Freeze-Dried Royal Jelly Proteins Enhanced the Testicular Development and Spermatogenesis in Pubescent Male Mice
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animals
2.3. Preparation and Dissolving of Freeze-Dried Royal Jelly
2.4. Sample Collection
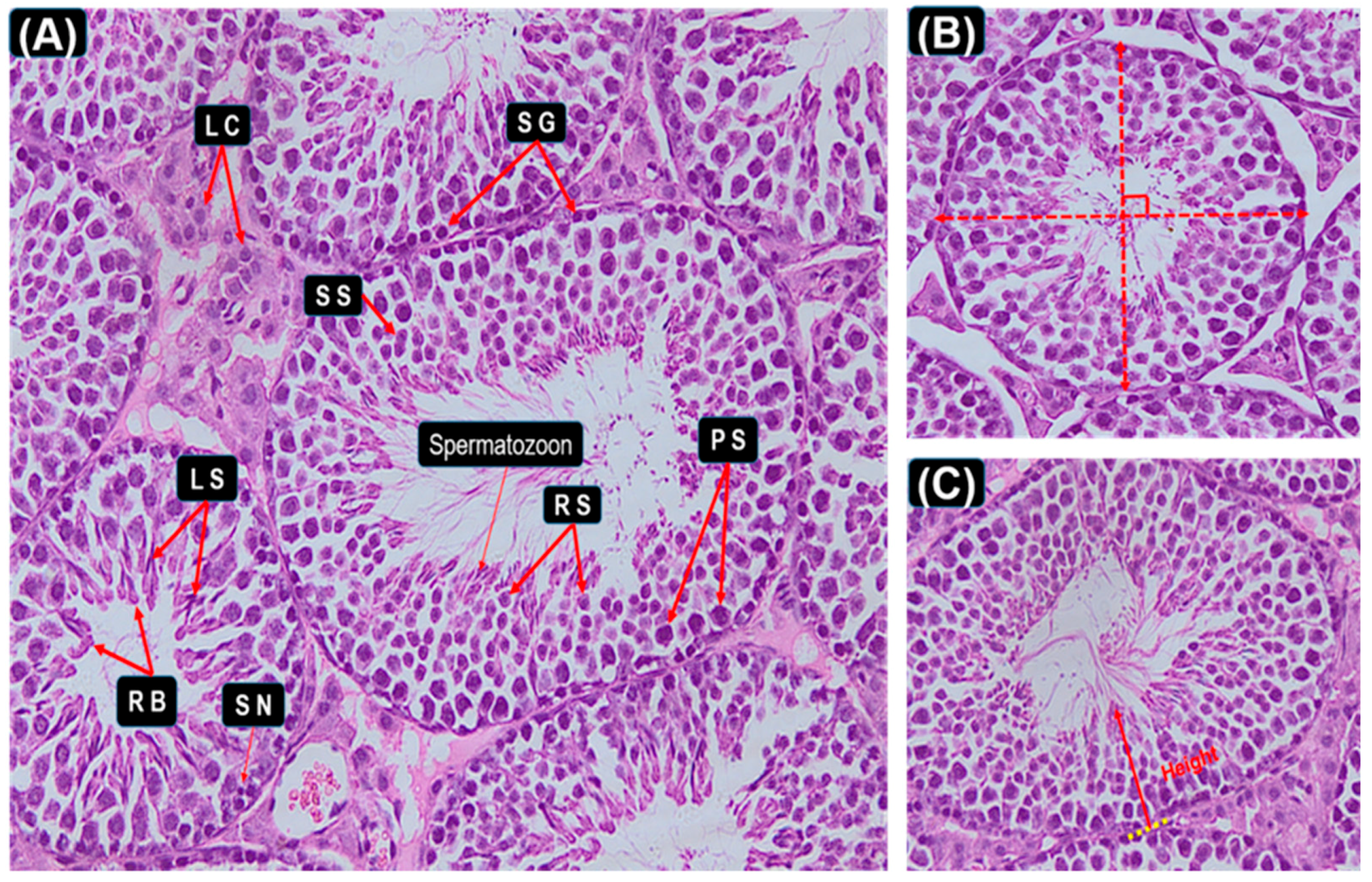
2.5. Histological Study of Testis
2.6. Reproduction Development Assesment
2.7. Immune Histochemistry (IHC) Analysis
2.8. Tunnel Assay
2.9. Hormones Determination in PNDs 21 and PNDs 35
2.10. Statistical Analysis
3. Results
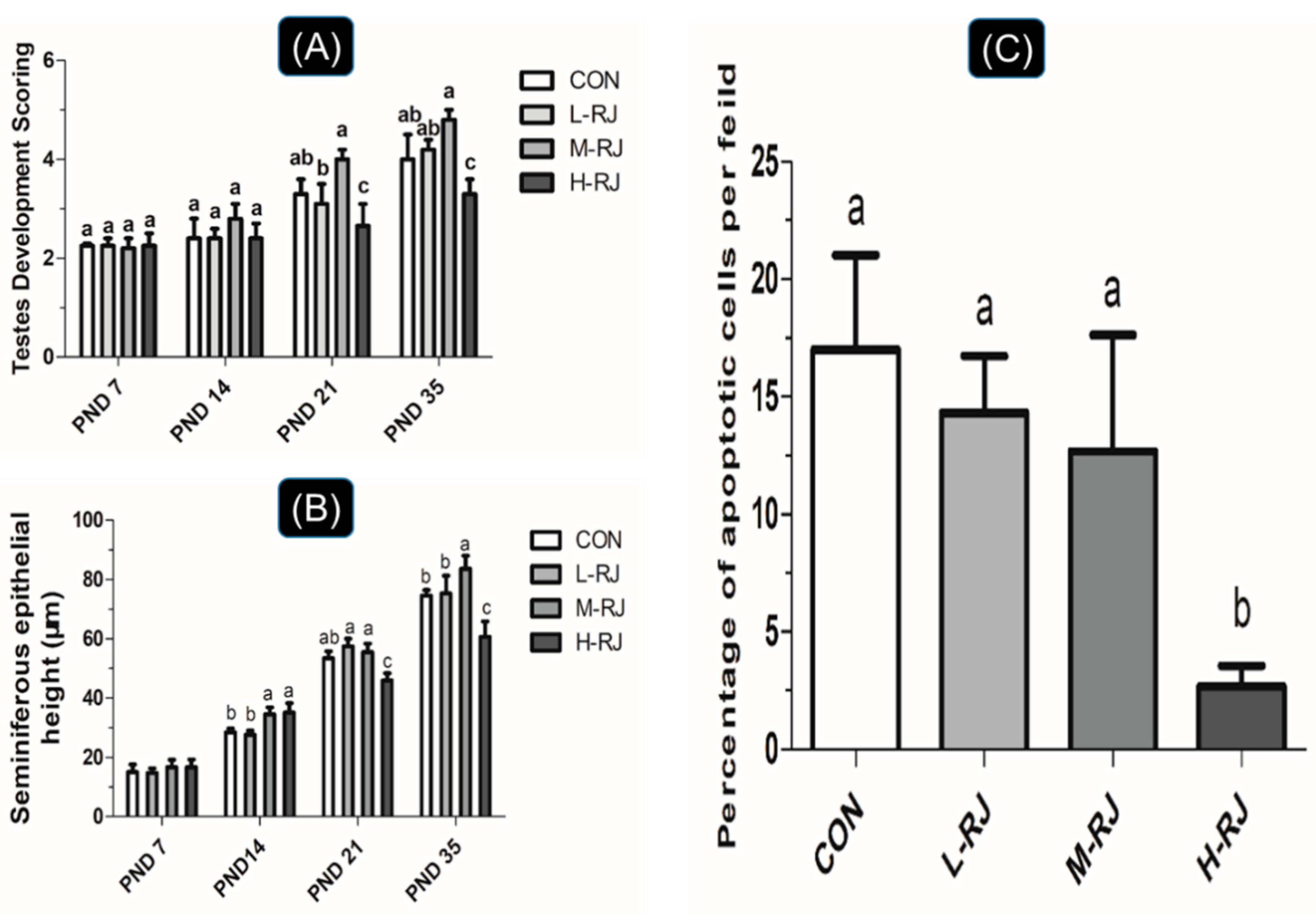
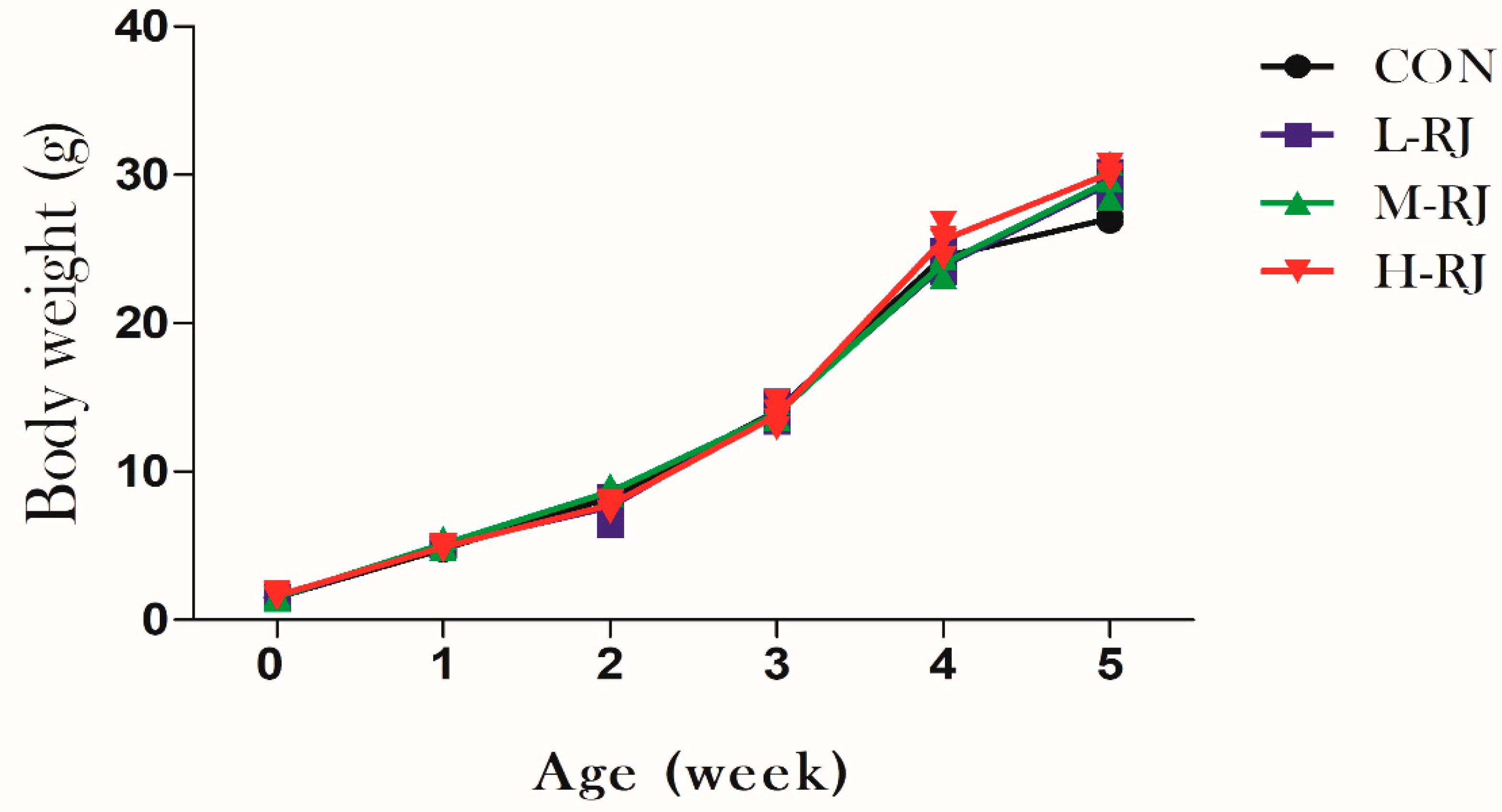
3.1. Grwoth Performance
3.2. Organs Weight Measurment
3.3. Seminiferous Epithelial Height and Seminiferous Tubule Diameter Measurements
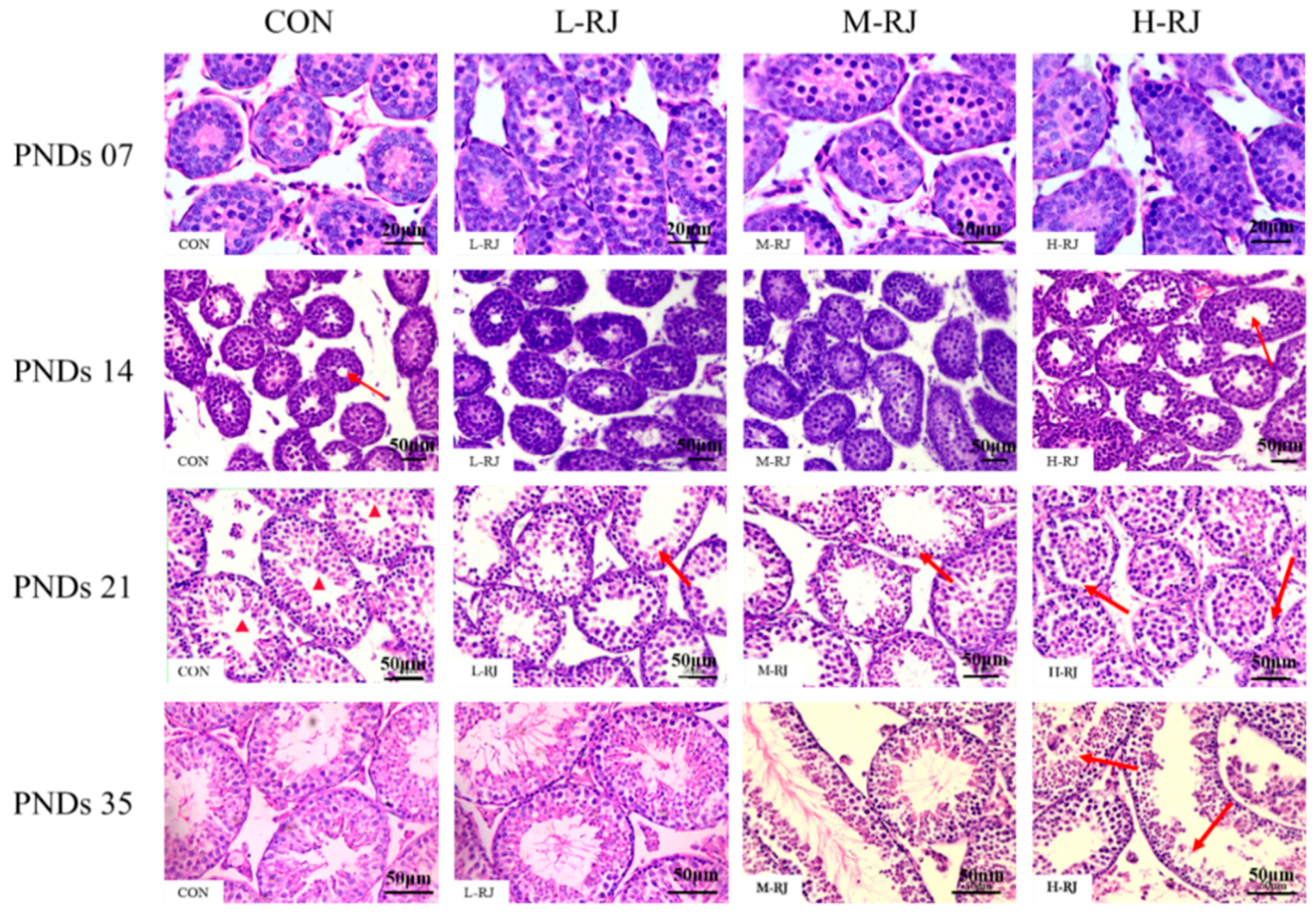
3.4. Histopathological Examination of the Testis
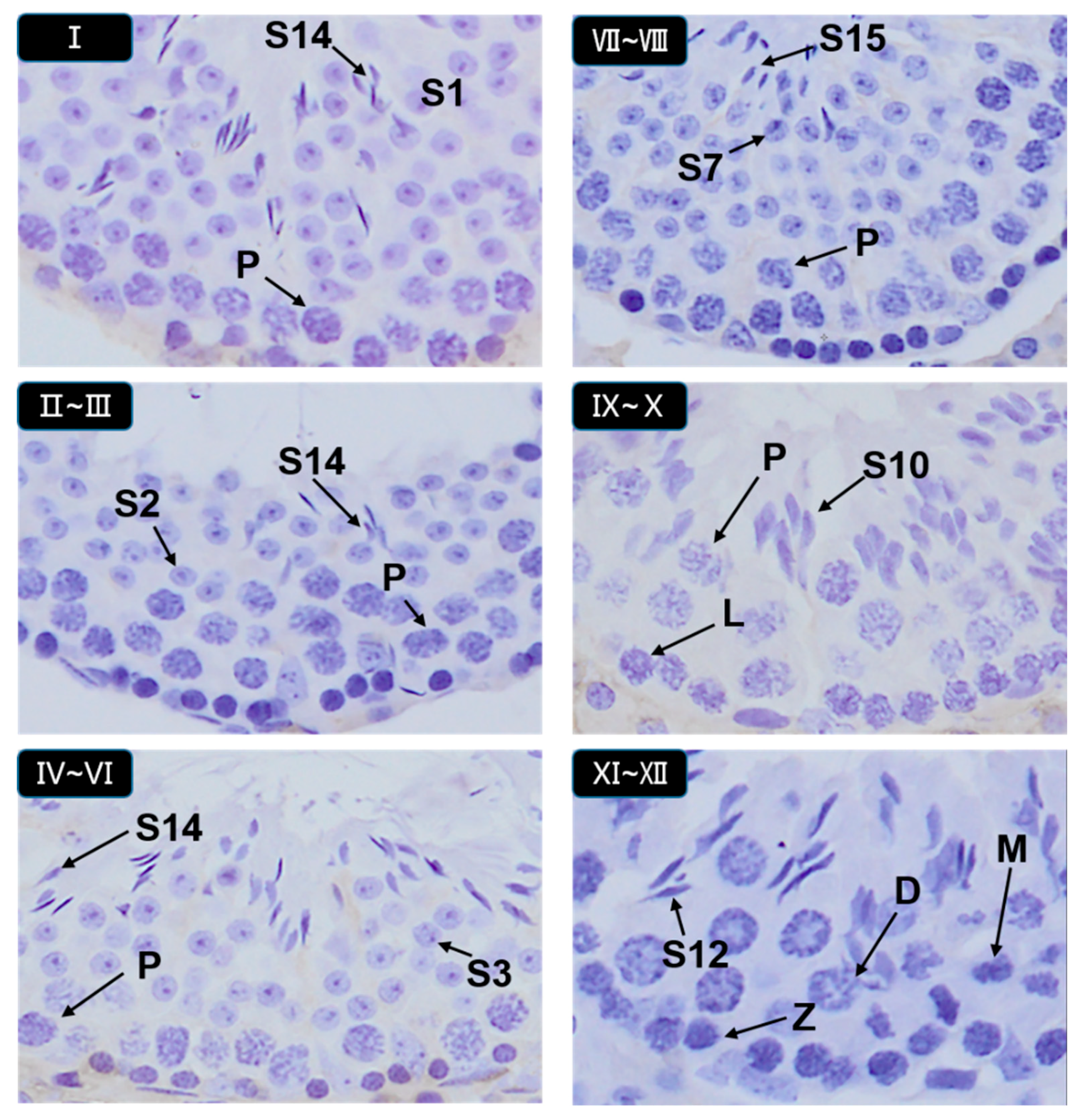
3.5. Histopathological Stages and Characteristics of Germ Cell
3.6. Graphic Explanation of Testis Scoring Method
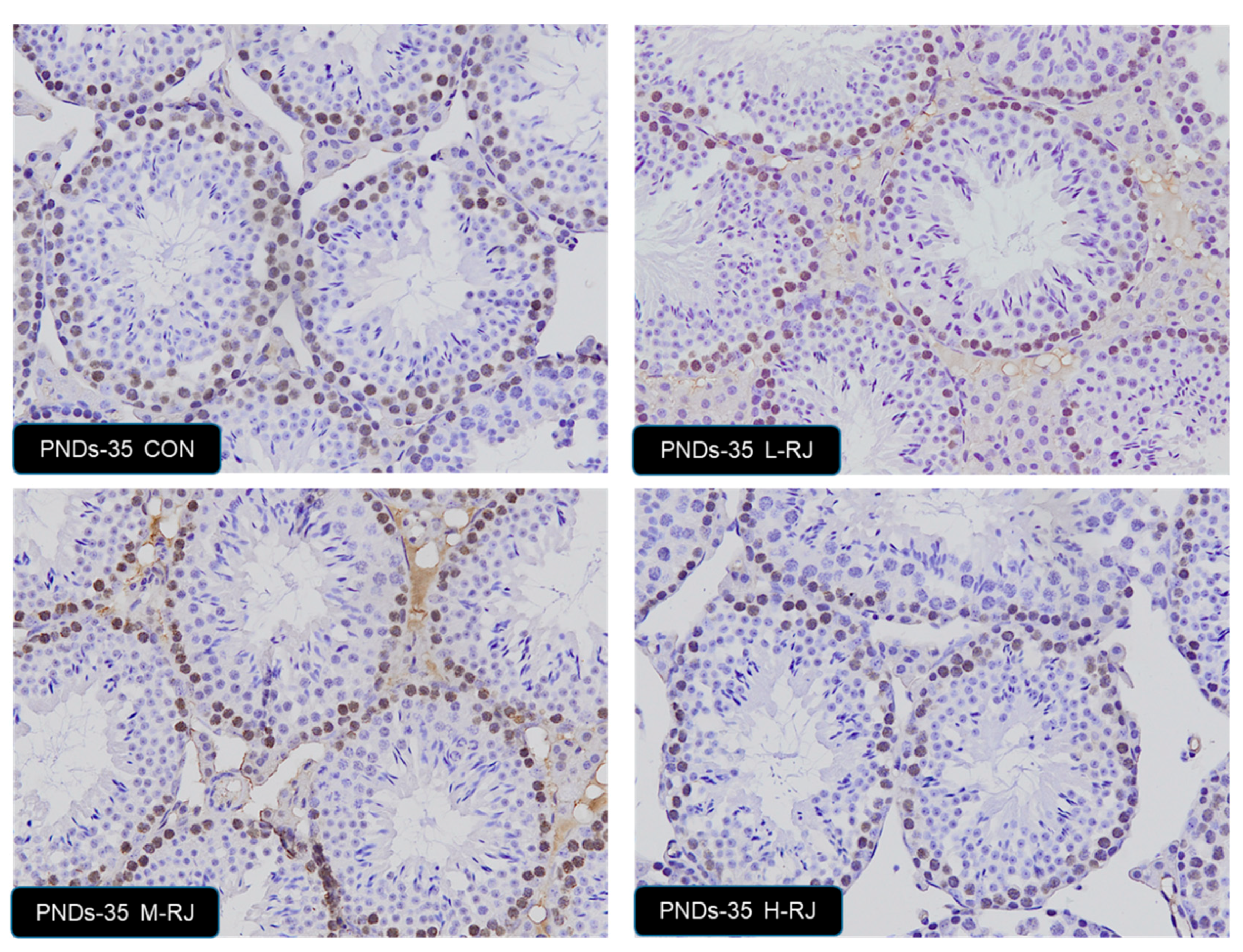
3.7. Effect of Oral Administration of Royal Jelly on Proliferation in Germ Cells
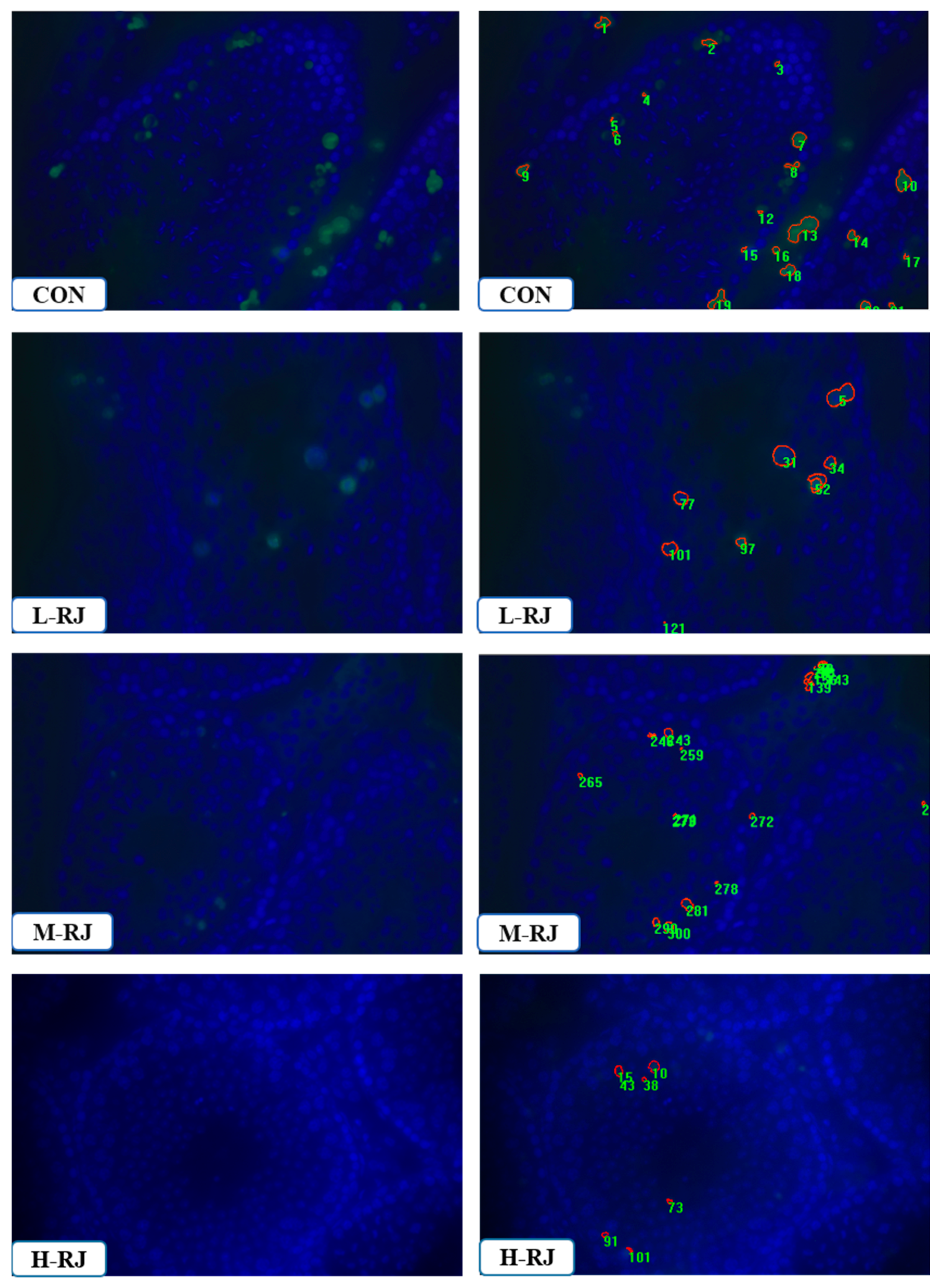
3.8. Administration of RJ Decreases Apoptosis in the Testis
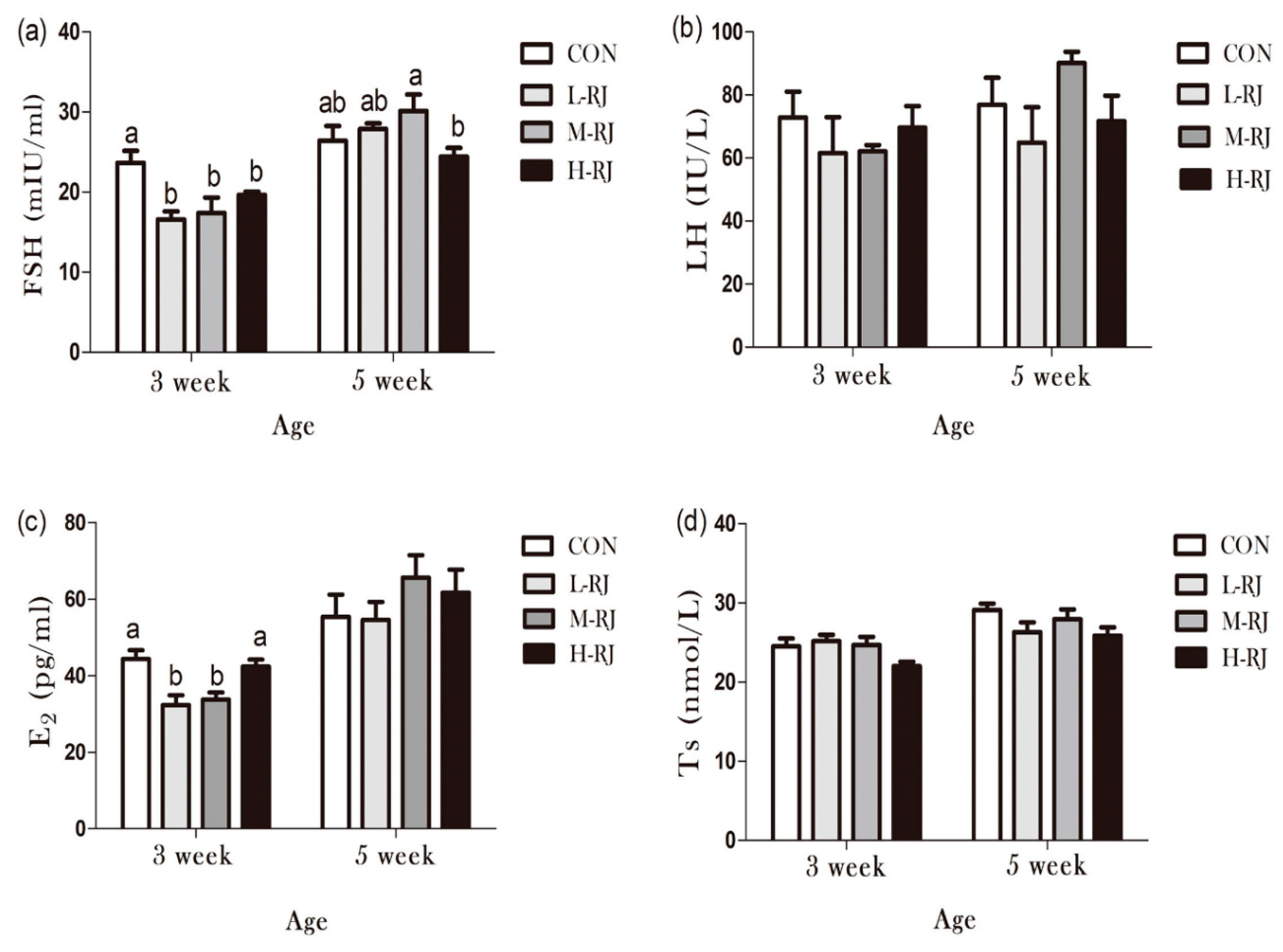
3.9. Determination of Sexual Hormones Concentration
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liming, W.; Jinhui, Z.; Xiaofeng, X.; Yi, L.; Jing, Z. Fast determination of 26 amino acids and their content changes in royal jelly during storage using ultra-performance liquid chromatography. J. Food Compos. Anal. 2009, 22, 242–249. [Google Scholar] [CrossRef]
- Drapeau, M.D.; Albert, S.; Kucharski, R.; Prusko, C.; Maleszka, R. Evolution of the Yellow/Major Royal Jelly Protein family and the emergence of social behavior in honey bees. Genome Res. 2006, 16, 1385–1394. [Google Scholar] [CrossRef] [PubMed]
- Fratini, F.; Cilia, G.; Mancini, S.; Felicioli, A. Royal Jelly: An ancient remedy with remarkable antibacterial properties. Microbiol. Res. 2016, 192, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Fujita, T.; Kozuka-Hata, H.; Ao-Kondo, H.; Kunieda, T.; Oyama, M.; Kubo, T. Proteomic analysis of the royal jelly and characterization of the functions of its derivation glands in the honeybee. J. Proteome Res. 2012, 12, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Vucevic, D.; Melliou, E.; Vasilijic, S.; Gasic, S.; Ivanovski, P.; Chinou, I.; Colic, M. Fatty acids isolated from royal jelly modulate dendritic cell-mediated immune response in vitro. Int. Immunopharmacol. 2007, 7, 1211–1220. [Google Scholar] [CrossRef]
