Behavioural Differences in Dogs with Atopic Dermatitis Suggest Stress Could Be a Significant Problem Associated with Chronic Pruritus
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. cAD Phenotype Characterisation
2.3. The C-BARQ
- Stranger-directed aggression (severity scale): Dog shows threatening or aggressive responses to strangers approaching or invading the dog’s or the owner’s personal space, territory, or home range.
- Owner-directed aggression (severity scale): Dog shows threatening or aggressive responses to the owner or other members of the household when challenged, manhandled, stared at, stepped over, or when approached while in possession of food or objects.
- Dog-directed aggression (severity scale): Dog shows threatening or aggressive responses when approached directly by unfamiliar dogs.
- Family dog aggression (severity scale): Dog shows aggressive or threatening responses to other familiar dogs in the same household.
- Stranger-directed fear (severity scale): Dog shows fearful or wary responses when approached directly by strangers.
- Non-social fear (severity scale): Dog shows fearful or wary responses to sudden or loud noises, traffic, and unfamiliar objects and situations.
- Dog-directed fear (severity scale): Dog shows fearful or wary responses when approached directly by unfamiliar dogs.
- Touch sensitivity (severity scale): Dog shows fearful or wary responses to potentially painful or uncomfortable procedures, including bathing, grooming, nail-clipping, and veterinary examinations.
- Separation-related behaviour (frequency scale): Dog vocalises and/or is destructive when separated from the owner, often accompanied or preceded by behavioural and autonomic signs of anxiety, including restlessness, loss of appetite, trembling, and excessive salivation.
- Attachment/attention-seeking (frequency scale): Dog maintains close proximity to the owner or other members of the household, solicits affection or attention, and displays agitation when the owner gives attention to third parties.
- Trainability (frequency scale): Dog shows a willingness to attend to the owner and obeys simple commands. Dog is not easily distracted, tends to be a fast learner, responds positively to correction, and will fetch or retrieve objects.
- Chasing (frequency scale): Dog chases cats, birds, and/or other small animals, given the opportunity.
- Excitability (severity scale): Dog displays strong reaction to potentially exciting or arousing events, such as going for walks or car trips, doorbells, the arrival of visitors, and the owner arriving home; has difficulty calming down after such events.
- Energy level (frequency scale): Dog is energetic, “always on the go”, and/or playful.
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Favrot, C. Clinical signs and diagnosis of canine atopic dermatitis. Eur. J. Companion Anim. Pract. 2009, 19, 219–222. [Google Scholar]
- Reich, A.; Mędrek, K.; Szepietowski, J.C. Interplay of itch and psyche in psoriasis: An update. Acta Derm. Venereol. 2016, 96, 55–57. [Google Scholar] [CrossRef] [PubMed]
