The Role of Oxytocin in the Dog–Owner Relationship
Abstract
:Simple Summary
Abstract
1. Introduction
2. Material and Methods
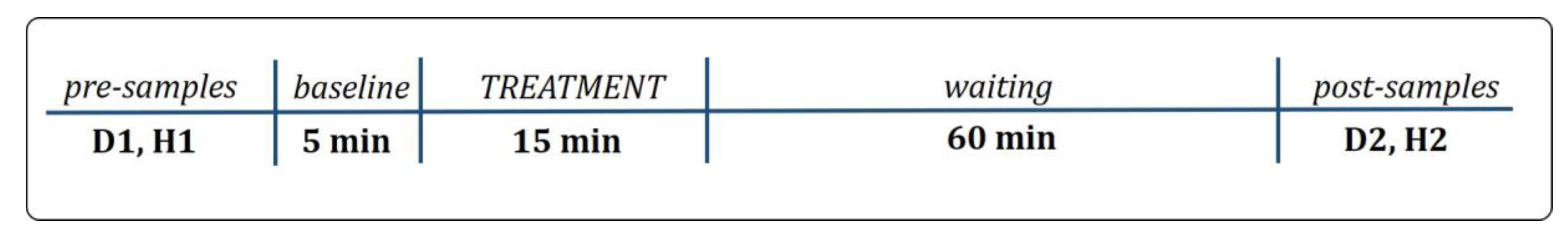
2.1. Study Design
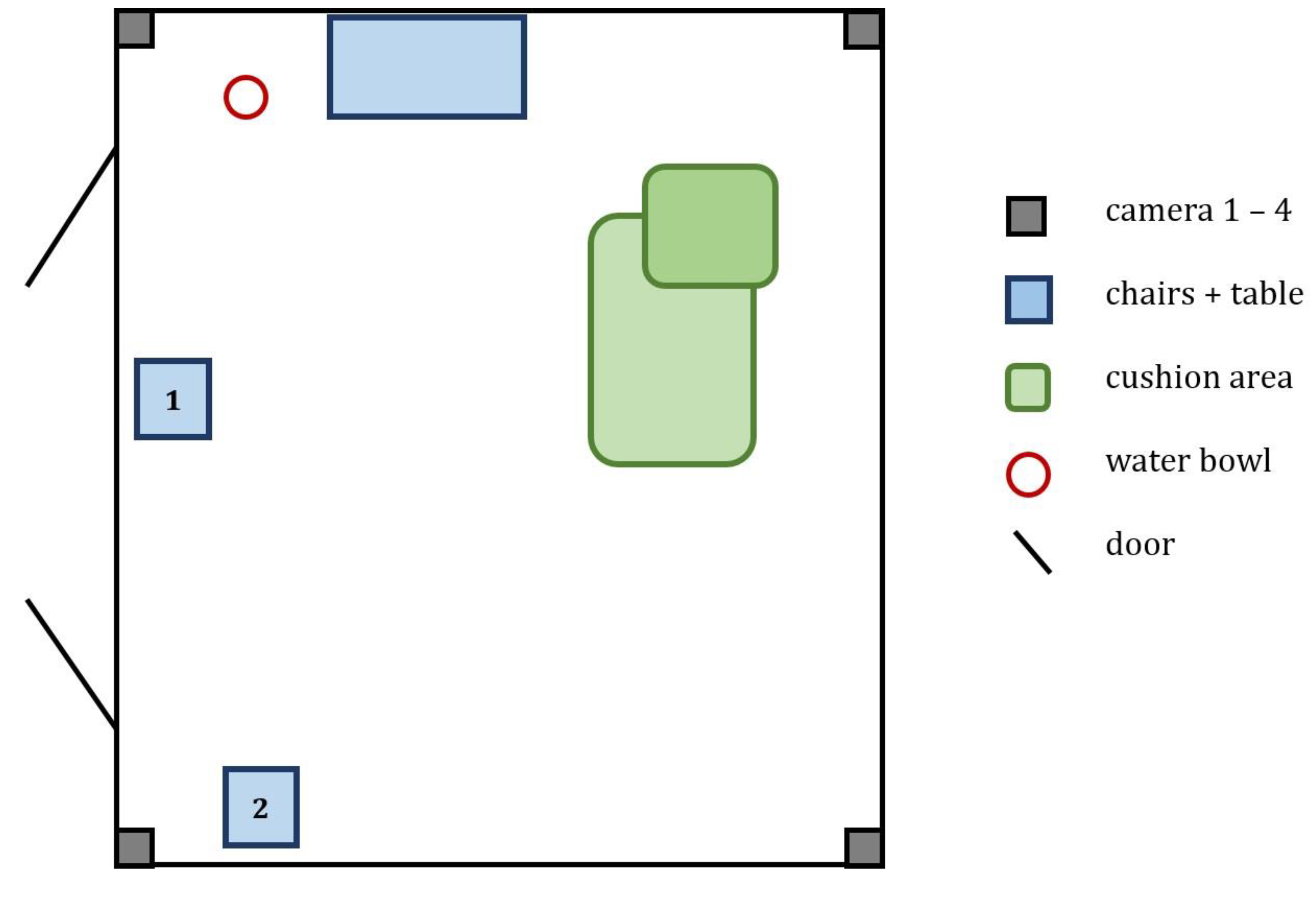
2.2. Experimental Setup
2.2.1. Pre-Testing Instructions to Owner and Pre-Sample Collection
2.2.2. Treatment Procedure
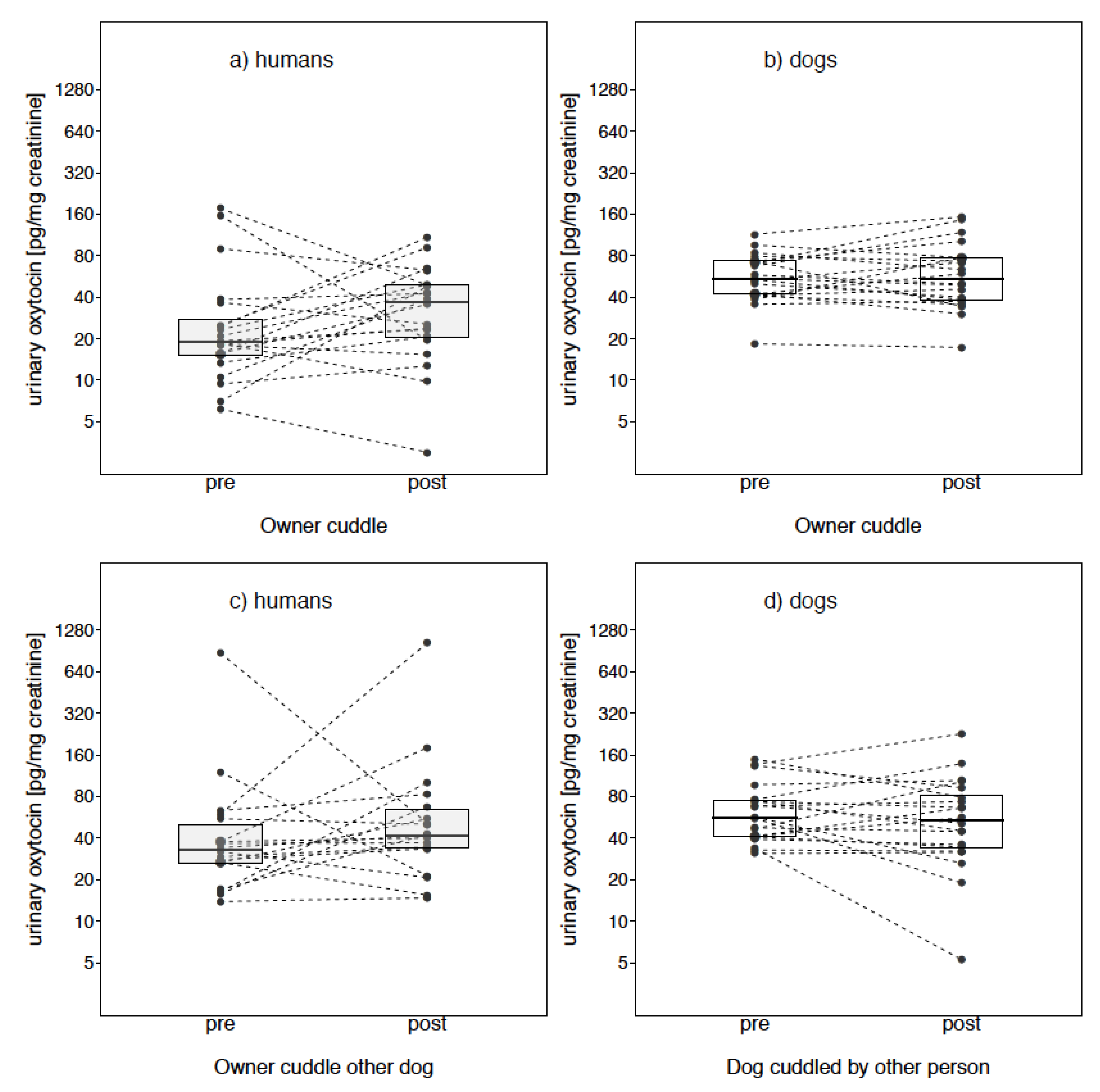
Owner Cuddle
Familiar Cuddle
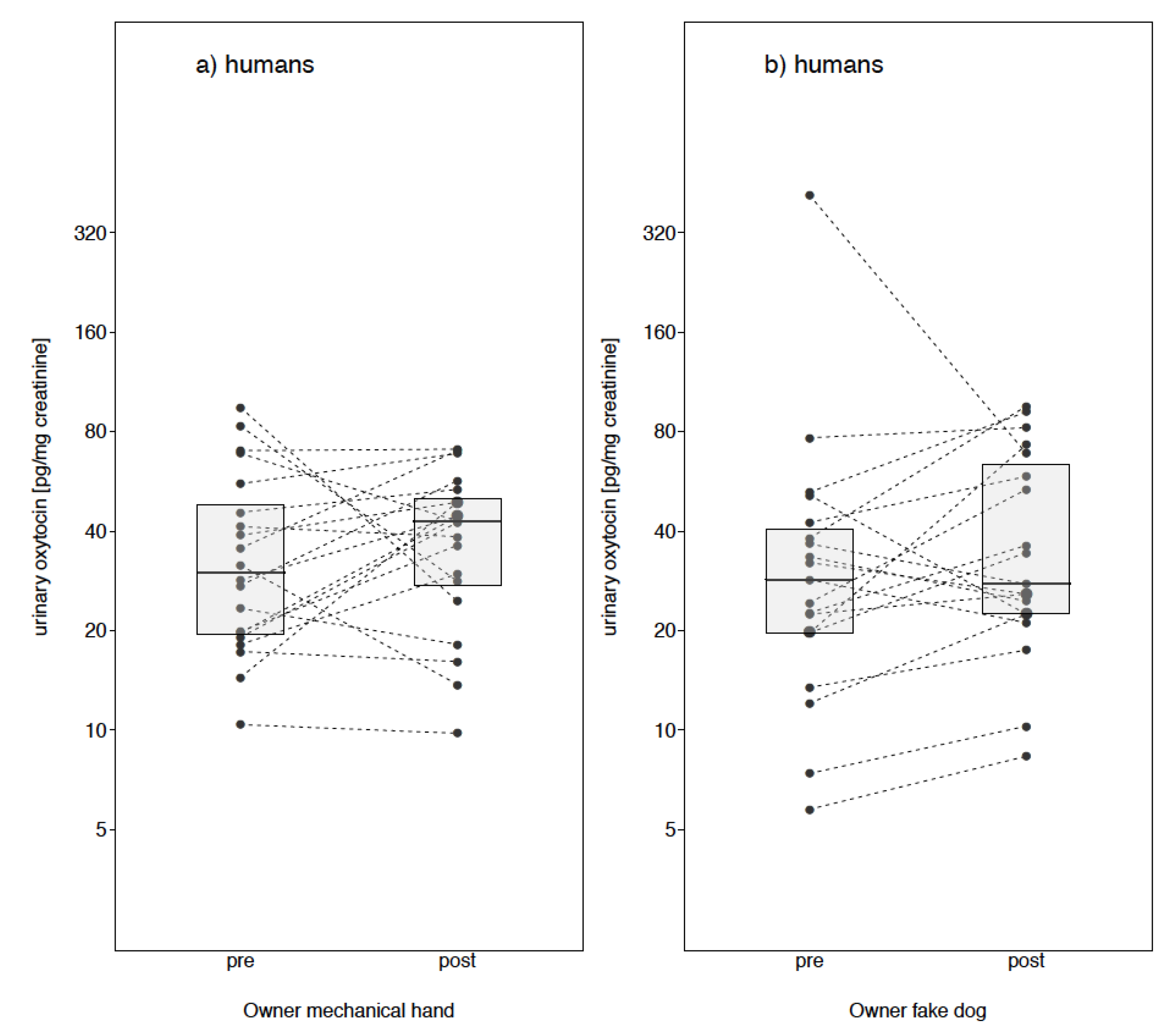
Owner Fake Dog
Familiar Fake Dog
Owner Cuddle Other Dog
Owner Mechanical Cuddle
2.2.3. Urine Sample Collection and Treatment
2.2.4. Attachment Questionnaire
2.2.5. Behavior Coding
2.2.6. Laboratory Analyses
2.2.7. Statistical Analyses
Models on OT Variance in Dogs
Models on OT Variance in Owners
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Anacker, A.M.J.; Beery, A.K. Life in groups: The roles of oxytocin in mammalian sociality. Front. Behav. Neurosci. 2013, 7, 185. [Google Scholar] [CrossRef] [PubMed]
- Gimpl, G.; Fahrenholz, F. The oxytocin receptor system: Structure, function, and regulation. Physiol. Rev. 2001, 81, 629–683. [Google Scholar] [CrossRef] [PubMed]
- Kirsch, P. Oxytocin modulates neural circuitry for social cognition and fear in humans. J. Neurosci. 2005, 25, 11489–11493. [Google Scholar] [CrossRef] [PubMed]
- Insel, T.R.; Young, L.J. The neurobiology of attachment. Nat. Rev. Neurosci. 2001, 2, 129–136. [Google Scholar] [CrossRef]
- Graustella, A.J.; MacLeod, C. A critical review of the influence of oxytocin nasal spray on social cognition in humans: Evidence and future directions. Horm. Behav. 2012, 61, 410–418. [Google Scholar] [CrossRef]
