Dietary Supplementation of 25-Hydroxycholecalciferol Improves Livability in Broiler Breeder Hens-Amelioration of Cardiac Pathogenesis and Hepatopathology
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Strategy
2.2. Animal Management
2.3. Necropsy, Tissue Collection, and Blood pH and Gas Parameter Analysis
2.4. ECG Analysis and Morphologic Indicators of Cardiac Dysfunction
2.5. TG Content, Hepatic Histological and Fibrosis, and Cardiac Calcineurin Activity Analysis
2.6. Western Blot Analysis
2.7. Statistics
3. Results
3.1. Incidence of Cardiac Pathologies at Necropsy
3.2. Heart and Liver Weight in Hens Experiencing SD and in Hens of Planned Sampling
3.3. Cardiac Hypertrophic Remodeling
3.4. Hepatic Steatosis and Fibrosis
3.5. Systemic Hypoxia, Acidosis, and Cardiac HIF-1α Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Yu, M.W.; Robinson, F.E.; Robblee, A.R. Effect of feed allowance during rearing and breeding on female broiler breeders. 1. Growth and carcass characteristics. Poult. Sci. 1992, 71, 1739–1749. [Google Scholar] [CrossRef] [PubMed]
- Griffin, H.D.; Goddard, C. Rapidly growing broiler (meat-type) chickens: Their origin and use for comparative studies of the regulation of growth. Int. J. Biochem. 1994, 26, 19–28. [Google Scholar] [CrossRef]
- McGovern, R.H.; Feddes, J.J.; Robinson, F.E.; Hanson, J.A. Growth performance, carcass characteristics, and the incidence of ascites in broilers in response to feed restriction and litter oiling. Poult. Sci. 1999, 78, 522–528. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.E.; McMurtry, J.P.; Walzem, R.L. Overfeeding-induced ovarian dysfunction in broiler breeder hens is associated with lipotoxicity. Poult Sci. 2006, 85, 70–81. [Google Scholar] [CrossRef]