- Reich, A.; Hrehorow, E.; Szepietowski, J.C. Pruritus is an important factor negatively influencing the well-being of psoriatic patients. Acta Derm. Venereol. 2010, 90, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Tey, H.L.; Wallengren, J.; Yosipovitch, G. Psychosomatic factors in pruritus. Clin. Dermatol. 2013, 31, 31–40. [Google Scholar] [CrossRef]
- Linek, M.; Favrot, C. Impact of canine atopic dermatitis on the health-related quality of life of affected dogs and quality of life of their owners. Vet. Dermatol. 2010, 21, 456–462. [Google Scholar] [CrossRef]
- Noli, C.; Colombo, S.; Cornegliani, L.; Ghibaudo, G.; Persico, P.; Vercelli, A.; Galzerano, M. Quality of life of dogs with skin disease and of their owners. Part 2: Administration of a questionnaire in various skin diseases and correlation to efficacy of therapy. Vet. Dermatol. 2011, 22, 344–351. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Yamazaki, S.; Hayashino, Y.; Takahashi, O.; Tokuda, Y.; Shimbo, T.; Fukui, T.; Hinohara, S.; Miyachi, Y.; Fukuhara, S. Association Between Frequency of Pruritic Symptoms and Perceived Psychological Stress. Arch. Dermatol. 2009, 145, 1384–1388. [Google Scholar] [CrossRef]
- Chida, Y.; Hamer, M.; Steptoe, A. A Bidirectional Relationship Between Psychosocial Factors and Atopic Disorders: A Systematic Review and Meta-Analysis. Psychosom. Med. 2008, 70, 102–116. [Google Scholar] [CrossRef]
- Yaghmaie, P.; Koudelka, C.W.; Simpson, E.L. Mental health comorbidity in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2013, 131, 428–433. [Google Scholar] [CrossRef]
- Li, J.C.; Fishbein, A.; Singam, V.; Patel, K.R.; Zee, P.C.; Attarian, H.; Cella, D.; Silverberg, J.I. Sleep Disturbance and Sleep-Related Impairment in Adults with Atopic Dermatitis. Dermatitis 2018, 29, 270–277. [Google Scholar] [CrossRef]
- Timonen, M.; Jokelainen, J.; Hakko, H.; Silvennoinen-Kassinen, S.; Meyer-Rochow, V.B.; Herva, A.; Räsänen, P. Atopy and depression: Results from the Northern Finland 1966 Birth Cohort Study. Mol. Psychiatry 2003, 8, 738–744. [Google Scholar] [CrossRef] [PubMed]
- Capitano, J.P. Nonhuman Primate Personality and Immunity: Mechanisms of Health and Disease. In Personality and Temperament in Nonhuman Primates; Weiss, A., King, J.E., Murray, L., Eds.; Springer: New York, NY, USA, 2011. [Google Scholar]
- Lopes, P.C. Why are behavioral and immune traits linked? Horm. Behav. 2017, 88, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Maddock, C.; Pariante, C.M. How does stress affect you? An overview of stress, immunity, depression and disease. Epidemiol. Psichiatr. Soc. 2001, 10, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Marin, M.-F.F.; Lord, C.; Andrews, J.; Juster, R.-P.P.; Sindi, S.; Arsenault-Lapierre, G.; Fiocco, A.J.; Lupien, S.J. Chronic stress, cognitive functioning and mental health. Neurobiol. Learn. Mem. 2011, 96, 583–595. [Google Scholar] [CrossRef] [PubMed]