- Cao, L.-F.; Zheng, H.-Q.; Pirk, C.W.; Hu, F.-L.; Xu, Z.-W. High royal jelly-producing honeybees (Apis mellifera ligustica)(Hymenoptera: Apidae) in China. J. Econ. Entomol. 2016, 109, 510–514. [Google Scholar] [CrossRef]
- Nakaya, M.; Onda, H.; Sasaki, K.; Yukiyoshi, A.; Tachibana, H.; Yamada, K. Effect of royal jelly on bisphenol A-induced proliferation of human breast cancer cells. Biosci. Biotechnol. Biochem. 2007, 71, 253–255. [Google Scholar] [CrossRef]
- Hidaka, S.; Okamoto, Y.; Uchiyama, S.; Nakatsuma, A.; Hashimoto, K.; Ohnishi, T.; Yamaguchi, M. Royal jelly prevents osteoporosis in rats: Beneficial effects in ovariectomy model and in bone tissue culture model. Evid.-Based Complement. Altern. Med. 2006, 3, 339–348. [Google Scholar] [CrossRef]
- Annie, S.; Prabhu, R.; Malini, S. Activity of Wedelia calendulacea Less. in post-menopausal osteoporosis. Phytomedicine 2006, 13, 43–48. [Google Scholar] [CrossRef]
- Kim, D.H.; Lee, H.J.; Lee, Y.; Ryu, K.H.; Jung, B.-I.; Song, Y.S.; Ryu, J.-H. New estrogenic compounds isolated from Broussonetia kazinoki. Bioorganic Med. Chem. Lett. 2010, 20, 3764–3767. [Google Scholar]
- Suzuki, K.-M.; Isohama, Y.; Maruyama, H.; Yamada, Y.; Narita, Y.; Ohta, S.; Araki, Y.; Miyata, T.; Mishima, S. Estrogenic activities of fatty acids and a sterol isolated from royal jelly. Evid.-Based Complement. Altern. Med. 2008, 5, 295–302. [Google Scholar] [CrossRef]
- Mishima, S.; Suzuki, K.-M.; Isohama, Y.; Kuratsu, N.; Araki, Y.; Inoue, M.; Miyata, T. Royal jelly has estrogenic effects in vitro and in vivo. J. Ethnopharmacol. 2005, 101, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Bincoletto, C.; Eberlin, S.; Figueiredo, C.A.; Luengo, M.B.; Queiroz, M.L. Effects produced by Royal Jelly on haematopoiesis: Relation with host resistance against Ehrlich ascites tumour challenge. Int. Immunopharmacol. 2005, 5, 679–688. [Google Scholar] [CrossRef] [PubMed]
- Kohno, K.; Okamoto, I.; Sano, O.; Arai, N.; Iwaki, K.; Ikeda, M.; Kurimoto, M. Royal jelly inhibits the production of proinflammatory cytokines by activated macrophages. Biosci. Biotechnol. Biochem. 2004, 68, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Šver, L.; Oršolić, N.; Tadić, Z.; Njari, B.; Valpotic, I.; Bašic, I. A royal jelly as a new potential immunomodulator in rats and mice. Comp. Immunol. Microbiol. Infect. Dis. 1996, 19, 31–38. [Google Scholar] [CrossRef]
- Lees, P.; Aliabadi, F.S. Rational dosing of antimicrobial drugs: Animals versus humans. Int. J. Antimicrob. Agents 2002, 19, 269–284. [Google Scholar] [CrossRef]