- Pan, Y.E.; Liu, Z.C.; Chang, C.J.; Xie, Y.L.; Chen, C.Y.; Chen, C.F.; Walzem, R.L.; Chen, S.E. Ceramide accumulation and upregulation of proinflammatory interleukin-1β exemplify lipotoxicity to mediate declines of reproductive efficacy of broiler hens. Domest. Anim. Endocrinol. 2012, 42, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.L.; Pan, Y.E.; Chang, C.J.; Tang, P.C.; Huang, Y.F.; Walzem, R.L.; Chen, S.E. Palmitic acid in chicken granulosa cell death-lipotoxic mechanisms mediate reproductive inefficacy of broiler breeder hens. Theriogenology 2012, 78, 1917–1928. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.C.; Xie, Y.L.; Chang, C.J.; Su, C.M.; Chen, Y.H.; Huang, S.Y.; Walzem, R.L.; Chen, S.E. Feed intake alters immune cell functions and ovarian infiltration in broiler hens-implications for reproductive performance. Biol. Reprod. 2014, 90, 134. [Google Scholar] [CrossRef]
- Walzem, R.L.; Chen, S.E. Obesity-induced dysfunctions in female reproduction: Lessons from birds and mammals. Adv. Nutr. 2014, 5, 199–206. [Google Scholar] [CrossRef]
- Chen, C.Y.; Lin, H.Y.; Chen, Y.W.; Ko, Y.J.; Liu, Y.J.; Chen, Y.H.; Walzem, R.L.; Chen, S.E. Obesity-associated cardiac pathogenesis in broiler breeder hens: Pathological adaption of cardiac hypertrophy. Poult. Sci. 2017, 96, 2428–2437. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Huang, Y.F.; Ko, Y.J.; Liu, Y.J.; Chen, Y.H.; Walzem, R.L.; Chen, S.E. Obesity-associated cardiac pathogenesis in broiler breeder hens: Development of metabolic cardiomyopathy. Poult. Sci. 2017, 96, 2438–2446. [Google Scholar] [CrossRef]
- Chen, S.; Law, C.S.; Grigsby, C.L.; Olsen, K.; Hong, T.T.; Zhang, Y.; Yeghiazarians, Y.; Gardner, D.G. Cardiomyocyte-specific deletion of the vitamin D receptor gene results in cardiac hypertrophy. Circulation 2011, 124, 1838–1847. [Google Scholar] [CrossRef] [PubMed]
