Thermotolerance of Broiler Chicks Ingesting Dietary Betaine and/or Creatine
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Location, Birds, and Experimental Procedure
2.2. Growth Performance Study
2.3. Slaughter Procedure and Carcass Traits Measurements
2.3.1. Meat Quality Measurements
2.3.2. Blood Serum Measurements
2.4. Heat Stress
2.5. Statistical Analysis
3. Results
3.1. Broiler Growth Performance
3.2. Breast Meat Quality and Color Values
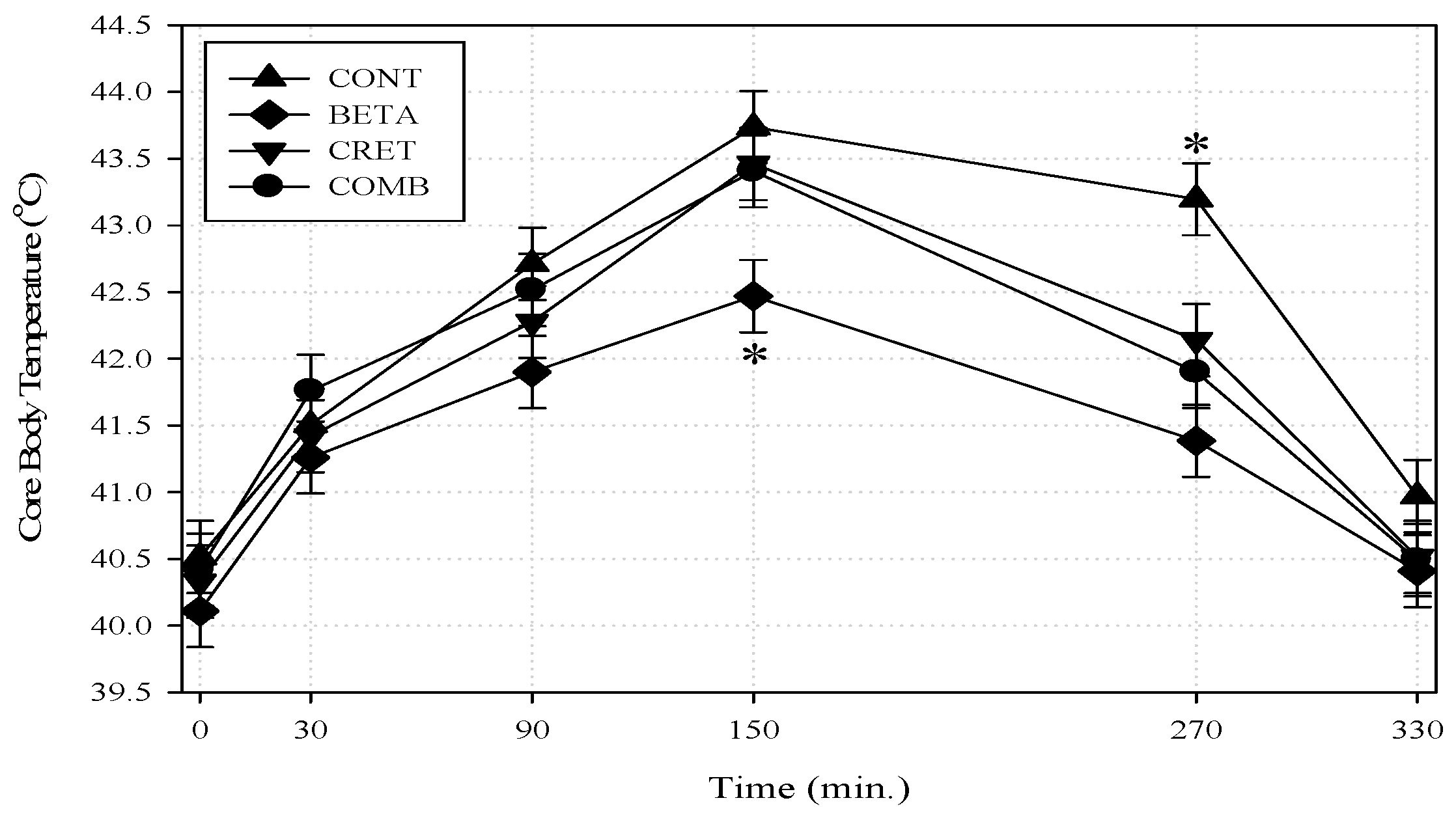
3.3. Thermoregulation and Hemogramic Profile
4. Discussion
4.1. Broiler Growth Performance
4.2. Breast Meat Quality and Color Values
4.3. Thermoregulation and Hemogramic Profile
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hossain, M.A.; Suvo, K.B.; Islam, M.M. Performance and economic suitability of three fast–growing broiler strains raised under farming condition in Bangladesh. Int. J. Agric. Res. Innov. Technol. 2011, 1, 37–43. [Google Scholar] [CrossRef]
- Al-Dawood, A.; Büscher, W. Air velocity produced by different types of mixing and ceiling fans to reduce heat stress in poultry houses. Int. J. Agric. For. 2014, 4, 145–153. [Google Scholar]
- Al-Dawood, A. Applications of acute phase proteins as biomarkers in poultry. Bull. Fac. Agric. Cairo Univ. 2016, 67, 193–212. [Google Scholar]
- Al-Ruwaili, M.; Herzallah, S.; Al-Dmoor, H.; Al-Atiyat, R. Effect of broiler commercial strains on total and free cholesterol levels of chicken muscle tissues. Glob. Vet. 2014, 12, 381–383. [Google Scholar]
- Husna, A.; Badruzzaman, A.T.M.; Runa, N.Y.; Yesmin, S.; Runa, N.S.; Rahman, M.A.; Mia, M.M. Evaluation of productive performance of selected broiler strains under field condition at Sylhet district of Bangladesh. Ann. Vet. Anim. Sci. 2017, 4, 104–109. [Google Scholar]
- Department of Statistics, Amman Agricultural Surveys, Jordan. Jordan Statistical Yearbook; Department of Statistics, Amman Agricultural Surveys, Jordan: Amman, Jordan, 2016; p. 282.
- Attia, Y.; Hassan, R.A.; Shehatta, M.H.; Abd El-Hady, S.B. Growth, carcass quality and serum constituents of slow growing chicks as affected by betaine addition to diets containing 2. Different levels of methionine. Int. J. Poult. Sci. 2005, 4, 856–865. [Google Scholar]
- Zhan, X.A.; Li, J.X.; Xu, Z.R.; Zhao, R.Q. Effects of methionine and betaine supplementation on growth performance, carcass composition and metabolism of lipids in male broilers. Br. Poult. Sci. 2006, 47, 576–580. [Google Scholar] [CrossRef]
- Michiels, J.; Maertens, L.; Buyse, J.; Lemme, A.; Rademacher, M.; Dierick, N.A.; de Smet, S. Supplementation of guanidinoacetic acid to broiler diets: Effects on performance, carcass characteristics, meat quality and energy metabolism. Poult. Sci. 2012, 91, 402–412. [Google Scholar] [CrossRef]