- Kimura, Y. Antitumor and antimetastatic actions of various natural products. Stud. Nat. Prod. Chem. 2008, 34, 35–76. [Google Scholar]
- Zahmatkesh, E.; Najafi, G.; Nejati, V.; Heidari, R. Protective effect of royal jelly on the sperm parameters and testosterone level and lipid peroxidation in adult mice treated with oxymetholone. Avicenna J. Phytomedicine 2014, 4, 43. [Google Scholar]
- Mahdivand, N.; Najafi, G.; Nejati, V.; Shalizar-Jalali, A.; Rahmani, F. Royal jelly protects male rats from heat stress-induced reproductive failure. Andrologia 2019, 51, e13213. [Google Scholar] [CrossRef]
- Elnagar, S.A. Royal jelly counteracts bucks’“summer infertility”. Anim. Reprod. Sci. 2010, 121, 174–180. [Google Scholar] [CrossRef]
- Yang, A.; Zhou, M.; Zhang, L.; Xie, G.; Chen, H.; Liu, Z.; Ma, W. Influence of royal jelly on the reproductive function of puberty male rats. Food Chem. Toxicol. 2012, 50, 1834–1840. [Google Scholar] [CrossRef] [PubMed]
- Delkhoshe-Kasmaie, F.; Malekinejad, H.; Khoramjouy, M.; Rezaei-Golmisheh, A.; Janbaze-Acyabar, H. Royal jelly protects from taxol-induced testicular damages via improvement of antioxidant status and up-regulation of E2f1. Syst. Biol. Reprod. Med. 2014, 60, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Tamura, S.; Kono, T.; Harada, C.; Yamaguchi, K.; Moriyama, T. Estimation and characterisation of major royal jelly proteins obtained from the honeybee Apis merifera. Food Chem. 2009, 114, 1491–1497. [Google Scholar] [CrossRef]
- Isidorov, V.; Isidorova, A.; Sczczepaniak, L.; Czyżewska, U. Gas chromatographic–mass spectrometric investigation of the chemical composition of beebread. Food Chem. 2009, 115, 1056–1063. [Google Scholar] [CrossRef]
- Isidorov, V.; Czyżewska, U.; Jankowska, E.; Bakier, S. Determination of royal jelly acids in honey. Food Chem. 2011, 124, 387–391. [Google Scholar] [CrossRef]
- Moutsatsou, P.; Papoutsi, Z.; Kassi, E.; Heldring, N.; Zhao, C.; Tsiapara, A.; Melliou, E.; Chrousos, G.P.; Chinou, I.; Karshikoff, A. Fatty acids derived from royal jelly are modulators of estrogen receptor functions. PLoS ONE 2010, 5, e15594. [Google Scholar] [CrossRef]
- Atanassova, N.N.; Walker, M.; McKinnell, C.; Fisher, J.S.; Sharpe, R.M. Evidence that androgens and oestrogens, as well as follicle-stimulating hormone, can alter Sertoli cell number in the neonatal rat. J. Endocrinol. 2005, 184, 107–117. [Google Scholar] [CrossRef]
- Jensen, K.C.; Mariappan, M.R.; Putcha, G.V.; Husain, A.; Chun, N.; Ford, J.M.; Schrijver, I.; Longacre, T.A. Microsatellite instability and mismatch repair protein defects in ovarian epithelial neoplasms in patients 50 years of age and younger. Am. J. Surg. Pathol. 2008, 32, 1029–1037. [Google Scholar] [CrossRef]