- Glenn, D.J.; Cardema, M.C.; Gardner, D.G. Amplification of lipotoxic cardiomyopathy in the VDR gene knockout mouse. J. Steroid Biochem. Mol. Biol. 2016, 164, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.W.; Lee, T.I.; Chang, C.J.; Lien, G.S.; Kao, Y.H.; Chao, T.F.; Chen, Y.J. Potential of vitamin D in treating diabetic cardiomyopathy. Nutr. Res. 2015, 35, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Qu, H.; Wang, H.; Ji, B.; Ding, Y.; Liu, D.; Duan, Y.; Liang, H.; Peng, C.; Xiao, X.; et al. 1,25-Dihydroxyvitamin-D3 prevents the development of diabetic cardiomyopathy in type 1 diabetic rats by enhancing autophagy via inhibiting the β-catenin/TCF4/GSK-3β/mTOR pathway. J. Steroid. Biochem. Mol. Biol. 2017, 168, 71–90. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.C.; Kong, J.; Wei, M.; Chen, Z.F.; Liu, S.Q.; Cao, L.P. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J. Clin. Investig. 2002, 110, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Brewer, L.C.; Michos, E.D.; Reis, J.P. Vitamin D in atherosclerosis, vascular disease, and endothelial function. Curr. Drug. Targets 2011, 12, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Kataoka, M.; Isobe, S.; Yamamoto, T.; Shirakawa, K.; Endo, J.; Satoh, T.; Hakamata, Y.; Kobayashi, E.; Sano, M.; et al. Therapeutic impact of dietary vitamin D supplementation for preventing right ventricular remodeling and improving survival in pulmonary hypertension. PLoS ONE 2017, 12, e0180615. [Google Scholar] [CrossRef] [PubMed]