- Hammen, C.; Kim, E.Y.; Eberhart, N.K.; Brennan, P.A. Chronic and acute stress and the prediction of major depression in women. Depress Anxiety 2009, 26, 718–723. [Google Scholar] [CrossRef]
- Selye, H. The general adaptation syndrome and the diseases of adaptation. J. Clin. Endocrinol. Metab. 1946, 6, 117–230. [Google Scholar] [CrossRef]
- Tausk, F.A.; Nousari, H. Stress and the Skin. Arch. Dermatol. 2001, 137, 78–82. [Google Scholar] [CrossRef]
- Hendrix, S. Neuroimmune communication in skin: Far from peripheral. J. Investig. Dermatol. 2008, 128, 260–261. [Google Scholar] [CrossRef]
- Garg, A.; Chren, M.M.; Sands, L.P.; Matsui, M.S.; Marenus, K.D.; Feingold, K.R.; Elias, P.M. Psychological stress perturbs epidermal permeability barrier homeostasis: Implications for the pathogenesis of stress-associated skin disorders. Arch. Dermatol. 2001, 137, 53–59. [Google Scholar] [CrossRef]
- Gil, K.M.; Keefe, F.J.; Sampson, H.A.; McCaskill, C.C.; Rodin, J.; Crisson, J.E. The relation of stress and family environment to atopic dermatitis symptoms in children. J. Psychosom. Res. 1987, 31, 673–684. [Google Scholar] [CrossRef]
- Kodama, A.; Horikawa, T.; Suzuki, T.; Ajiki, W.; Takashima, T.; Harada, S.; Ichihashi, M. Effect of stress on atopic dermatitis: Investigation in patients after the Great Hanshin Earthquake. J. Allergy Clin. Immunol. 1999, 104, 173–176. [Google Scholar] [CrossRef]
- Denda, M.; Tsuchiya, T.; Hosoi, J.; Koyama, J. Immobilization-induced and crowded environment-induced stress delay barrier recovery in murine skin. Br. J. Dermatol. 1998, 138, 780–785. [Google Scholar] [CrossRef] [PubMed]
- Denda, M.; Tsuchiya, T.; Elias, P.M.; Feingold, K.R. Stress alters cutaneous permeability barrier homeostasis. Am. J. Physiol. Integr. Comp. Physiol. 2000, 278, R367–R372. [Google Scholar] [CrossRef] [PubMed]
- Michel, F.B. Psychology of the allergic patient. Allergy 1994, 49, 28–30. [Google Scholar] [CrossRef] [PubMed]
- Runeson, R.; Wahlstedt, K.; Norback, D. Pilot Study of Personality Traits Assessed by the Karolinska Scales of Personality (Ksp) in Asthma, Atopy, and Rhinitis. Percept. Mot. Skills 2011, 113, 909–920. [Google Scholar] [CrossRef]
- Dreschel, N.A. The effects of fear and anxiety on health and lifespan in pet dogs. Appl. Anim. Behav. Sci. 2010, 125, 157–162. [Google Scholar] [CrossRef]
- Nettle, D.; Bateson, M. The evolutionary origins of mood and its disorders. Curr. Biol. 2012, 22, R712–R721. [Google Scholar] [CrossRef]
- Duric, V.; Clayton, S.; Leong, M.L.; Yuan, L.-L. Comorbidity Factors and Brain Mechanisms Linking Chronic Stress and Systemic Illness. Neural Plast. 2016, 2016, 5460732. [Google Scholar] [CrossRef]
- Harvey, N.D.; Shaw, S.C.; Blott, S.C.; Vàzquez-Diosdado, J.A.; England, G.C.W. Development and validation of a new standardised data collection tool to aid in the diagnosis of canine skin allergies. Sci. Rep. 2019, 9, 3039. [Google Scholar] [CrossRef]