- De Dreu, C.K.W.; Greer, L.L.; Handgraaf, M.J.J.; Shalvi, S.; van Kleef, G.A.; Baas, M.; Velden, F.S.T.; van Dijk, E.; Feith, S.W.W. The neuropeptide oxytocin regulates parochial altruism in intergroup conflict among humans. Science 2010, 328, 1408–1411. [Google Scholar] [CrossRef]
- Samuni, L.; Preis, A.; Mundry, R.; Deschner, T.; Crockford, C.; Wittig, R.M. Oxytocin reactivity during intergroup conflict in wild chimpanzees. Proc. Natl. Acad. Sci. USA 2017, 114, 268–273. [Google Scholar] [CrossRef]
- Coria-Avila, G.A.; Manzo, J.; Garcia, L.I.; Carrillo, P.; Miquel, M.; Pfaus, J.G. Neurobiology of social attachments. Neurosci. Biobehav. Rev. 2014, 43, 173–182. [Google Scholar] [CrossRef] [Green Version]
- Crockford, C.; Deschner, T.; Ziegler, T.E.; Wittig, R.M. Endogenous peripheral oxytocin measures can give insight into the dynamics of social relationships: A review. Front. Behav. Neurosci. 2014, 8, 68. [Google Scholar] [CrossRef]
- Feldman, R. Oxytocin and social affiliation in humans. Horm. Behav. 2012, 61, 380–391. [Google Scholar] [CrossRef]
- Crockford, C.; Wittig, R.M.; Langergraber, K.; Ziegler, T.E.; Zuberbuhler, K.; Deschner, T. Urinary oxytocin and social bonding in related and unrelated wild chimpanzees. Proc. R. Soc. B Biol. Sci. 2013, 280, 20122765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dale, R.; Range, F.; Stott, L.; Kotrschal, K.; Marshall-Pescini, S. The influence of social relationship on food tolerance in wolves and dogs. Behav. Ecol. Sociobiol. 2017, 71, 107. [Google Scholar] [CrossRef] [PubMed]
- Topál, J.; Miklósi, Á.; Csányi, V.; Dóka, A. Attachment behavior in dogs (Canis familiaris): A new application of Ainsworth’s (1969) Strange Situation Test. J. Comp. Psychol. 1998, 112, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Nagasawa, M.; Mogi, K.; Kikusui, T. Attachment between humans and dogs. Jpn. Psychol. Res. 2009, 51, 209–221. [Google Scholar] [CrossRef]