- Pilz, S.; Tomaschitz, A.; Drechsler, C.; Dekker, J.M.; März, W. Vitamin D deficiency and myocardial diseases. Mol. Nutr. Food Res. 2010, 54, 1103–1113. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.Y.; Chung, T.K.; Chen, Y.H.; Walzem, R.L.; Chen, S.E. Dietary supplementation of 25-hydroxycholecalciferol improves livability in broiler breeder hens. Poult. Sci. 2019. [Google Scholar] [CrossRef] [PubMed]
- Julian, R.J. Physiological, management and environmental triggers of the ascites syndrome: A review. Avian Pathol. 2000, 29, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Olkowski, A.A.; Nain, C.; Wojnarowicz, C.; Laarveld, B.; Alcorn, J.; Ling, B.B. Comparative study of myocardial high energy phosphate substrate content in slow and fast growing chicken and in chickens with heart failure and ascites. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2007, 148, 230–238. [Google Scholar] [CrossRef] [PubMed]
- McDonald, H.; Peart, J.; Kurniawan, N.; Galloway, G.; Royce, S.; Samuel, C.S.; Chen, C. Hexarelin treatment preserves myocardial function and reduces cardiac fibrosis in a mouse model of acute myocardial infarction. Physiol. Rep. 2018, 6, e13699. [Google Scholar] [CrossRef] [PubMed]
- Maillet, M.; van Berlo, J.H.; Molkentin, J.D. Molecular basis of physiological heart growth: Fundamental concepts and new players. Nat. Rev. Mol. Cell Biol. 2013, 14, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Wideman, R.F.; Rhoads, D.D.; Erf, G.F.; Anthony, N.B. Pulmonary arterial hypertension (ascites syndrome) in broilers: A review. Poult. Sci. 2013, 92, 64–83. [Google Scholar] [CrossRef] [PubMed]
- Rance, K.A.; McEntee, G.M.; McDevitt, R.M. Genetic and phenotypic relationships between and within support and demand tissues in a single line of broiler chicken. Br. Poult. Sci. 2002, 43, 518–527. [Google Scholar] [CrossRef]
