards. Acta Herpetol. 2011, 6, 229–236. [Google Scholar] [CrossRef]
- Ciofi, C.; Puswati, J.; Winana, D.; Boer, M.E.D.; Chelazzi, G.; Sastrawan, P. Preliminary Analysis of Home Range Structure in the Komodo Monitor, Varanus komodoensis. Copeia 2007, 2007, 462–470. [Google Scholar] [CrossRef]
- Boyle, S.A.; Lourenço, W.C.; Silva, L.R.D.; Smith, A.T. Home Range Estimates Vary with Sample Size and Methods. Folia Primatol. 2009, 80, 33–42. [Google Scholar] [CrossRef]
- Van Beest, F.M.; Rivrud, I.M.; Loe, L.E.; Milner, J.M.; Mysterud, A. What determines variation in home range size across spatiotemporal scales in a large browsing herbivore? J. Anim. Ecol. 2001, 80, 771–785. [Google Scholar] [CrossRef]
- Row, J.R.; Blouin-Demers, G. Thermal quality influences habitat selection at multiple spatial scales in milksnakes. Écoscience 2006, 13, 443–450. [Google Scholar] [CrossRef]
- Burgman, M.A.; Fox, J.C. Bias in species range estimates from minimum convex polygons: Implications for conservation and options for improved planning. Anim. Conserv. 2003, 6, 19–28. [Google Scholar] [CrossRef]
- Nilsen, E.B.; Pedersen, S.; Linnell, J.D.C. Can minimum convex polygon home ranges be used to draw biologically meaningful conclusions? Ecol. Res. 2008, 23, 635–639. [Google Scholar] [CrossRef]
- Row, J.R.; Blouin-Demers, G. Kernels Are Not Accurate Estimators of Home-Range Size for Herpetofauna. Copeia 2006, 2006, 797–802. [Google Scholar] [CrossRef]
- Mitchell, A. The ESRI Guide to GIS Analysis. Volume 2: Spatial Measurements & Statistics; ESRI Press: Redlands, CA, USA, 2005. [Google Scholar]
- Mayhew, S. A Dictionary of Geography; Oxford University Press: New York, NY, USA, 2015. [Google Scholar]
- Documentation of ArcGIS Desktop. Available online: http://desktop.arcgis.com/en/documentation/ (accessed on 31 May 2019).
- Fu, P.; Rich, P.M. A geometric solar radiation model with applications in agriculture and forestry. Comput. Electron. Agric. 2002, 37, 25–35. [Google Scholar] [CrossRef]
- Gent, A.H.; Spellerberg, I.F. Movement rates of the smooth snake Coronella austriaca (Colubridae) A radio telemetric study. Herpetol. J. 1993, 3, 140–146. [Google Scholar]
- Hosmer, D.W., Jr.; Lemeshow, S.; Sturdivant, R.X. Applied Logistic Regression; John Wiley & Sons: Hoboken, NJ, USA, 2013; pp. 227–242. ISBN 978-1-118-54838-7. [Google Scholar] [CrossRef]
- Environmental Systems Research Institute. ArcGIS Desktop: Release 10.2; Environmental Systems Research Institute: Redlands, CA, USA, 2013. [Google Scholar]
- QGIS. Geographic Information System: Release 3.0. Open Source Geospatial Foundation Project. Technical documentation. Available online: https://www.qgis.org/en/docs/index.html (accessed on 1 August 2019).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; ISBN 3-900051-07-0. Available online: http://www.R-project.org (accessed on 31 May 2019).
- Technical documentation of Python Language, version 2.7. Available online: https://docs.python.org/2/reference/index.html (accessed on 01 May 2019).
- Shine, R.; Webb, J.K.; Fitzgerald, M.; Sumner, J. The impact of bush-rock removal on an endangered snake species, Hoplocephalus bungaroides (Serpentes: Elapidae). Wildl. Res. 1998, 25, 285–295. [Google Scholar] [CrossRef]
- Visagie, L.; Mouton, P.; Bauwens, D. Experimental analysis of grouping behaviour in cordylid lizards. Herpetol. J. 2005, 15, 91–96. [Google Scholar]
- Alfermann, D.; Bohme, W. Populationsstruktur und Raumnutzung der Schlingnatter auf Freileitungstrassen in Wäldern Freilandökologische Untersuchungen unter Zuhilfenahme künstlicher Verstecke (KV) und der Radiotelemetrie. Z. Feldherpetol. 2009, 15, 373–392. [Google Scholar]
- Madsen, T. Movements, Home Range Size and Habitat Use of Radio-Tracked Grass Snakes (Natrix natrix) in Southern Sweden. Copeia 1984, 1984, 707–713. [Google Scholar] [CrossRef]
- Shine, R. Intraspecific Variation in Thermoregulation, Movements and Habitat Use by Australian Blacksnakes, Pseudechis porphyriacus (Elapidae). J. Herpetol. 1987, 21, 165–177. [Google Scholar] [CrossRef]
- Goddard, P. Limited movements areas and spatial behaviour in the smooth snake in southern England. In Proceedings of the European Herpetological Symposium CWLP Oxford; Coborn, J., Ed.; Cotswold Wild Life Park: Burford, UK, 1981; pp. 25–40. [Google Scholar]
- Smith, N.D. The Ecology of the Slow-Worm (Anguis fragilis L.) in Southern England. Ph.D. Thesis, University of Southampton, Southampton, UK, 1990. [Google Scholar]
- Reinert, H.K. Habitat Selection in Snakes. In Snakes: Ecology and Behavior; Seigel, R.A., Collins, J.T., Eds.; McGraw-Hill: New York, NY, USA, 1993; pp. 201–240. [Google Scholar]
| Group | Character of the Distribution | ANN Ratio (p-Value) |
|---|---|---|
| All snakes | dispersed | 1.735 (<0.01) |
| Adult unique snakes | dispersed | 1.440 (<0.05) |
| Juvenile unique snakes | dispersed | 4.305 (<0.01) |
| All lizards | clustered | 0.338 (<0.01) |
| Adult unique lizards | clustered | 0.687 (<0.01) |
| Juvenile unique lizards | random | 0.824 (>0.1) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolanek, A.; Bury, S.; Turniak, E.; Szymanowski, M. Age-Dependent Utilization of Shelters and Habitat in Two Reptile Species with Contrasting Intraspecific Interactions. Animals 2019, 9, 995. https://doi.org/10.3390/ani9110995
Kolanek A, Bury S, Turniak E, Szymanowski M. Age-Dependent Utilization of Shelters and Habitat in Two Reptile Species with Contrasting Intraspecific Interactions. Animals. 2019; 9(11):995. https://doi.org/10.3390/ani9110995
Chicago/Turabian StyleKolanek, Aleksandra, Stanisław Bury, Edyta Turniak, and Mariusz Szymanowski. 2019. "Age-Dependent Utilization of Shelters and Habitat in Two Reptile Species with Contrasting Intraspecific Interactions" Animals 9, no. 11: 995. https://doi.org/10.3390/ani9110995
APA StyleKolanek, A., Bury, S., Turniak, E., & Szymanowski, M. (2019). Age-Dependent Utilization of Shelters and Habitat in Two Reptile Species with Contrasting Intraspecific Interactions. Animals, 9(11), 995. https://doi.org/10.3390/ani9110995