- Handlin, L.; Hydbring-Sandberg, E.; Nilsson, A.; Ejdebäck, M.; Jansson, A.; Uvnäs-Moberg, K. Short-term interaction between dogs and their owners: Effects on oxytocin, cortisol, insulin and heart rate—An exploratory study. Anthrozoos 2011, 24, 301–315. [Google Scholar] [CrossRef]
- Thielke, L.E.; Udell, M.A.R. The role of oxytocin in relationships between dogs and humans and potential applications for the treatment of separation anxiety in dogs: Oxytocin and separation anxiety. Biol. Rev. 2017, 92, 378–388. [Google Scholar] [CrossRef]
- Buttner, A.P. Neurobiological underpinnings of dogs’ human-like social competence: How interactions between stress response systems and oxytocin mediate dogs’ social skills. Neurosci. Biobehav. Rev. 2016, 71, 198–214. [Google Scholar] [CrossRef]
- Prato-Previde, E.; Spiezio, C.; Sabatini, F.; Custance, D.M. Is the dog-human relationship an attachment bond? An observational study using Ainsworth’s strange situation. Behaviour 2003, 140, 225–254. [Google Scholar] [CrossRef]
- Horn, L.; Huber, L.; Range, F. The importance of the secure base effect for domestic dogs—Evidence from a manipulative problem-solving task. PLoS ONE 2013, 8, e65296. [Google Scholar] [CrossRef]
- Gácsi, M.; Maros, K.; Sernkvist, S.; Faragó, T.; Miklósi, Á. Human analogue safe haven effect of the owner: Behavioural and heart rate response to stressful social stimuli in dogs. PLoS ONE 2013, 8, e58475. [Google Scholar] [CrossRef]
- Stoeckel, L.E.; Palley, L.S.; Gollub, R.L.; Niemi, S.M.; Evins, A.E. Patterns of brain activation when mothers view their own child and dog: An fMRI study. PLoS ONE 2014, 9, e107205. [Google Scholar] [CrossRef] [PubMed]