- Xue, W.; Cai, L.; Tan, Y.; Thistlethwaite, P.; Kang, Y.J.; Li, X.; Feng, W. Cardiac-specific overexpression of HIF-1α prevents deterioration of glycolytic pathway and cardiac remodeling in streptozotocin-induced diabetic mice. Am. J. Pathol. 2010, 177, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Ryan, Z.C.; Craig, T.A.; Folmes, C.D.; Wang, X.; Lanza, I.R.; Schaible, N.S.; Salisbury, J.L.; Nair, K.S.; Terzic, A.; Sieck, G.C.; et al. 1, α, 25-dihydroxyvitamin D3 regulates mitochondrial oxygen consumption and dynamics in human skeletal muscle cells. J. Biol. Chem. 2016, 291, 1514–1528. [Google Scholar] [CrossRef] [PubMed]
- Moller, S.; Bernardi, M. Interactions of the heart and the liver. Eur. Heart J. 2013, 34, 2804–2811. [Google Scholar] [CrossRef] [PubMed]
- Anstee, Q.M.; Mantovani, A.; Tilg, H.; Targher, G. Risk of cardiomyopathy and cardiac arrhythmias in patients with nonalcoholic fatty liver disease. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 425–439. [Google Scholar] [CrossRef] [PubMed]
- Bugianesi, E. Nonalcoholic fatty liver disease (NAFLD) and cardiac lipotoxicity: Another piece of the puzzle. Hepatology 2008, 47, 2–4. [Google Scholar] [CrossRef]
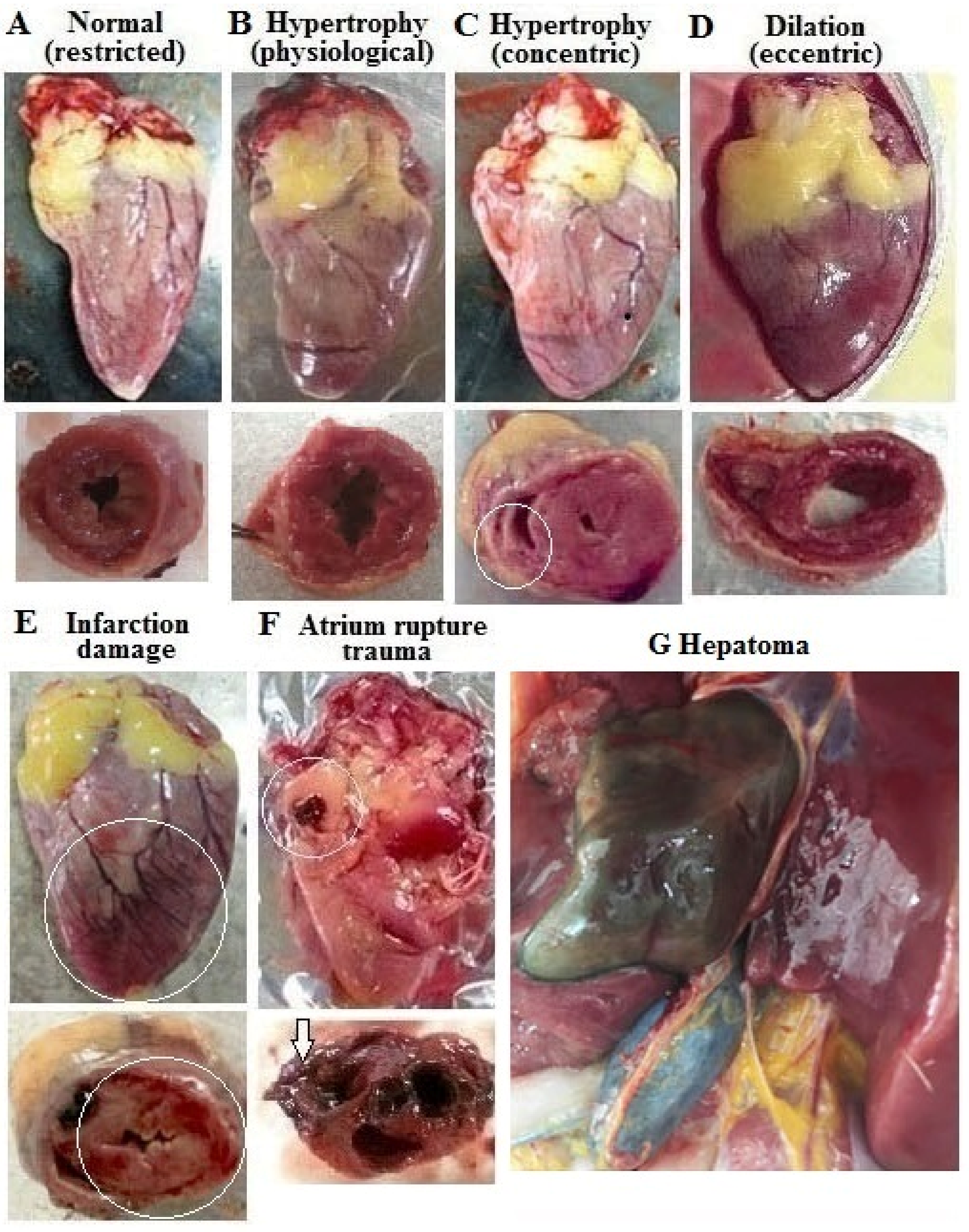
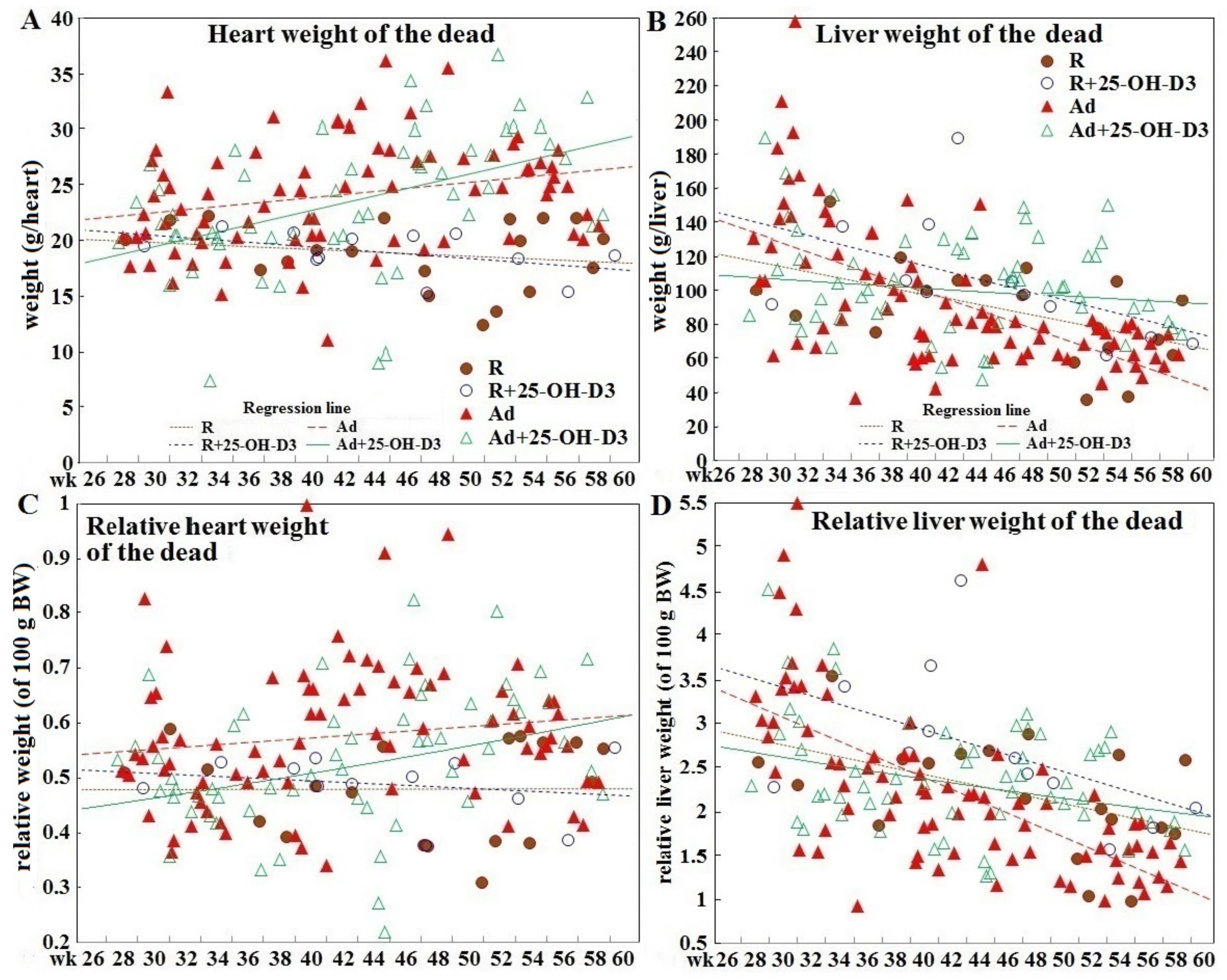

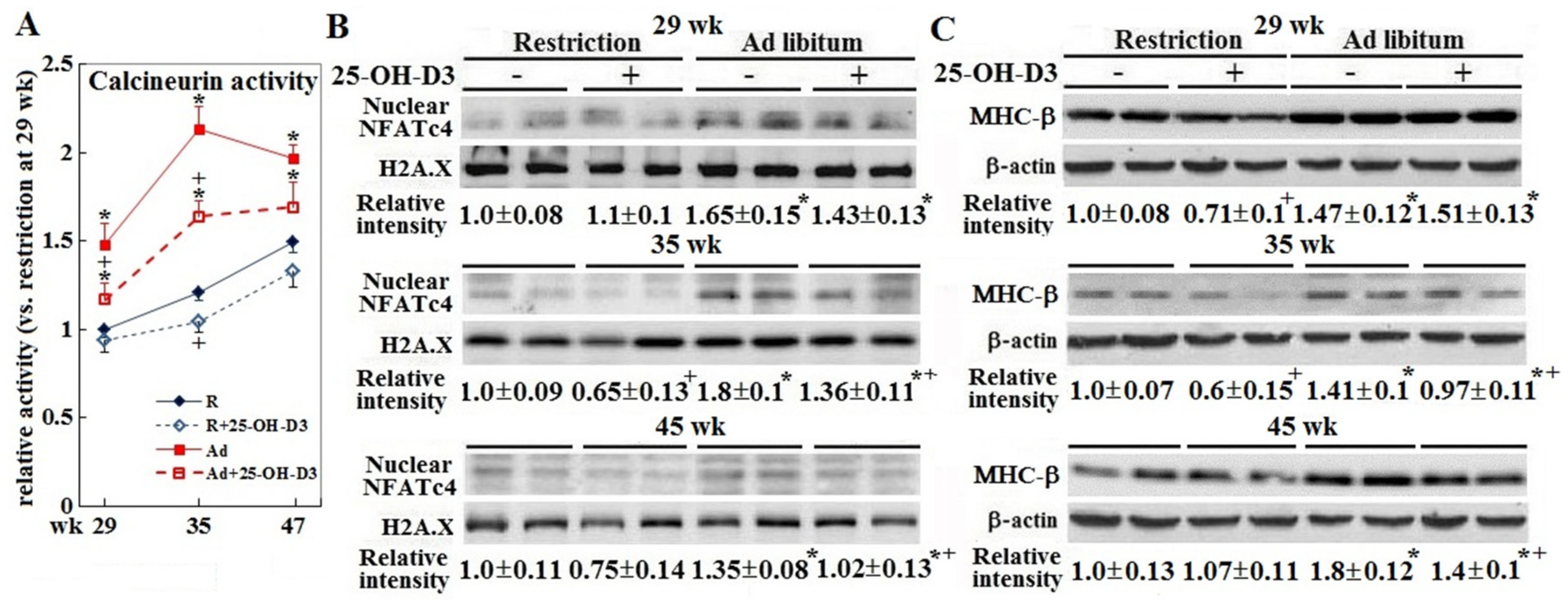
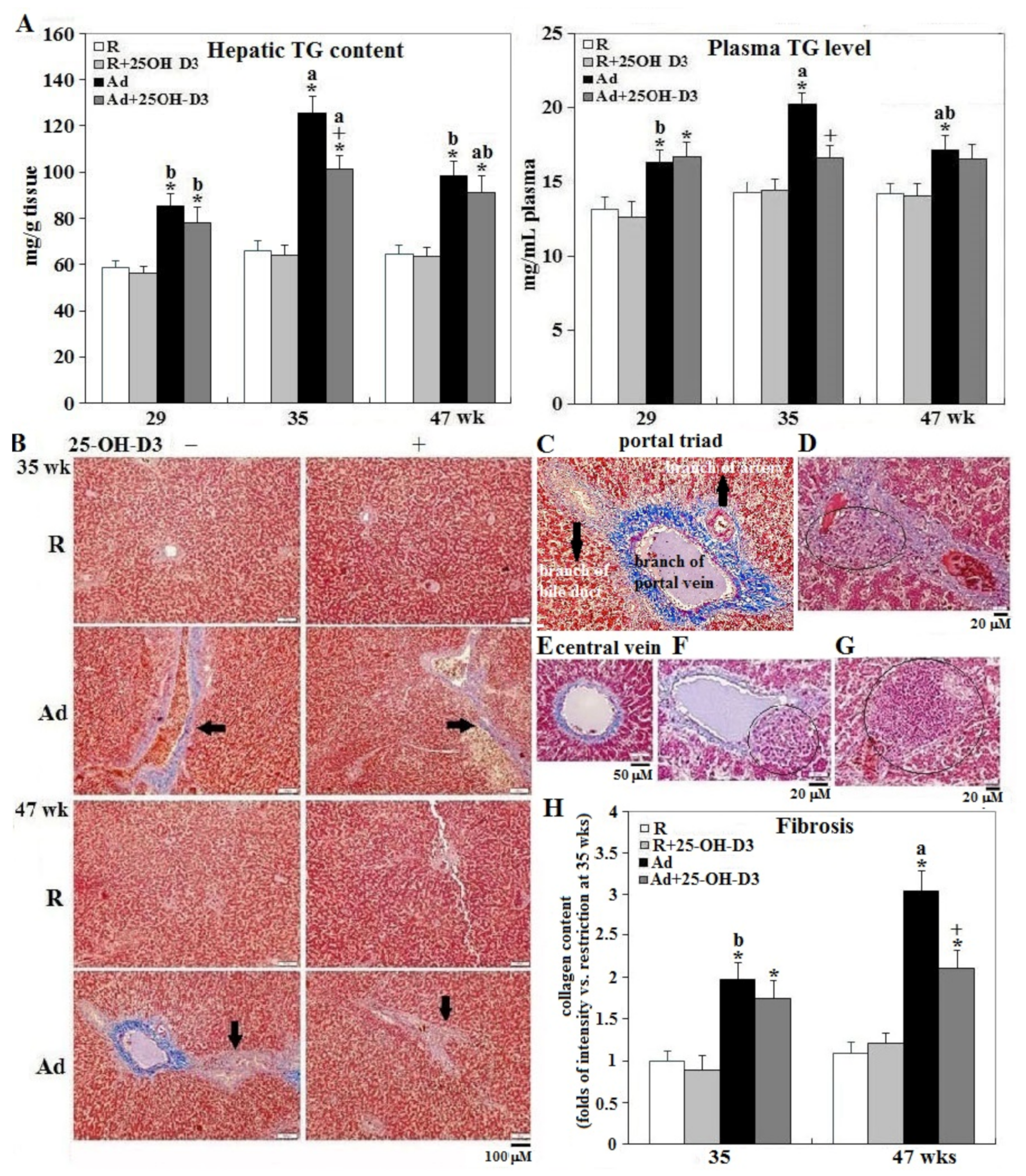

| Incidence | Restriction | Restriction +25-OH-D3 | Ad libitum | Ad libitum +25-OH-D3 |
|---|---|---|---|---|
| Sudden death mortalities 1,2 Gross cardiac pathologies 3 | 15/19 (78.9%) | 9/12 (75%) | 73/78 (93.6%) | 52/57 (91.2%) |
| Chi square (χ2)= 46.14, p < 0.0001 by Ad 2.34, p < 0.126 by 25-OH-D3 | ||||
| Hens sampled for tissue collection 1,2 Gross cardiac pathologies 3 | 4/18 (22.2%) | 4/18 (22.2%) | 14/18 (77.8%) | 10/18 (55.6%) |