- Carvalho, C.M.C.; Fernandes, E.A.; de Carvalho, A.P.; Maciel, M.P.; Caires, R.M.; Fagundes, N.S. Effect of creatine addition in feeds containing animal meals on the performance and carcass yield of broilers. Rev. Bras. Ciência Avícola 2013, 15, 269–275. [Google Scholar] [CrossRef]
- Cholewa, J.M.; Guimaraes-Ferreira, L.; Zanchi, N.E. Effects of betaine on performance and body composition: A review of recent findings and potential mechanisms. Amino Acids 2014, 46, 1785–1793. [Google Scholar] [CrossRef]
- Hruby, M.; Ombabi, A.; Schlagheck, A. Natural betaine maintains layer performance in methionine/choline chloride reduced diets. In Proceedings of the 15th European Symposium on Poultry Nutrition, Balatonfüred, Hungary, 25–29 September 2005. [Google Scholar]
- Eklund, M.; Mosenthin, R.; Piepho, H.P. Effects of betaine and condensed molasses solubles on ileal and total tract nutrient digestibilities in piglets. Acta Agric. Scand Sect. A Anim. Sci. 2006, 56, 83–90. [Google Scholar] [CrossRef]
- Young, J.F.; Bertram, H.C.; Rosenvold, K.; Lindahl, G.; Oksbjerg, N. Dietary creatine monohydrate affects quality attributes of Duroc but not Landrace pork. Meat Sci. 2005, 70, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Hultman, E.; Soderlund, K.; Timmons, J.A.; Cederblad, G.; Greenhaff, P.L. Muscle creatine loading in men. J. Appl. Physiol. 1996, 81, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Lara, L.J.; Rostagno, M.H. Impact of heat stress on poultry production. Animals 2013, 3, 356–369. [Google Scholar] [CrossRef] [PubMed]
- Al-Dawood, A. Adoption of agricultural innovations: Investigating current status and barriers to adoption of heat stress management in small ruminants in Jordan. Am.-Eurasian J. Agric. Environ. Sci. 2015, 15, 388–398. [Google Scholar]
- Silanikove, N.; Koluman, N. Impact of climate change on the dairy industry in temperate zones: Predications on the overall negative impact and on the positive role of dairy goats in adaptation to earth warming. Small Rumin. Res. 2015, 123, 27–34. [Google Scholar] [CrossRef]
- Virden, W.S.; Kidd, M.T. Physiological stress in broilers: Ramifications on nutrient digestibility and responses. J. Appl. Poult. Res. 2009, 18, 338–347. [Google Scholar] [CrossRef]
- Rezaei, M.; Moghaddam, H.N.; Reza, J.P.; Kermanshahi, H. The effect of dietary protein and lysine levels on broiler performance and carcass characteristics and N excretion. Int. J. Poult. Sci. 2004, 3, 148–152. [Google Scholar]
- Hudspeth, J.; Lyon, C.E.; Lyon, B.G.; Mercuri, A.J. Weights of broiler parts as related to carcass weights and type of cut. J. Food Sci. 1973, 38, 145–150. [Google Scholar] [CrossRef]
- Abdullah, A.; Matarneh, S. Broiler performance and the effects of carcass weight, broiler sex and postchill carcass aging duration on breast fillet quality characteristics. J. Appl. Poult. Res. 2010, 19, 46–58. [Google Scholar] [CrossRef]