- Mitsui, S.; Yamamoto, M.; Nagasawa, M.; Mogi, K.; Kikusui, T.; Ohtani, N.; Ohta, M. Urinary oxytocin as a noninvasive biomarker of positive emotion in dogs. Horm. Behav. 2011, 60, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Odendaal, J.S.J.; Meintjes, R.A. Neurophysiological correlates of affiliative behaviour between humans and dogs. Vet. J. 2003, 165, 296–301. [Google Scholar] [CrossRef]
- Nagasawa, M.; Kikusui, T.; Onaka, T.; Ohta, M. Dog’s gaze at its owner increases owner’s urinary oxytocin during social interaction. Horm. Behav. 2009, 55, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Rehn, T.; Handlin, L.; Uvnäs-Moberg, K.; Keeling, L.J. Dogs’ endocrine and behavioural responses at reunion are affected by how the human initiates contact. Physiol. Behav. 2014, 124, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Romero, T.; Nagasawa, M.; Mogi, K.; Hasegawa, T.; Kikusui, T. Oxytocin promotes social bonding in dogs. Proc. Natl. Acad. Sci. USA 2014, 111, 9085–9090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagasawa, M.; Mitsui, S.; En, S.; Ohtani, N.; Ohta, M.; Sakuma, Y.; Onaka, T.; Mogi, K.; Kikusui, T. Oxytocin-gaze positive loop and the coevolution of human–dog bonds. Science 2015, 348, 333–336. [Google Scholar] [CrossRef]
- Miller, S.C.; Kennedy, C.C.; DeVoe, D.C.; Hickey, M.; Nelson, T.; Kogan, L. An examination of changes in oxytocin levels in men and women before and after interaction with a bonded dog. Anthrozoös 2009, 22, 31–42. [Google Scholar] [CrossRef]
- Grebe, N. From Norway with Love: A Study of Oxytocin, Social Bonding, and Life-History Trade-Offs. Ph.D. Thesis, University of New Mexico, Albuquerque, NM, USA, 2016. [Google Scholar]