| Chi square (χ2)= 18.94, p < 0.0001 by Ad 0.266, p < 0.606 by 25-OH-D3 | ||||
| Cardiac arrhythmia 4 | 3/18 (16.7%) | 4/18 (22.2%) | 9/18 (50%) | 8/18 (44.4%) |
| Chi square (χ2)= 6.25, p < 0.012 by Ad 0.0001, p < 0.999 by 25-OH-D3 | ||||
| Pooled R and R+25OH-D3 | Pooled Ad and Ad+25OH-D3 | |
|---|---|---|
| Relative liver vs. heart weight 1 | r = 0.39, p < 0.035 | r = 0.14, p < 0.16 |
| Liver vs. heart weight 1 | r = 0.46, p < 0.013 | r = 0.27, p < 0.008 |
| Normal | Pathological Hypertrophy | |
|---|---|---|
| Body weight (kg) | ||
| at age of 35 weeks | 3.54 ± 0.05 | 3.77 ± 0.05 * |
| at age of 47 weeks | 3.78 ± 0.03 | 3.86 ± 0.55 |
| Liver weight (g) | ||
| at age of 35 weeks | 57.1 ± 2.1 | 71.1 ± 3.1 * |
| at age of 47 weeks | 59.7 ± 1.2 | 71.2 ± 3.0 * |
| Relative liver weight (g/100g BW, %) | ||
| at age of 35 weeks | 1.61 ± 0.06 | 1.89 ± 0.06 * |
| at age of 47 weeks | 1.58 ± 0.03 | 1.84 ± 0.06 * |
| Heart weight (g) | ||
| at age of 35 weeks | 12.1 ± 0.18 | 13.7 ± 0.18 * |
| at age of 47 weeks | 13.6 ± 0.22 | 14.8 ± 0.18 * |
| Relative heart weight (g/100 g BW, %) | ||
| at age of 35 weeks | 0.34 ± 0.004 | 0.36 ± 0.008 |
| at age of 47 weeks | 0.36 ± 0.005 | 0.38 ± 0.005 * |
| Hepatic TG content (mg/g tissue) | ||
| at age of 35 weeks | 59.4 ± 2.0 | 79.1 ± 3.4 * |
| at age of 47 weeks | 59.6 ± 2.1 | 75.1 ± 3.0 * |
| Plasma TG level (mg/mL) | ||
| at age of 35 weeks | 13.3 ± 0.45 | 16.8 ± 0.63 * |
| at age of 47 weeks | 13.2 ± 0.40 | 16.2 ± 0.50 * |
| Incidence of arrhythmic ECG | ||
| at age of 35 weeks | 1/10 | 3/4 |
| at age of 47 weeks | 0/10 | 3/4 |
| pH value | ||
| at age of 35 weeks | 7.34 ± 0.011 | 7.28 ± 0.024 * |
| at age of 47 weeks | 7.32 ± 0.009 | 7.25 ± 0.016 * |