- Okano, T.; Ishiniwa, H.; Onuma, M.; Shindo, J.; Yokohata, Y.; Tamaoki, M. Effects of environmental radiation on testes and spermatogenesis in wild large Japanese field mice (Apodemus speciosus) from Fukushima. Sci. Rep. 2016, 6, 23601. [Google Scholar] [CrossRef]
- Mustafa, S.; Wei, Q.; Ennab, W.; Lv, Z.; Nazar, K.; Siyal, F.A.; Rodeni, S.; Kavita, N.M.; Shi, F. Resveratrol Ameliorates Testicular Histopathology of Mice Exposed to Restraint Stress. Animals 2019, 9, 743. [Google Scholar] [CrossRef]
- Hamdard, E.; Shi, Z.; Lv, Z.; Zahir, A.; Wei, Q.; Rahmani, M.M.; Shi, F. Denatonium Benzoate-Induces Oxidative Stress in the Heart and Kidney of Chinese Fast Yellow Chickens by Regulating Apoptosis, Autophagy, Antioxidative Activities and Bitter Taste Receptor Gene Expressions. Animals 2019, 9, 701. [Google Scholar] [CrossRef] [PubMed]
- Oakberg, E.F. A description of spermiogenesis in the mouse and its use in analysis of the cycle of the seminiferous epithelium and germ cell renewal. Am. J. Anat. 1956, 99, 391–413. [Google Scholar] [CrossRef] [PubMed]
- Orsi, A.M.; Ferreira, A.L. Definition of the stages of the cycle of the seminiferous epithelium of the opossum (Didelphis azarae, Temminck, 1825). Cells Tissues Organs 1978, 100, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Hogarth, C.A.; Griswold, M.D. The key role of vitamin A in spermatogenesis. J. Clin. Investig. 2010, 120, 956–962. [Google Scholar] [CrossRef] [PubMed]
- Afonso, C.L.; Amarasinghe, G.K.; Bányai, K.; Bào, Y.; Basler, C.F.; Bavari, S.; Bejerman, N.; Blasdell, K.R.; Briand, F.-X.; Briese, T. Taxonomy of the order Mononegavirales: Update 2016. Arch. Virol. 2016, 161, 2351–2360. [Google Scholar] [CrossRef]
- Johnsen, S.G. Testicular biopsy score count–a method for registration of spermatogenesis in human testes: Normal values and results in 335 hypogonadal males. Horm. Res. Paediatr. 1970, 1, 2–25. [Google Scholar] [CrossRef]
- Klein, A.M.; Nakagawa, T.; Ichikawa, R.; Yoshida, S.; Simons, B.D. Mouse germ line stem cells undergo rapid and stochastic turnover. Cell Stem Cell 2010, 7, 214–224. [Google Scholar] [CrossRef]
- Ramadan, M.F.; Al-Ghamdi, A. Bioactive compounds and health-promoting properties of royal jelly: A review. J. Funct. Foods 2012, 4, 39–52. [Google Scholar] [CrossRef]
- Guo, H.; Kouzuma, Y.; Yonekura, M. Structures and properties of antioxidative peptides derived from royal jelly protein. Food Chem. 2009, 113, 238–245. [Google Scholar] [CrossRef]