- Hritcu, L.D.; Horhogea, C.; Ciobica, A.; Spataru, M.C.; Spataru, C.; Kis, A. Conceptual replication of canine serum oxytocin increase following a positive dog-human interaction. Rev. Chim. 2019, 70, 1579–1581. [Google Scholar]
- Powell, L.; Edwards, K.M.; Bauman, A.; Guastella, A.J.; Drayton, B.; Stamatakis, E.; McGreevy, P. Canine endogenous oxytocin responses to dog-walking and affiliative human–dog interactions. Animals 2019, 9, 51. [Google Scholar] [CrossRef]
- MacLean, E.L.; Gesquiere, L.R.; Gee, N.R.; Levy, K.; Martin, W.L.; Carter, C.S. Effects of affiliative human–animal interaction on dog salivary and plasma oxytocin and vasopressin. Front. Psychol. 2017, 8, 1606. [Google Scholar] [CrossRef] [PubMed]
- Uvnäs-Moberg, K.; Handlin, L.; Petersson, M. Self-soothing behaviors with particular reference to oxytocin release induced by non-noxious sensory stimulation. Front. Psychol. 2015, 5, 1529. [Google Scholar] [CrossRef] [PubMed]
- Kekecs, Z.; Szollosi, A.; Palfi, B.; Szaszi, B.; Kovacs, K.J.; Dienes, Z.; Aczel, B. Commentary: Oxytocin-gaze positive loop and the coevolution of human–dog bonds. Front. Neurosci. 2016, 10, 155. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.P.; Garrity, T.F.; Stallones, L. Psychometric evaluation of the lexington attachment to pets scale (Laps). Anthrozoös 1992, 5, 160–175. [Google Scholar] [CrossRef]
- Schaebs, F.S.; Marshall-Pescini, S.; Range, F.; Deschner, T. Analytical validation of an enzyme immunoassay for the measurement of urinary oxytocin in dogs and wolves. Gen. Comp. Endocrinol. 2019, 281, 73–82. [Google Scholar] [CrossRef]
- Schaebs, F.S.; Marshall-Pescini, S.; Range, F.; Deschner, T. Urinary oxytocin in humans—An analytical validation. In preparation.