- Colombo, S.; Hill, P.B.; Shaw, D.J.; Thoday, K.L. Effectiveness of low dose immunotherapy in the treatment of canine atopic dermatitis: A prospective, double-blinded, clinical study. Vet. Dermatol. 2005, 16, 162–170. [Google Scholar] [CrossRef]
- Duffy, D.L.; Serpell, J.A. Predictive validity of a method for evaluating temperament in young guide and service dogs. Appl. Anim. Behav. Sci. 2012, 138, 99–109. [Google Scholar] [CrossRef]
- McGreevy, P.D.; Georgevsky, D.; Carrasco, J.; Valenzuela, M.; Duffy, D.L.; Serpell, J.A. Dog Behavior Co-Varies with Height, Bodyweight and Skull Shape. PLoS ONE 2013, 8, e80529. [Google Scholar] [CrossRef] [PubMed]
- Storey, J.D.; Bass, A.J.; Dabney, A.; David, R. Q-value estimation for false discovery rate control. Medicine 2004, 344, 48. [Google Scholar]
- Dabney, A.; Storey, J. Bioconductor’s Qvalue Package. Available online: https://www.bioconductor.org/packages/release/bioc/vignettes/qvalue/inst/doc/qvalue.pdf (accessed on 15 October 2019).
- Klinck, M.P.; Shofer, F.S.; Reisner, I.R. Association of pruritus with anxiety or aggression in dogs. J. Am. Vet. Med. Assoc. 2008, 233, 1105–1111. [Google Scholar] [CrossRef]
- Notari, L. Stress in veterinary behavioural medicine. In BSAVA Manual of Canine and Feline Behavioural Medicine; British Small Animal Veterinary Association: Gloucester, UK, 2009; pp. 136–145. ISBN 978-1-905319-87-9. [Google Scholar]
- Kuhne, F.; Hößler, J.C.; Struwe, R. Emotions in dogs being petted by a familiar or unfamiliar person: Validating behavioural indicators of emotional states using heart rate variability. Appl. Anim. Behav. Sci. 2014, 161, 113–120. [Google Scholar] [CrossRef]
- Overall, K. Manual of Clinical Behavioral Medicine for Dogs and Cats; Mosby Elsevier Inc.: St Louis, MO, USA, 2013. [Google Scholar]
- Overall, K.L.; Dunham, A.E. Clinical features and outcome in dogs and cats with obsessive-compulsive disorder: 126 cases (1989–2000). J. Am. Vet. Med. Assoc. 2002, 221, 1445–1452. [Google Scholar] [CrossRef]
- Packer, R.M.A.; McGreevy, P.D.; Pergande, A.; Volk, H.A. Negative effects of epilepsy and antiepileptic drugs on the trainability of dogs with naturally occurring idiopathic epilepsy. Appl. Anim. Behav. Sci. 2018, 200, 106–113. [Google Scholar] [CrossRef]
- Heller, M.M.; Lee, E.S.; Koo, J.Y. Stress as an influencing factor in psoriasis. Ski. Ther. Lett 2011, 16, 1–4. [Google Scholar]
- Kitagaki, H.; Hiyama, H.; Kitazawa, T.; Shiohara, T. Psychological stress with long-standing allergic dermatitis causes psychodermatological conditions in mice. J. Investig. Dermatol. 2014, 134, 1561–1569. [Google Scholar] [CrossRef]
- Mills, D.; Karagiannis, C.; Zulch, H. Stress—Its Effects on Health and Behavior: A Guide for Practitioners Stress Health Behavior Arousal Emotions Stress audit. Vet. Clin. Small Anim. 2014, 44, 525–541. [Google Scholar] [CrossRef]
- Starling, M.; Branson, N.; Cody, D.; McGreevy, P.; Starling, M.J.; Branson, N.; Cody, D.; McGreevy, P.D. Conceptualising the Impact of Arousal and Affective State on Training Outcomes of Operant Conditioning. Animals 2013, 3, 300–317. [Google Scholar] [CrossRef]