- Ghanbari, E.; Nejati, V.; Khazaei, M. Antioxidant and protective effects of Royal jelly on histopathological changes in testis of diabetic rats. Int. J. Reprod. Biomed. 2016, 14, 519. [Google Scholar] [CrossRef]
- Pyrzanowska, J.; Wawer, A.; Joniec-Maciejak, I.; Piechal, A.; Blecharz-Klin, K.; Graikou, K.; Chinou, I.; Widy-Tyszkiewicz, E. Long-term administration of Greek Royal Jelly decreases GABA concentration in the striatum and hypothalamus of naturally aged Wistar male rats. Neurosci. Lett. 2018, 675, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Wellard, S.R.; Hopkins, J.; Jordan, P.W. A seminiferous tubule squash technique for the cytological analysis of spermatogenesis using the mouse model. J. Vis. Exp. 2018, 56453. [Google Scholar] [CrossRef] [PubMed]
- Kimoto, T.; Arai, S.; Kohguchi, M.; Aga, M.; Nomura, Y.; Micallef, M.J.; Kurimoto, M.; Mito, K. Apoptosis and suppression of tumor growth by artepillin C extracted from Brazilian propolis. Cancer Detect. Prev. 1998, 22, 506–515. [Google Scholar] [CrossRef] [PubMed]
- Shetty, J.; Marathe, G.; Dighe, R. Specific immunoneutralization of FSH leads to apoptotic cell death of the pachytene spermatocytes and spermatogonial cells in the rat. Endocrinology 1996, 137, 2179–2182. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Morinaga, H.; Hwang, V.; Fan, W.; Fernandez, M.O.; Varki, N.; Olefsky, J.M.; Webster, N.J. Free fatty acids induce Lhb mRNA but suppress Fshb mRNA in pituitary LβT2 gonadotropes and diet-induced obesity reduces FSH levels in male mice and disrupts the proestrous LH/FSH surge in female mice. Endocrinology 2013, 154, 2188–2199. [Google Scholar] [CrossRef] [PubMed]
- Bayer, R.J. Patterns of clonal diversity in the Antennaria rosea (Asteraceae) polyploid agamic complex. Am. J. Bot. 1990, 77, 1313–1319. [Google Scholar] [CrossRef]
- Graham, H.N. Green tea composition, consumption, and polyphenol chemistry. Prev. Med. 1992, 21, 334–350. [Google Scholar] [CrossRef]
- Álvarez-Hernández, G.; Lara-Valencia, F.; Reyes-Castro, P.; Rascón-Pacheco, R. An analysis of spatial and socio-economic determinants of tuberculosis in Hermosillo, Mexico, 2000–2006. Int. J. Tuberc. Lung Dis. 2010, 14, 708–713. [Google Scholar]
- Hariharan, S.; Johnson, C.P.; Bresnahan, B.A.; Taranto, S.E.; McIntosh, M.J.; Stablein, D. Improved graft survival after renal transplantation in the United States, 1988 to 1996. N. Eng. J. Med. 2000, 342, 605–612. [Google Scholar] [CrossRef]
- De Lamirande, E.; Gagnon, C. Impact of reactive oxygen species on spermatozoa: A balancing act between beneficial and detrimental effects. Hum. Reprod. 1995, 10, 15–21. [Google Scholar] [CrossRef]