- Sams, A.; Janky, D. The influence of brine chilling on tenderness of hot-boned, chill-boned and age-boned broiler breast fillets. Poult. Sci. 1986, 65, 1316–1321. [Google Scholar] [CrossRef]
- Horcada, A.; Beriain, M.J.; Purroy, A.; Lizaso, G.; Chasco, J. Effect of sex on meat quality of Spanish lamb breeds (Lacha and Rasa Aragonesa). Anim. Sci. 1998, 67, 541–547. [Google Scholar] [CrossRef]
- SAS Institute Inc. SAS/STAT® 12.1 User’s Guide; SAS Institute Inc.: Cary, NC, USA, 2012. [Google Scholar]
- Littell, R.C.; Milliken, G.A.; Stroup, W.W.; Wolfinger, R.D.; Schabenberger, O. SAS for Mixed Models; SAS Institute: Cary, NC, USA, 2006. [Google Scholar]
- Esteve-Garcia, E.; Mack, S. The effect of DL-methionine and betaine on growth performance and carcass characteristics in broilers. Anim. Feed Sci. Technol. 2000, 87, 85–93. [Google Scholar] [CrossRef]
- Pillai, P.B.; Fanatico, A.C.; Beers, K.W.; Blair, M.E.; Emmert, J.L. Homocysteine remethylation in young broilers fed varying levels of methionine, choline and betaine. Poult. Sci. 2006, 85, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, J.L.; Gao, T.; Lin, M.; Wang, X.F.; Zhu, X.D.; Gao, F.; Zhou, G.H. Effects of dietary supplementation with creatine monohydrate during the finishing period on growth performance, carcass traits, meat quality and muscle glycolytic potential of broilers subjected to transport stress. Animals 2014, 8, 1955–1962. [Google Scholar] [CrossRef]
- Remus, J. Betaine for increased breast meat yield in turkeys. World Poult. 2001, 17, 14–15. [Google Scholar]
- Hassan, R.A.; Attia, Y.A.; El-Ganzory, E.H. Growth, carcass quality and serum constituents of slow growing chicks as affected by betaine addition to diets containing 1. Different levels of choline. Int. J. Poult. Sci. 2005, 4, 840–850. [Google Scholar]
- Eklund, M.; Bauer, E.; Wamatu, J.; Mosenthin, R. Potential nutritional and physiological functions of betaine in livestock. Nutr. Res. Rev. 2005, 18, 31–48. [Google Scholar] [CrossRef]
- Iraki, J.; Fitschen, P.; Espinar, S.; Helms, E. Nutrition recommendations for bodybuilders in the off-season: A narrative review. Sports 2019, 7, 154. [Google Scholar] [CrossRef] [PubMed]
- James, B.W.; Goodband, R.D.; Unruh, J.A.; Tokach, M.D.; Nelssen, J.L.; Dritz, S.S.; O’Quinn, P.R.; Andrews, B.S. Effect of creatine monohydrate on finishing pig growth performance, carcass characteristics and meat quality. Anim. Feed Sci. Technol. 2002, 96, 135–145. [Google Scholar] [CrossRef]
- Konca, Y.; Kirkpinar, F.; Mert, S.; Yaylak, E. Effects of betaine on performance, carcass, bone and blood characteristics of broilers during natural summer temperatures. J. Anim. Vet. Adv. 2008, 7, 930–937. [Google Scholar]