| pCO2 (mmHg) | ||
| at age of 35 weeks | 46.0 ± 1.26 | 53.1 ± 1.84 * |
| at age of 47 weeks | 49.0 ± 1.34 | 53.6 ± 2.85 |
| pO2 (mmHg) | ||
| at age of 35 weeks | 52.1 ± 1.48 | 46.8 ± 1.80 * |
| at age of 47 weeks | 50.5 ± 1.29 | 45.7 ± 2.58 |
| HbO2 saturation (%) | ||
| at age of 35 weeks | 77.7 ± 1.54 | 73.7 ± 1.87 * |
| at age of 47 weeks | 75.7 ± 1.36 | 72.8 ± 1.46 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, H.-Y.; Chou, P.-C.; Chen, Y.-H.; Lai, L.-S.; Chung, T.K.; Walzem, R.L.; Huang, S.-Y.; Chen, S.-E. Dietary Supplementation of 25-Hydroxycholecalciferol Improves Livability in Broiler Breeder Hens-Amelioration of Cardiac Pathogenesis and Hepatopathology. Animals 2019, 9, 770. https://doi.org/10.3390/ani9100770
Lin H-Y, Chou P-C, Chen Y-H, Lai L-S, Chung TK, Walzem RL, Huang S-Y, Chen S-E. Dietary Supplementation of 25-Hydroxycholecalciferol Improves Livability in Broiler Breeder Hens-Amelioration of Cardiac Pathogenesis and Hepatopathology. Animals. 2019; 9(10):770. https://doi.org/10.3390/ani9100770
Chicago/Turabian StyleLin, Hsuan-Yu, Pao-Chia Chou, Yu-Hui Chen, Lih-Shiuh Lai, Thau Kiong Chung, Rosemary L. Walzem, San-Yuan Huang, and Shuen-Ei Chen. 2019. "Dietary Supplementation of 25-Hydroxycholecalciferol Improves Livability in Broiler Breeder Hens-Amelioration of Cardiac Pathogenesis and Hepatopathology" Animals 9, no. 10: 770. https://doi.org/10.3390/ani9100770
APA StyleLin, H.-Y., Chou, P.-C., Chen, Y.-H., Lai, L.-S., Chung, T. K., Walzem, R. L., Huang, S.-Y., & Chen, S.-E. (2019). Dietary Supplementation of 25-Hydroxycholecalciferol Improves Livability in Broiler Breeder Hens-Amelioration of Cardiac Pathogenesis and Hepatopathology. Animals, 9(10), 770. https://doi.org/10.3390/ani9100770