- Iliadou, P.K.; Tsametis, C.; Kaprara, A.; Papadimas, I.; Goulis, D.G. The Sertoli cell: Novel clinical potentiality. Hormones 2015, 14, 504–514. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Ding, Z.; Hong, Z.; Zou, Z.; Feng, Y.; Zhu, R.; Ma, J.; Ge, X.; Li, C.; Yao, B. Spermatogenesis improved by suppressing the high level of endogenous gonadotropins in idiopathic non-obstructive azoospermia: A case control pilot study. Reprod. Biol. Endocrinol. 2018, 16, 91. [Google Scholar] [CrossRef] [PubMed]
- De Gendt, K.; Atanassova, N.; Tan, K.A.; de França, L.R.; Parreira, G.G.; McKinnell, C.; Sharpe, R.M.; Saunders, P.T.; Mason, J.I.; Hartung, S. Development and function of the adult generation of Leydig cells in mice with Sertoli cell-selective or total ablation of the androgen receptor. Endocrinology 2005, 146, 4117–4126. [Google Scholar] [CrossRef] [PubMed]
- Seyyedi, F.; Rafiean-Kopaei, M.; Miraj, S. Comparison of the effects of vaginal royal jelly and vaginal estrogen on quality of life, sexual and urinary function in postmenopausal women. J. Clin. Diagn. Res. 2016, 10, QC01. [Google Scholar] [CrossRef]
- Joseph, A.; Hess, R.A.; Schaeffer, D.J.; Ko, C.; Hudgin-Spivey, S.; Chambon, P.; Shur, B.D. Absence of estrogen receptor alpha leads to physiological alterations in the mouse epididymis and consequent defects in sperm function. Biol. Reprod. 2010, 82, 948–957. [Google Scholar] [CrossRef]
- Serge, C.; Rex, H. Oestrogens and spermatogenesis. Philos. Trans. R. Soc. 2010, 365, 1517–1535. [Google Scholar]
- Hayes, T.B.; Anderson, L.L.; Beasley, V.R.; De Solla, S.R.; Iguchi, T.; Ingraham, H.; Kestemont, P.; Kniewald, J.; Kniewald, Z.; Langlois, V.S. Demasculinization and feminization of male gonads by atrazine: Consistent effects across vertebrate classes. J. Steroid Biochem. Mol. Biol. 2011, 127, 64–73. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, X.; Zhang, J.; Wu, K. Impact of Endocrine-Disrupting Chemicals on Reproductive Function in Zebrafish (Danio rerio). Reproduction Domest. Anim. 2015, 50, 1–6. [Google Scholar] [CrossRef]
- Gray, M.A.; Metcalfe, C.D. Induction of testis-ova in Japanese medaka (Oryzias latipes) exposed to p-nonylphenol. Environ. Toxicol. Chem. Int. J. 1997, 16, 1082–1086. [Google Scholar]
- Metcalfe, N.B.; Monaghan, P. Growth versus lifespan: Perspectives from evolutionary ecology. Exp. Gerontol. 2003, 38, 935–940. [Google Scholar] [CrossRef]
- Brolo, A.G.; Arctander, E.; Gordon, R.; Leathem, B.; Kavanagh, K.L. Nanohole-enhanced Raman scattering. Nano Lett. 2004, 4, 2015–2018. [Google Scholar] [CrossRef]