- Olff, M.; Frijling, J.L.; Kubzansky, L.D.; Bradley, B.; Ellenbogen, M.A.; Cardoso, C.; Bartz, J.A.; Yee, J.R.; van Zuiden, M. The role of oxytocin in social bonding, stress regulation and mental health: An update on the moderating effects of context and interindividual differences. Psychoneuroendocrinology 2013, 38, 1883–1894. [Google Scholar] [CrossRef] [Green Version]
- Cardoso, C.; Kingdon, D.; Ellenbogen, M.A. A meta-analytic review of the impact of intranasal oxytocin administration on cortisol concentrations during laboratory tasks: Moderation by method and mental health. Psychoneuroendocrinology 2014, 49, 161–170. [Google Scholar] [CrossRef]
- Philipp, T.; Christine, H.; Sonja, E.; Elisabeth, B.; Pathik, W.; Claudia, B. Oxytocin pathways in the intergenerational transmission of maternal early life stress. Neurosci. Biobehav. Rev. 2017, 73, 293–308. [Google Scholar]
- Baayen, R.H. Analyzing Linguistic Data: A Practical Introduction to Statistics Using R.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2008; ISBN 978-0-521-88259-0. [Google Scholar]
- Mundry, R. Statistical issues and assumptions of phylogenetic generalized least squares. In Modern Phylogenetic Comparative Methods and Their Application in Evolutionary Biology; Garamszegi, L.Z., Ed.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 131–153. ISBN 978-3-662-43549-6. [Google Scholar]
- Schielzeth, H. Simple means to improve the interpretability of regression coefficients: Interpretation of regression coefficients. Methods Ecol. Evol. 2010, 1, 103–113. [Google Scholar] [CrossRef]
- Field, A.P. Discovering Statistics Using SPSS: (and Sex, Drugs and Rock “n” Roll), 3rd ed.; SAGE Publications: Los Angeles, CA, USA, 2009; ISBN 978-1-84787-906-6. [Google Scholar]
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 3rd ed.; Sage: Thousand Oaks, CA, USA, 2019. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67. [Google Scholar] [CrossRef]
- Dobson, A.J.; Barnett, A.G. An introduction to generalized linear models. In Chapman & Hall/CRC Texts in Statistical Science Series, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2008; ISBN 978-1-58488-950-2. [Google Scholar]
- Forstmeier, W.; Schielzeth, H. Cryptic multiple hypotheses testing in linear models: Overestimated effect sizes and the winner’s curse. Behav. Ecol. Sociobiol. 2011, 65, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Barr, D.J.; Levy, R.; Scheepers, C.; Tily, H.J. Random effects structure for confirmatory hypothesis testing: Keep it maximal. J. Mem. Lang. 2013, 68, 255–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Schielzeth, H.; Forstmeier, W. Conclusions beyond support: Overconfident estimates in mixed models. Behav. Ecol. 2009, 20, 416–420. [Google Scholar] [CrossRef] [PubMed]
- Kovács, K.; Kis, A.; Kanizsár, O.; Hernádi, A.; Gácsi, M.; Topál, J. The effect of oxytocin on biological motion perception in dogs (Canis familiaris). Anim. Cogn. 2016, 19, 513–522. [Google Scholar] [CrossRef]
- Bence, M.; Marx, P.; Szantai, E.; Kubinyi, E.; Ronai, Z.; Banlaki, Z. Lessons from the canine Oxtr gene: Populations, variants and functional aspects: Lessons from canine Oxtr variants. Genes Brain Behav. 2017, 16, 427–438. [Google Scholar] [CrossRef]
- Cimarelli, G.; Turcsán, B.; Bánlaki, Z.; Range, F.; Virányi, Z. Dog owners’ interaction styles: Their components and associations with reactions of pet dogs to a social threat. Front. Psychol. 2016, 7, 1979. [Google Scholar] [CrossRef]
- Handlin, L.; Nilsson, A.; Ejdebäck, M.; Hydbring-Sandberg, E.; Uvnäs-Moberg, K. Associations between the psychological characteristics of the human–dog relationship and oxytocin and cortisol levels. Anthrozoös 2012, 25, 215–228. [Google Scholar] [CrossRef]
- Higuchi, T.; Honda, K.; Fukuoka, T.; Negoro, H.; Wakabayashi, K. Release of oxytocin during suckling and parturition in the rat. J. Endocrinol. 1985, 105, 339–346. [Google Scholar] [CrossRef]