- Eysenck, M.W. Anxiety, learning, and memory: A reconceptualization. J. Res. Personal. 1979, 13, 363–385. [Google Scholar] [CrossRef]
- Silverberg, J.I. Associations between atopic dermatitis and other disorders. F1000Research 2018, 7, 303. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Dong, Y.; Xu, Z.; Gompf, H.S.; Ward, S.A.P.; Xue, Z.; Miao, C.; Zhang, Y.; Chamberlin, N.L.; Xie, Z. Sleep disturbance induces neuroinflammation and impairment of learning and memory. Neurobiol. Dis. 2012, 48, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Nolen, T.M. Sedative effects of antihistamines: Safety, performance, learning, and quality of life. Clin. Ther. 1997, 19, 39–55. [Google Scholar] [CrossRef]
- Packer, R.M.A.; Law, T.H.; Davies, E.; Zanghi, B.; Pan, Y.; Volk, H.A. Effects of a ketogenic diet on ADHD-like behavior in dogs with idiopathic epilepsy. Epilepsy Behav. 2016, 55, 62–68. [Google Scholar] [CrossRef]
- Harvey, N.D.; Shaw, S.C.; Craigon, P.J.; Blott, S.C.; England, G.C.W. Environmental risk factors for canine atopic dermatitis: A retrospective large-scale study in Labrador and golden retrievers. Vet. Dermatol. 2019, 30, 396-e119. [Google Scholar] [CrossRef]
- McGreevy, P.D.; Wilson, B.J.; Mansfield, C.S.; Brodbelt, D.C.; Church, D.B.; Dhand, N.; Soares Magalhães, R.J.; O’Neill, D.G. Labrador retrievers under primary veterinary care in the UK: Demography, mortality and disorders. Canine Genet. Epidemiol. 2018, 5, 8. [Google Scholar] [CrossRef]
- Lofgren, S.E.; Wiener, P.; Blott, S.C.; Sanchez-Molano, E.; Woolliams, J.A.; Clements, D.N.; Haskell, M.J. Management and personality in Labrador Retriever dogs. Appl. Anim. Behav. Sci. 2014, 156, 44–53. [Google Scholar] [CrossRef]
- Hoffman, C.L.; Chen, P.; Serpell, J.A.; Jacobson, K.C. Do Dog Behavioral Characteristics Predict the Quality of the Relationship between Dogs and Their Owners? Hum. Anim. Interact. Bull. 2013, 1, 20–37. [Google Scholar]
- Serpell, J.A. Evidence for an association between pet behavior and owner attachment levels. Appl. Anim. Behav. Sci. 1996, 47, 49–60. [Google Scholar] [CrossRef]
- Salman, M.D.; Hutchison, J.; Ruch-Gallie, R.; Kogan, L.; New, J.C.; Kass, P.H.; Scarlett, J.M. Behavioral Reasons for Relinquishment of Dogs and Cats to 12 Shelters. J. Appl. Anim. Welf. Sci. 2000, 3, 93–106. [Google Scholar] [CrossRef]
| CBARQ Score | Wording |
|---|---|
| Escapes | Escapes or would escape from home or garden given the chance. |
| Rolls in faeces | Rolls in animal droppings or other ‘smelly’ substances. |
| Coprophagia | Eats own or other animals’ droppings or faeces. |
| Chews | Chews inappropriate objects. |
| Mounts | Mounts objects, furniture, or people. |
| Begs for food | Begs persistently for food when people are eating. |
| Steals food | Steals food |
| Nervous on stairs | Nervous or frightened on stairs. |
| Pulls on leash | Pulls excessively hard when on the leash. |
| Urine marks | Urinates against objects/furnishings in your home. |