- Taylor, A.E.; Keevil, B.; Huhtaniemi, I.T. Mass spectrometry and immunoassay: How to measure steroid hormones today and tomorrow. Eur. J. Endocrinol. 2015, 173, D1–D12. [Google Scholar] [CrossRef] [PubMed]
- Chandolia, R.K.; Weinbauer, G.F.; Simoni, M.; Behre, H.M.; Nieschlag, E. Comparative effects of chronic administration of the non-steroidal antiandrogens flutamide and Casodex on the reproductive system of the adult male rat. Eur. J. Endocrinol. 1991, 125, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, R.; Maddocks, S.; Millar, M.; Kerr, J.; Saunders, P.; McKinnell, C. Testosterone and Spermatogenesis Identification of Stage-Specific, Androgen-Regulated Proteins Secreted by Adult Rat Seminiferous Tubules. J. Androl. 1992, 13, 172–184. [Google Scholar]
- Muffly, K.E.; Nazian, S.J.; Cameron, D.F. Effects of follicle-stimulating hormone on the junction-related Sertoli cell cytoskeleton and daily sperm production in testosterone-treated hypophysectomized rats. Biol. Reprod. 1994, 51, 158–166. [Google Scholar] [CrossRef]
- Singh, J.; O’Neill, C.; Handelsman, D.J. Induction of spermatogenesis by androgens in gonadotropin-deficient (hpg) mice. Endocrinology 1995, 136, 5311–5321. [Google Scholar] [CrossRef]
- Yang, N.Y.; Zhou, D.; Chung, R.C.; Li, C.W.; Fong, K.N. Rehabilitation interventions for unilateral neglect after stroke: A systematic review from 1997 through 2012. Front. Hum. Neurosci. 2013, 7, 187. [Google Scholar] [CrossRef]
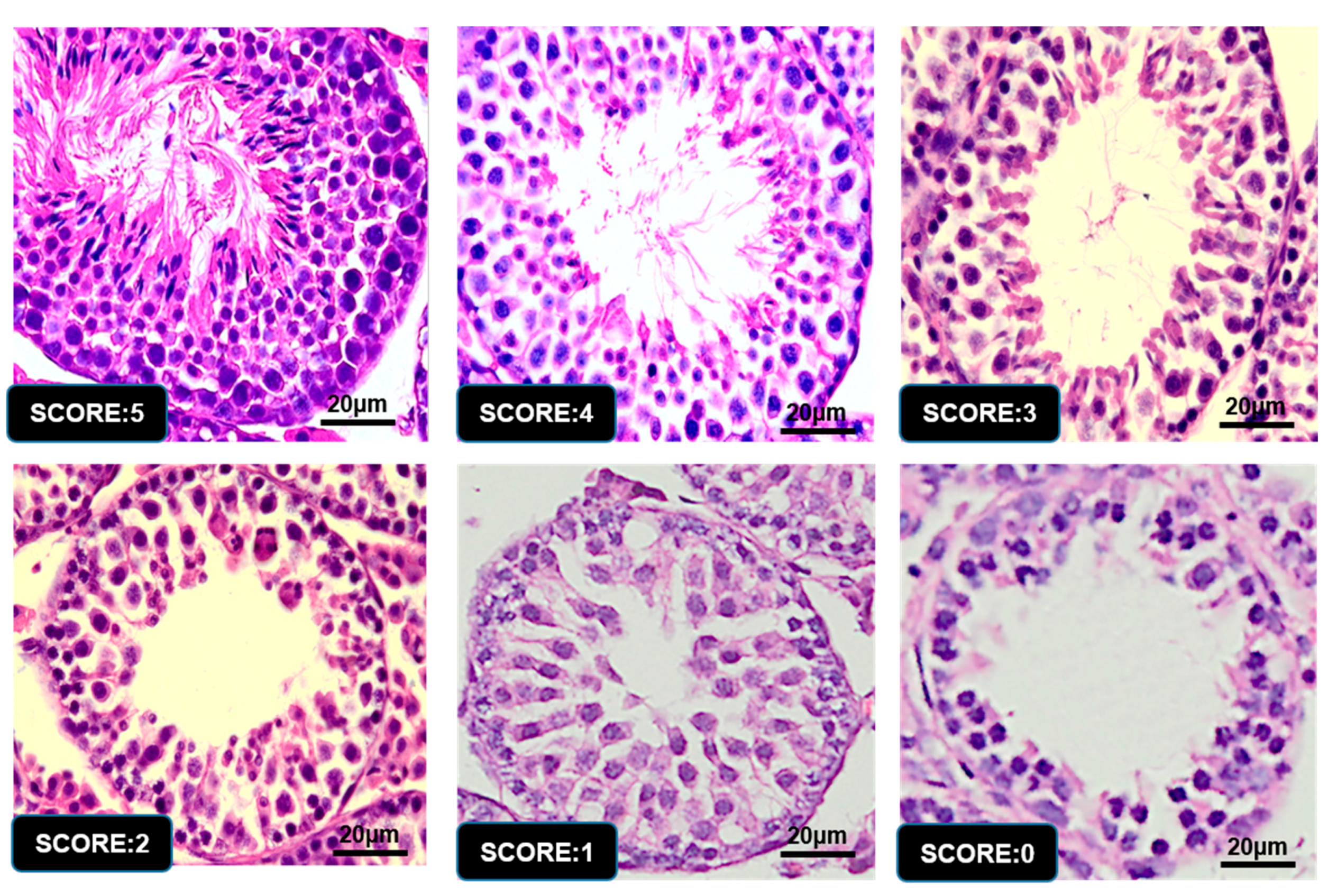
| 5 | Complete spermatogenesis and perfect tubules |
| 4 | Spermatozoa present with disorganized spermatogenesis |
| 3 | No spermatozoa, but long spermatids present |
| 2 | No long spermatids present, but round spermatids present |
| 1 | No spermatids, but spermatocytes present |
| 0 | Only spermatogonia present |
| Group | Day | Testis (mg) | Epididymis (mg) | Spleen (mg) | Liver (mg) | Kidney (mg) | pH |
|---|---|---|---|---|---|---|---|
| PNDs-07 | 16.66 ± 1.50 | 8.21 ± 0.02 | 34.61 ± 1.56 a | 200 ± 0.02 | 73.94 ± 3.96 | 3.49 ± 0.21 | |
| Control | PNDs-14 | 33.48 ± 1.26 b | 13.12 ± 0.33 | 39.27 ± 3.83 a | 250 ± 0.02 b | 104.10 ± 2.64 b | 4.31 ± 0.07 |
| PNDs-21 | 77.16 ± 2.72 bc | 16.96 ± 0.64 | 120 ± 0.01 b | 850 ± 0.03 b | 205.64 ± 7.88 b | 5.05 ± 0.26 a | |
| PNDs-35 | 166.54 ± 7.22 b | 38.94 ± 1.60 | 100 ± 0.01 b | 1480 ± 0.11 | 380 ± 0.02 | 3.59 ± 0.05 c | |
| PNDs-07 | 15.02 ± 0.92 | 11.21 ± 0.01 | 30.10 ± 1.23 b | 210 ± 0.01 | 78.58 ± 2.00 | 3.67 ± 0.24 | |
| Low dose | PNDs-14 | 35.00 ± 1.78 b | 14.12 ± 0.31 | 41.16 ± 3.22 a | 260 ± 0.01 b | 108.60 ± 5.64 ab | 4.38 ± 0.06 |