- Onaka, T.; Yagi, K.; Hamamura, M. Vasopressin secretion: Suppression after light and tone stimuli previously paired with footshocks in rats. Exp. Brain Res. 1988, 71, 291–297. [Google Scholar] [CrossRef]
- Onaka, T.; Yagi, K. Differential effects of naloxone on neuroendocrine responses to fear-related emotional stress. Exp. Brain Res. 1990, 81, 53–58. [Google Scholar] [CrossRef]
- McCullough, M.E.; Churchland, P.S.; Mendez, A.J. Problems with measuring peripheral oxytocin: Can the data on oxytocin and human behavior be trusted? Neurosci. Biobehav. Rev. 2013, 37, 1485–1492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacDonald, K.; Feifel, D. Oxytocin’s role in anxiety: A critical appraisal. Brain Res. 2014, 1580, 22–56. [Google Scholar] [CrossRef] [PubMed]
- Landgraf, R.; Neumann, I.; Schwarzberg, H. Central and peripheral release of vasopressin and oxytocin in the conscious rat after osmotic stimulation. Brain Res. 1988, 457, 219–225. [Google Scholar] [CrossRef]
- Wotjak, C.T.; Ganster, J.; Kohl, G.; Holsboer, F.; Landgraf, R.; Engelmann, M. Dissociated central and peripheral release of vasopressin, but not oxytocin, in response to repeated swim stress: New insights into the secretory capacities of peptidergic neurons. Neuroscience 1998, 85, 1209–1222. [Google Scholar] [CrossRef]
- Ross, H.E.; Cole, C.D.; Smith, Y.; Neumann, I.D.; Landgraf, R.; Murphy, A.Z.; Young, L.J. Characterization of the oxytocin system regulating affiliative behavior in female prairie voles. Neuroscience 2009, 162, 892–903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.-L.; Yuan, Y.; Yang, J.; Wang, C.-H.; Pan, Y.-J.; Lu, L.; Wu, Y.-Q.; Wang, D.-X.; Lv, L.-X.; Li, R.-R.; et al. The interaction between the oxytocin and pain modulation in headache patients. Neuropeptides 2013, 47, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Carson, D.S.; Berquist, S.W.; Trujillo, T.H.; Garner, J.P.; Hannah, S.L.; Hyde, S.A.; Sumiyoshi, R.D.; Jackson, L.P.; Moss, J.K.; Strehlow, M.C.; et al. Cerebrospinal fluid and plasma oxytocin concentrations are positively correlated and negatively predict anxiety in children. Mol. Psychiatry 2015, 20, 1085–1090. [Google Scholar] [CrossRef] [PubMed]
- Robinson, I.C.A.F.; Jones, P.M. Oxytocin and neurophysin in plasma and CSF during suckling in the guinea-pig. Neuroendocrinology 1982, 34, 59–63. [Google Scholar] [CrossRef]
- Amico, J.A.; Challinor, S.M.; Cameron, J.L. Pattern of oxytocin concentrations in the plasma and cerebrospinal fluid of lactating rhesus monkeys (Macaca mulatto): Evidence for functionally independent oxytocinergic pathways in primates. J. Clin. Endocrinol. Metab. 1990, 71, 1531–1535. [Google Scholar] [CrossRef]
- Kagerbauer, S.M.; Martin, J.; Schuster, T.; Blobner, M.; Kochs, E.F.; Landgraf, R. Plasma oxytocin and vasopressin do not predict neuropeptide concentrations in human cerebrospinal fluid. J. Neuroendocrinol. 2013, 25, 668–673. [Google Scholar] [CrossRef]
- Striepens, N.; Kendrick, K.M.; Hanking, V.; Landgraf, R.; Wüllner, U.; Maier, W.; Hurlemann, R. Elevated cerebrospinal fluid and blood concentrations of oxytocin following its intranasal administration in humans. Sci. Rep. 2013, 3, 3440. [Google Scholar] [CrossRef] [PubMed]
- Valstad, M.; Alvares, G.A.; Egknud, M.; Matziorinis, A.M.; Andreassen, O.A.; Westlye, L.T.; Quintana, D.S. The correlation between central and peripheral oxytocin concentrations: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2017, 78, 117–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amico, J.A.; Ulbrecht, J.S.; Robinson, A.G. Clearance studies of oxytocin in humans using radioimmunoassay measurements of the hormone in plasma and urine. J. Clin. Endocrinol. Metab. 1987, 64, 340–345. [Google Scholar] [CrossRef]
- Feldman, R.; Gordon, I.; Zagoory-Sharon, O. Maternal and paternal plasma, salivary, and urinary oxytocin and parent-infant synchrony: Considering stress and affiliation components of human bonding: Oxytocin and parent-infant synchrony. Dev. Sci. 2011, 14, 752–761. [Google Scholar] [CrossRef] [PubMed]