| Emotional urination | Urinates when approached, petted, handled or picked up. |
| Urination when left alone | Urinates when left alone at night, or during the daytime. |
| Defaecation when left alone | Defaecates when left alone at night, or during the daytime. |
| Hyperactive/restless | Hyperactive, restless, has trouble settling down. |
| Compulsive staring | Stares intently at nothing visible. |
| Snaps at flies | Snaps at (invisible) flies. |
| Tail chasing | Chases own tail/hind end. |
| Shadow chasing | Chases/follows shadows, light spots, etc. |
| Barks persistently | Barks persistently when alarmed or excited. |
| Self-grooming | Licks him/herself excessively. |
| Allo-grooming | Licks people or objects excessively. |
| Other bizarre or repetitive behaviour | Displays other bizarre, strange, or repetitive behaviour(s) (Please describe) |
| C-BARQ Score | Predictors | Main Effect: Diagnosis (Case vs. Control) | Main Effect: Itch Severity (EPS) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| B | 95% CI | t | P | B | 95% CI | t | P | ||
| Trainability | Breed, Sex | −0.10 | −0.18 to −0.03 | −2.92 | 0.004 | −0.04 | −0.07 to −0.02 | −3.51 | <0.001 |
| Excitability | None | 0.09 | −0.20 to 0.19 | 1.59 | 0.112 | 0.05 | 0.01 to 0.09 | 2.65 | 0.008 |
| Attachment/attention-seeking | Age | 0.19 | 0.09 to 0.30 | 3.70 | <0.001 | 0.08 | 0.05 to 0.12 | 4.77 | <0.001 |
| Chasing | Breed, Age | 0.09 | −0.05 to 0.22 | 1.25 | 0.211 | 0.04 | −0.00 to 0.09 | 1.82 | 0.069 |
| Energy | Age | 0.07 | −0.05 to 0.20 | 1.14 | 0.254 | 0.03 | −0.01 to 0.07 | 1.31 | 0.191 |
| C-BARQ Score | Predictors | Main Effect: Diagnosis (Case vs. Control) | Main Effect: Itch Severity (EPS) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| B | 95% CI | Wald | P | B | 95% CI | Wald | P | ||
| Rolling | Breed, Sex, Neutered | 0.04 | −0.07 to 0.15 | 0.48 | 0.488 | 0.02 | −0.02 to 060 | 1.26 | 0.263 |
| Coprophagia | Sex, Neutered | 0.15 | 0.03 to 0.27 | 5.79 | 0.016 | 0.64 | 0.03 to 0.10 | 10.01 | 0.001 |
| Begging | Breed, Neutered | 0.25 | 0.14 to 0.36 | 20.49 | <0.001 | 0.09 | 0.06 to 0.13 | 26.73 | <0.001 |
| Pulls leash | Sex, Age | 0.25 | 0.13 to 0.37 | 17.36 | <0.001 | 0.08 | 0.04 to 0.12 | 16.49 | <0.001 |
| Self-grooms | None | 1.89 | 1.69 to 2.01 | 348.32 | <0.001 | 0.49 | 0.44 to 0.54 | 422.98 | <0.001 |
| Type | Scores | Predictors | Main Effect: Diagnosis (Case) | Main Effect: Itch Scale | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | Wald | P | OR | 95% CI | Wald | P | |||
| Fear/anxiety/aggression traits | ||||||||||
| Non-Social Fear | Breed | 1.04 | 0.79 to 1.37 | 0.08 | 0.785 | 1.03 | 0.93 to 1.13 | 0.28 | 0.599 | |
| Owner Dir. Agg. | 1.20 | 0.81 to 1.78 | 0.79 | 0.374 | 1.10 | 0.97 to 1.25 | 2.05 | 0.152 | ||
| Dog Dir. Fear | 1.27 | 0.97 to 1.66 | 2.93 | 0.087 | 1.06 | 0.97 to 1.17 | 1.75 | 0.186 | ||
| Dog Dir. Agg. | Age, Sex, Sex * Neutered | 1.14 | 0.86 to 1.51 | 0.81 | 0.368 | 1.05 | 0.95 to 1.16 | 0.98 | 0.323 | |