- Cromwell, G.L.; Lindemann, M.D.; Randolph, J.R.; Monegue, H.J.; Laurent, K.M.; Parker, G.R. Efficacy of betaine as a carcass modifier in finishing pigs fed normal and reduced energy diets. J. Anim. Sci. 1999, 77, 179. [Google Scholar]
- Matthews, J.O.; Southern, L.L.; Higbie, A.D.; Persica, M.A.; Bidner, T.D. Effects of betaine on growth, carcass characteristics, pork quality and plasma metabolites of finishing pigs. J. Anim. Sci. 2001, 79, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Enting, H.; Eissen, J.; de los Mozos, J.; del Álamo, A.G.; Ayala, P.P. TNI betaine improves broiler chicken performance and carcass quality under heat stress conditions. In Proceedings of the 16th European Symposium on Poultry Nutrition, Strasbourg, France, 26–30 August 2007. [Google Scholar]
- Hur, S.J.; Yang, H.S.; Park, B.; Joo, S.T. Effects of dietary glycine betaine on pork quality in different muscle types. Asian Aust. J. Anim. Sci. 2007, 20, 1754–1760. [Google Scholar] [CrossRef]
- St-Pierre, N.R.; Cobanov, B.; Schnitkey, G. Economic losses from heat stress by US livestock industries. J. Dairy Sci. 2003, 86, 52–77. [Google Scholar] [CrossRef]
- Quinteiro-Filho, W.M.; Ribeiro, A.; Ferraz-de-Paula, V.; Pinheiro, M.L.; Sakai, M.; Sá, L.R.; Ferreira, A.J.; Palermo-Neto, J. Heat stress impairs performance parameters, induces intestinal injury and decreases macrophage activity in broiler chickens. Poult. Sci. 2010, 89, 1905–1914. [Google Scholar] [CrossRef]
- Borges, S.A.; Fischer da Silva, A.V.; Majorka, A.; Hooge, D.M.; Cummings, K.R. Physiological responses of broiler chickens to heat stress and dietary electrolyte balance (sodium plus potassium minus chloride, milliequivalents per kilogram). Poult. Sci. 2004, 83, 1551–1558. [Google Scholar] [CrossRef]
- Aengwanich, W.; Suttajit, M. Effect of polyphenols extracted from Tamarind (Tamarindus indica L.) seed coat on physiological changes, heterophil/lymphocyte ratio, oxidative stress and body weight of broilers (Gallus domesticus) under chronic heat stress. Anim. Sci. J. 2010, 81, 264–270. [Google Scholar] [CrossRef]
| Ingredients (%) | Starter | Grower |
|---|---|---|
| Corn | 61.00 | 61.60 |
| Soybean Meal | 34.20 | 32.20 |
| Concentrate | 2.50 | 2.50 |
| Vegetable Oil | 0.80 | 2.20 |
| Limestone | 1.20 | 1.20 |
| Premixes | 0.10 | 0.10 |
| Antifungal | 0.20 | 0.20 |
| Total | 100.0 | 100.0 |
| Nutrients Composition (Calculated) | ||
| Metabolizable energy, kcal/kg | 3100 | 3150 |
| Crude Protein (%) | 22.50 | 21.50 |
| Lysine (%) | 1.46 | 1.28 |
| Methionine (%) | 0.52 | 0.49 |
| Calcium | 1.05 | 0.98 |
| Available Phosphorus | 0.51 | 0.46 |
| Item | Age (Week) | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| BW (g/week) | |||||
| CONT (B−/C−) | 173.40 | 469.00 | 909.80 | 1545.40 | 2195.80 |