- Lane, A.; Luminet, O.; Nave, G.; Mikolajczak, M. Is there a publication bias in behavioural intranasal oxytocin research on humans? Opening the file drawer of one laboratory. J. Neuroendocrinol. 2016, 28. [Google Scholar] [CrossRef]
- Colegrave, N.; Ruxton, G.D. Confidence intervals are a more useful complement to nonsignificant tests than are power calculations. Behav. Ecol. 2003, 14, 446–447. [Google Scholar] [CrossRef]
- Hoenig, J.M.; Heisey, D.M. The abuse of power: The pervasive fallacy of power calculations for data analysis. Am. Stat. 2001, 55, 19–24. [Google Scholar] [CrossRef]
- Beetz, A.; Uvnäs-Moberg, K.; Julius, H.; Kotrschal, K. Psychosocial and psychophysiological effects of human-animal interactions: The possible role of oxytocin. Front. Psychol. 2012, 3, 234. [Google Scholar] [CrossRef]
| Dog | Sex | Castration | Age (Years/Months) | Breed | Owner |
|---|---|---|---|---|---|
| Ch | female | Yes | 8/3 | Australian Shepherd | JS |
| Cl | female | Yes | 2/4 | Mixed breed | DB |
| Gu | female | No | 11/7 | Border Collie | FR |
| Hy | female | Yes | 8/3 | Mixed breed-likely labrador mix | JE |
| Lo | female | No | 3/9 | Mixed breed-likely herding mix | DB |
| Ti | female | Yes | 9/8 | Mixed breed-husky mix | SM |
| Tu | female | Yes | 2/7 | Mixed breed | JE |
| Fr | male | Yes | 2/8 | Mixed breed-likely terrier mix | GC |
| Li | male | No | 2/3 | Australian Shepherd | JS |
| Ki | male | Yes | 6/9 | Mixed breed | RD |
| Ma | male | No | 11/10 | Golden Retriever | SM |
| Mo | male | Yes | 3/6 | Mixed breed-likely labrador mix | AM |
| Ol | male | Yes | 10/5 | Mixed breed-likely herding mix | KG |
| Sc | male | Yes | 2 | Mixed breed-likely herding mix | SK |
| Tia | female | No | 5/2 | Border Collie | AG |
| Pa | female | Yes | 3/8 | American Staffordshire Terrier | SJ |
| Me | male | Yes | 8/6 | Border Collie | MK |
| Che | female | No | 4/3 | Australian Shepherd | CG |
| Ja | male | Yes | 8/5 | Labradoodle | HLJ |
| Jac | male | Yes | 6/10 | Mixed breed—Greece shepherd mix | KR |
| Category | Condition | Owner’s Dog | Owner | Dog Sample | Owner Sample |
|---|---|---|---|---|---|
| Social Conditions | Owner cuddle | Is being cuddled by their owner | Is cuddling their own dog | yes | yes |
| Familiar cuddle | Is being cuddled by the familiar person | Is quietly sitting in the room | yes | no | |
| Owner cuddle other dog | Dog-subject is absent, another ‘stooge/familiar’ dog takes its place | Is cuddling a familiar dog | no | yes | |
| Non-social Conditions | Owner fake dog | Is present but not given attention to | Is cuddling a fake dog | yes | yes |
| Familiar fake dog | Is present but not given attention to | Is quietly sitting in the room | yes | no | |
| Owner mechanical cuddle | Is being stroked by the artificial hand held by the owner. No social interaction. | Is stroking (but no other social interaction) own dog using an artificial hand | yes | yes |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marshall-Pescini, S.; Schaebs, F.S.; Gaugg, A.; Meinert, A.; Deschner, T.; Range, F. The Role of Oxytocin in the Dog–Owner Relationship. Animals 2019, 9, 792. https://doi.org/10.3390/ani9100792
Marshall-Pescini S, Schaebs FS, Gaugg A, Meinert A, Deschner T, Range F. The Role of Oxytocin in the Dog–Owner Relationship. Animals. 2019; 9(10):792. https://doi.org/10.3390/ani9100792
Chicago/Turabian StyleMarshall-Pescini, Sarah, Franka S. Schaebs, Alina Gaugg, Anne Meinert, Tobias Deschner, and Friederike Range. 2019. "The Role of Oxytocin in the Dog–Owner Relationship" Animals 9, no. 10: 792. https://doi.org/10.3390/ani9100792
APA StyleMarshall-Pescini, S., Schaebs, F. S., Gaugg, A., Meinert, A., Deschner, T., & Range, F. (2019). The Role of Oxytocin in the Dog–Owner Relationship. Animals, 9(10), 792. https://doi.org/10.3390/ani9100792