| PNDs-21 | 80.84 ± 2.62 ab | 17.68 ± 1.10 | 130 ± 0.02 b | 840 ± 0.02 b | 234.28 ± 4.38 a | 4.81 ± 0.07 b | |
| PNDs-35 | 163.70 ± 5.92 b | 40.20 ± 0.80 | 100 ± 0.02 b | 1510 ± 0.03 | 340 ± 0.027 | 4.91 ± 0.12 a | |
| Moderate Dose | PNDs-07 | 17.8 ± 0.62 b | 13.21 ± 0.25 | 22.97 ± 1.00 c | 150 ± 0.01 | 73.46 ± 3.02 | 3.84 ± 0.21 |
| PNDs-14 | 40.72 ± 2.24 a | 16.12 ± 0.43 | 48.76 ± 4.35 a | 320 ± 0.01 a | 118.60 ± 0.94 a | 4.37 ± 0.09 | |
| PNDs-21 | 81.20 ± 1.58 a | 19.16 ± 0.94 | 100 ± 0.01 b | 730 ± 0.02 c | 175.20 ± 3.18 c | 4.09 ± 0.43 b | |
| PNDs-35 | 173.34 ± 7.72 a | 47.20 ± 1.50 | 120 ± 0.02 ab | 1760 ± 0.09 | 400 ± 0.10 | 4.42 ± 0.05 b | |
| PNDs-07 | 16.52 ± 1.02 | 9.21 ± 0.26 | 32.36 ± 1.42 ab | 180 ± 0.01 | 70.06 ± 3.38 | 3.79 ± 0.22 | |
| High dose | PNDs-14 | 34.26 ± 1.30b | 15.12 ± 0.48 | 28.28 ± 2.65 b | 270 ± 0.01 b | 97.76 ± 6.62 b | 4.21 ± 0.06 |
| PNDs-21 | 79.36 ± 3.46 b | 18.60 ± 1.28 | 160 ± 0.01 a | 920 ± 0.01 a | 206.2 ± 6.22 b | 5.09 ± 0.16 a | |
| PNDs-35 | 125.34 ± 10.62 c | 41.80 ± 5.56 | 120 ± 0.01 a | 1650 ± 0.10 | 360 ± 0.04 | 4.40 ± 0.03 b |
| CON | L-RJ | M-RJ | H-RJ | p-Value | |
|---|---|---|---|---|---|
| PND 07 | 64.04 ± 0.68 | 63.52 ± 0.79 | 65.84 ± 1.01 | 63.86 ± 1.30 | 0.36 |
| PND 14 | 74.56 ± 1.31b | 74.84 ± 2.06b | 87.34 ± 1.08a | 70.90 ± 0.68c | 0.0003 |
| PND 21 | 153.17 ± 3.54a | 150.64 ± 2.72a | 152.27 ± 2.25a | 134.95 ± 1.89b | 0.002 |
| PND 35 | 189.99 ± 3.63ab | 185.67 ± 2.24b | 193.89 ± 3.37a | 173.10 ± 2.87c | 0.02 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Z.; Enayatullah, H.; Lv, Z.; Dai, H.; Wei, Q.; Shen, L.; Karwand, B.; Shi, F. Freeze-Dried Royal Jelly Proteins Enhanced the Testicular Development and Spermatogenesis in Pubescent Male Mice. Animals 2019, 9, 977. https://doi.org/10.3390/ani9110977
Shi Z, Enayatullah H, Lv Z, Dai H, Wei Q, Shen L, Karwand B, Shi F. Freeze-Dried Royal Jelly Proteins Enhanced the Testicular Development and Spermatogenesis in Pubescent Male Mice. Animals. 2019; 9(11):977. https://doi.org/10.3390/ani9110977
Chicago/Turabian StyleShi, Zhicheng, Hamdard Enayatullah, Zengpeng Lv, Hongjian Dai, Quanwei Wei, Lirong Shen, Babrak Karwand, and Fangxiong Shi. 2019. "Freeze-Dried Royal Jelly Proteins Enhanced the Testicular Development and Spermatogenesis in Pubescent Male Mice" Animals 9, no. 11: 977. https://doi.org/10.3390/ani9110977
APA StyleShi, Z., Enayatullah, H., Lv, Z., Dai, H., Wei, Q., Shen, L., Karwand, B., & Shi, F. (2019). Freeze-Dried Royal Jelly Proteins Enhanced the Testicular Development and Spermatogenesis in Pubescent Male Mice. Animals, 9(11), 977. https://doi.org/10.3390/ani9110977