| BETA (B+/C−) | 174.40 | 474.20 | 924.80 | 1512.20 | 2171.40 |
| CRET (B−/C+) | 176.30 | 490.40 | 934.60 | 1551.80 | 2228.00 |
| COMB (B+/C+) | 172.80 | 482.80 | 933.80 | 1544.00 | 2194.80 |
| SEM 3 | 3.87 | 8.03 | 16.70 | 20.08 | 28.13 |
| p-Value | |||||
| B | 0.75 | 0.80 | 0.6 | 0.07 | 0.32 |
| C | 0.87 | 0.08 | 0.33 | 0.65 | 0.34 |
| B × C | 0.57 | 0.44 | 0.64 | 0.18 | 0.88 |
| FCR (%) | |||||
| CONT (B−/C−) | 1.40 | 1.30a | 1.43 | 1.57 | 1.67 |
| BETA (B+/C−) | 1.37 | 1.27a | 1.41 | 1.58 | 1.68 |
| CRET (B−/C+) | 1.32 | 1.22b | 1.42 | 1.55 | 1.64 |
| COMB (B+/C+) | 1.40 | 1.22b | 1.43 | 1.55 | 1.64 |
| SEM 3 | 0.05 | 0.02 | 0.03 | 0.02 | 0.05 |
| p-Value | |||||
| B | 0.62 | 0.39 | 0.82 | 0.86 | 0.98 |
| C | 0.57 | <0.01 | 0.90 | 0.25 | 0.51 |
| B × C | 0.27 | 0.29 | 0.55 | 0.72 | 0.96 |
| FI (g/week) | |||||
| CONT (B−/C−) | 185.71 | 384.99 | 630.53 | 996.61 | 1084.56 |
| BETA (B+/C−) | 183.71 | 380.80 | 635.01 | 925.45 | 1102.00 |
| CRET (B−/C+) | 179.65 | 380.86 | 628.71 | 957.50 | 1108.50 |
| COMB (B+/C+) | 184.82 | 377.45 | 643.19 | 945.08 | 1057.78 |
| SEM 3 | 2.91 | 7.80 | 14.94 | 20.89 | 30.16 |
| p-Value | |||||
| B | 0.59 | 0.63 | 0.53 | 0.07 | 0.59 |
| C | 0.41 | 0.64 | 0.83 | 0.65 | 0.74 |
| B × C | 0.24 | 0.96 | 0.74 | 0.18 | 0.28 |
| BWG (g/week) | |||||
| CONT (B−/C−) | 133.40 | 295.60b | 440.80 | 635.60 | 650.40 |
| BETA (B+/C−) | 134.40 | 299.80b | 450.60 | 587.40 | 659.20 |
| CRET (B−/C+) | 136.30 | 314.10a | 444.20 | 617.20 | 676.20 |
| COMB (B+/C+) | 132.80 | 310.00a | 451.00 | 610.20 | 650.80 |
| SEM 3 | 3.87 | 5.99 | 12.12 | 14.50 | 23.84 |
| p-Value | |||||
| B | 0.75 | 0.99 | 0.50 | 0.08 | 0.73 |
| C | 0.87 | 0.03 | 0.8 | 0.88 | 0.72 |
| B × C | 0.57 | 0.50 | 0.90 | 0.17 | 0.48 |
| Variable 1 | Treatment Groups 2 | SEM 3 | p-Value | |||
|---|---|---|---|---|---|---|
| CONT | BETA | CRET | COMB | |||
| Carcass dressing (%) | 76.08 | 75.15 | 77.71 | 76.89 | 1.03 | 0.268 |
| pH | 6.03 | 6.03 | 6.03 | 6.08 | 0.02 | 0.267 |
| Cooking loss (%) | 26.02 | 26.11 | 26.11 | 27.97 | 0.95 | 0.415 |
| WHC (%) | 33.22 | 31.70 | 34.03 | 33.32 | 1.64 | 0.793 |
| SF (Kg/cm2) | 4.15 | 4.54 | 4.68 | 4.97 | 0.48 | 0.677 |
| Lightness | 46.37 | 45.86 | 45.67 | 47.17 | 6.0 | 0.330 |
| Redness | 31.57a | 26.74bc | 24.46c | 31.27ab | 1.6 | 0.011 |
| Yellowness | 19.36 | 19.33 | 19.65 | 19.38 | 2.4 | 0.805 |
| Parameter | Treatment Group 2 | Effect-Associated p-Value 4 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| CONT | BETA | CRET | COMB | SEM 3 | B | C | B × C | Time | |
| White blood cells (×103/µL) | NS | NS | NS | NS | |||||
| Pre–heat stress | 10.88 | 9.11 | 9.23 | 5.75 | 1.43 | ||||
| Post–heat stress | 6.69 | 9.73 | 8.67 | 7.20 | 1.27 | ||||
| Red blood cells (×106/µL) | NS | NS | NS | NS | |||||