| Stranger Dir. Fear | Age, Breed | 0.96 | 0.71 to 1.31 | 0.06 | 0.814 | 0.97 | 0.87 to 1.08 | 0.27 | 0.605 | |
| Family Dog Agg. | 0.79 | 0.56 to 1.11 | 1.85 | 0.174 | 0.99 | 0.89 to 1.12 | 0.00 | 0.953 | ||
| Sep. Rel. Beh. | 0.95 | 0.73 to 1.25 | 0.12 | 0.735 | 1.01 | 0.92 to 1.11 | 0.05 | 0.826 | ||
| Stair fear | 1.10 | 0.76 to 1.59 | 0.24 | 0.621 | 1.09 | 0.96 to 1.23 | 1.72 | 0.189 | ||
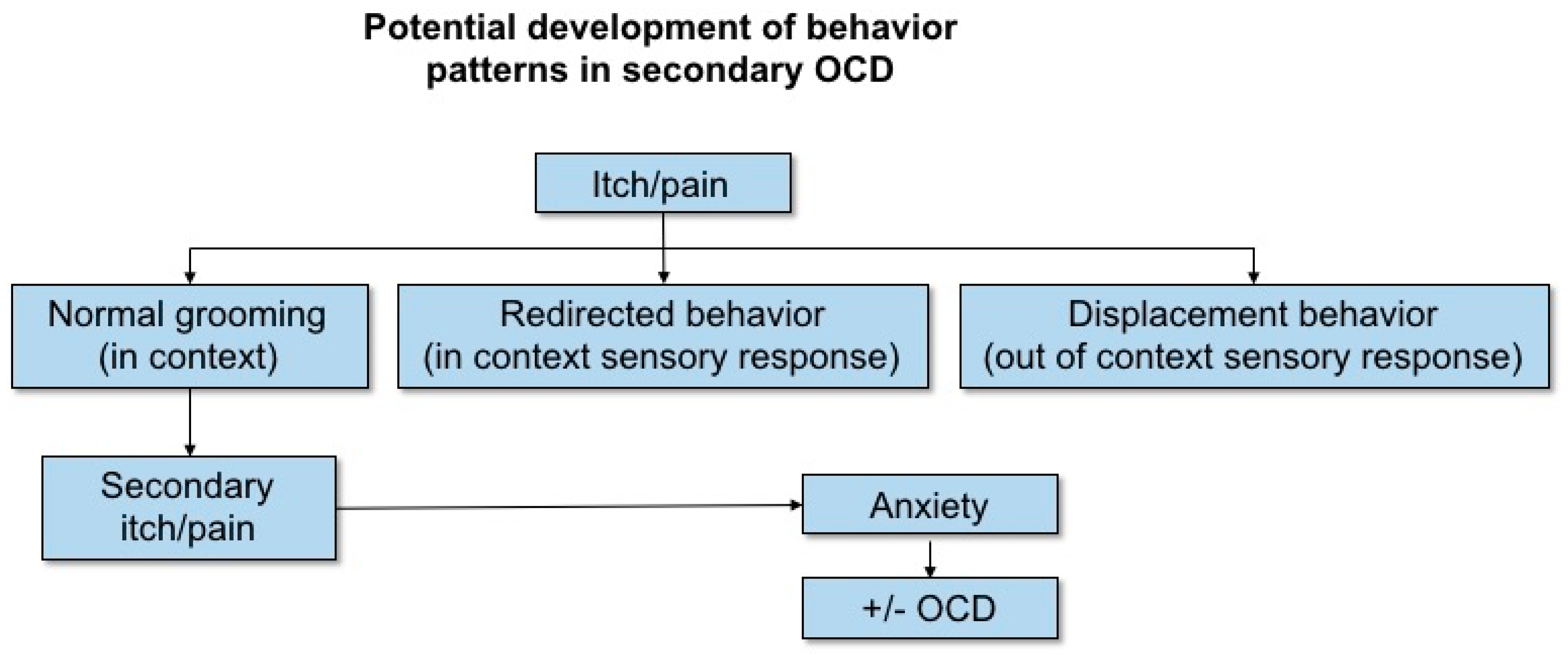
| Redirected/displacement behaviour | ||||||||||
| Chewing | Age | 1.40 | 1.06 to 1.85 | 5.64 | 0.018 | 1.14 | 1.04 to 1.25 | 7.53 | 0.006 | |
| Mounting | Breed, Sex | 2.14 | 1.28 to 3.55 | 8.55 * | 0.003 | 1.14 | 1.02 to 1.28 | 5.23 | 0.022 | |
| Hyper/restless | Age | 1.64 | 1.18 to 2.28 | 8.85 | 0.003 | 1.20 | 1.08 to 1.34 | 11.43 | 0.001 | |
| Steals food | Breed, Neutered | 1.18 | 0.89 to 1.57 | 1.39 | 0.238 | 1.14 | 1.04 to 1.25 | 7.35 | 0.007 | |
| Allo-grooming | Age, Breed | 1.88 | 1.41 to 2.52 | 18.14 | <0.001 | 1.27 | 1.15 to 1.40 | 22.68 | <0.001 | |
| Abnormal/repetitive behaviour | ||||||||||
| Staring | Neutered | 1.26 | 0.92 to 1.72 | 2.04 | 0.153 | 1.09 | 0.99 to 1.21 | 2.82 | 0.093 | |
| Snaps at flies | 0.79 | 0.54 to 1.17 | 1.38 | 0.240 | 0.94 | 0.83 to 1.08 | 0.71 | 0.401 | ||
| Chases tail | Age, Breed | 0.97 | 0.72 to 1.31 | 0.05 | 0.830 | 1.05 | 0.95 to 1.16 | 0.81 | 0.369 | |
| Chases shadows | Age, Breed | 1.27 | 0.90 to 1.79 | 1.82 | 0.178 | 1.09 | 0.97 to 1.23 | 2.15 | 0.142 | |
| Other Rep. Beh. | 2.73 | 1.79 to 4.15 | 21.91 | <0.001 | 1.44 | 1.27 to 1.64 | 10.81 | <0.001 | ||
| Other/problem behaviour | ||||||||||
| Touch Sens. | 1.38 | 1.05 to 1.82 | 5.39 | 0.020 | 1.12 | 1.02 to 1.23 | 6.06 | 0.014 | ||
| Barking | Breed | 1.24 | 0.94 to 1.63 | 2.37 | 0.124 | 1.09 | 0.99 to 1.20 | 3.76 | 0.052 | |
| Escapes | 1.21 | 0.92 to 1.59 | 1.87 | 0.171 | 1.11 | 1.01 to 1.22 | 4.90 | 0.027 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harvey, N.D.; Craigon, P.J.; Shaw, S.C.; Blott, S.C.; England, G.C.W. Behavioural Differences in Dogs with Atopic Dermatitis Suggest Stress Could Be a Significant Problem Associated with Chronic Pruritus. Animals 2019, 9, 813. https://doi.org/10.3390/ani9100813
Harvey ND, Craigon PJ, Shaw SC, Blott SC, England GCW. Behavioural Differences in Dogs with Atopic Dermatitis Suggest Stress Could Be a Significant Problem Associated with Chronic Pruritus. Animals. 2019; 9(10):813. https://doi.org/10.3390/ani9100813
Chicago/Turabian StyleHarvey, Naomi D., Peter J. Craigon, Stephen C. Shaw, Sarah C. Blott, and Gary C.W. England. 2019. "Behavioural Differences in Dogs with Atopic Dermatitis Suggest Stress Could Be a Significant Problem Associated with Chronic Pruritus" Animals 9, no. 10: 813. https://doi.org/10.3390/ani9100813
APA StyleHarvey, N. D., Craigon, P. J., Shaw, S. C., Blott, S. C., & England, G. C. W. (2019). Behavioural Differences in Dogs with Atopic Dermatitis Suggest Stress Could Be a Significant Problem Associated with Chronic Pruritus. Animals, 9(10), 813. https://doi.org/10.3390/ani9100813