| Pre–heat stress | 2.57 | 2.49 | 2.60 | 2.83 | 0.31 | ||||
| Post–heat stress | 2.34 | 2.91 | 2.41 | 2.75 | 0.22 | ||||
| Hemoglobin (g/dL) | NS | NS | NS | NS | |||||
| Pre–heat stress | 10.88 | 11.45 | 11.71 | 11.98 | 0.57 | ||||
| Post–heat stress | 11.39 | 10.27 | 10.73 | 10.38 | 0.50 | ||||
| Hematocrit (%) | NS | NS | NS | ** | |||||
| Pre–heat stress | 31.96 | 31.50 | 31.63 | 33.17 | 1.82 | ♦ | |||
| Post–heat stress | 32.30 | 28.00 | 28.63 | 28.67 | 1.61 | ||||
| (MCV; fL) | NS | NS | NS | NS | |||||
| Pre–heat stress | 127.92 | 125.18 | 123.91 | 121.90 | 12.72 | ||||
| Post–heat stress | 143.41 | 106.25 | 119.47 | 107.70 | 9.00 | ||||
| (MCH; pg) | NS | NS | NS | NS | |||||
| Pre–heat stress | 42.81 | 45.42 | 45.84 | 44.22 | 4.11 | ♦ | |||
| Post–heat stress | 50.51 | 38.94 | 44.98 | 39.27 | 3.63 | ||||
| (MCHC; g/dL) | NS | NS | NS | NS | |||||
| Pre–heat stress | 34.26 | 36.32 | 37.05 | 36.10 | 1.44 | ||||
| Post–heat stress | 35.43 | 36.79 | 37.58 | 36.45 | 1.28 | ||||
| Thrombocytes (×103/µL) | NS | NS | NS | NS | |||||
| Pre–heat stress | 26.04 | 24.36 | 21.40 | 22.67 | 4.62 | ||||
| Post–heat stress | 23.09 | 23.28 | 18.97 | 22.67 | 4.08 | ||||
| Heterocytes (H; %) | NS | * | NS | NS | |||||
| Pre–heat stress | 50.00 | 54.10 | 60.75 | 61.50 | 4.45 | ||||
| Post–heat stress | 50.00 | 51.60 | 52.18 | 51.00 | 3.93 | ||||
| Lymphocytes (L; %) | NS | * | NS | * | |||||
| Pre–heat stress | 42.56 | 38.00 | 32.50 | 33.17 | 4.63 | ||||
| Post–heat stress | 43.56 | 42.00 | 41.07 | 41.67 | 4.09 | ||||
| Monocytes (%) | NS | NS | NS | NS | |||||
| Pre–heat stress | 5.89 | 6.30 | 6.00 | 5.17 | 0.37 | ||||
| Post–heat stress | 5.89 | 5.80 | 6.00 | 6.67 | 0.38 | ||||
| H:L ratio | NS | * | NS | * | |||||
| Pre–heat stress | 1.23 | 1.59 | 2.03 | 1.90 | 0.36 | ||||
| Post–heat stress | 1.20 | 1.18 | 1.45 | 1.28 | 0.32 | ||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Tamimi, H.; Mahmoud, K.; Al-Dawood, A.; Nusairat, B.; Bani Khalaf, H. Thermotolerance of Broiler Chicks Ingesting Dietary Betaine and/or Creatine. Animals 2019, 9, 742. https://doi.org/10.3390/ani9100742
Al-Tamimi H, Mahmoud K, Al-Dawood A, Nusairat B, Bani Khalaf H. Thermotolerance of Broiler Chicks Ingesting Dietary Betaine and/or Creatine. Animals. 2019; 9(10):742. https://doi.org/10.3390/ani9100742
Chicago/Turabian StyleAl-Tamimi, Hosam, Kamel Mahmoud, Amani Al-Dawood, Basheer Nusairat, and Hussam Bani Khalaf. 2019. "Thermotolerance of Broiler Chicks Ingesting Dietary Betaine and/or Creatine" Animals 9, no. 10: 742. https://doi.org/10.3390/ani9100742
APA StyleAl-Tamimi, H., Mahmoud, K., Al-Dawood, A., Nusairat, B., & Bani Khalaf, H. (2019). Thermotolerance of Broiler Chicks Ingesting Dietary Betaine and/or Creatine. Animals, 9(10), 742. https://doi.org/10.3390/ani9100742





