Assessment of Commercially Available Immunoassays to Measure Glucocorticoid Metabolites in African Grey Parrot (Psittacus Erithacus) Droppings: A Ready Tool for Non-Invasive Monitoring of Stress
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Birds
2.2. Sample Collection
2.3. HLR Count
2.4. Methods Assessment and Selection
2.5. Analytical Validation of the Selected Procedure
2.6. Biological Assessment of the Procedure and Statistical Procedures
3. Results
3.1. Methods Assessment and Selection
3.1.1. Extraction Buffers Influence on EIA Key Parameters
3.1.2. Corticosterone Recovery in Extraction Buffers
3.1.3. Corticosterone “Virtual Recovery” in African Grey Parrots’ Droppings
3.1.4. Relative Accuracy of Each Methods Combination
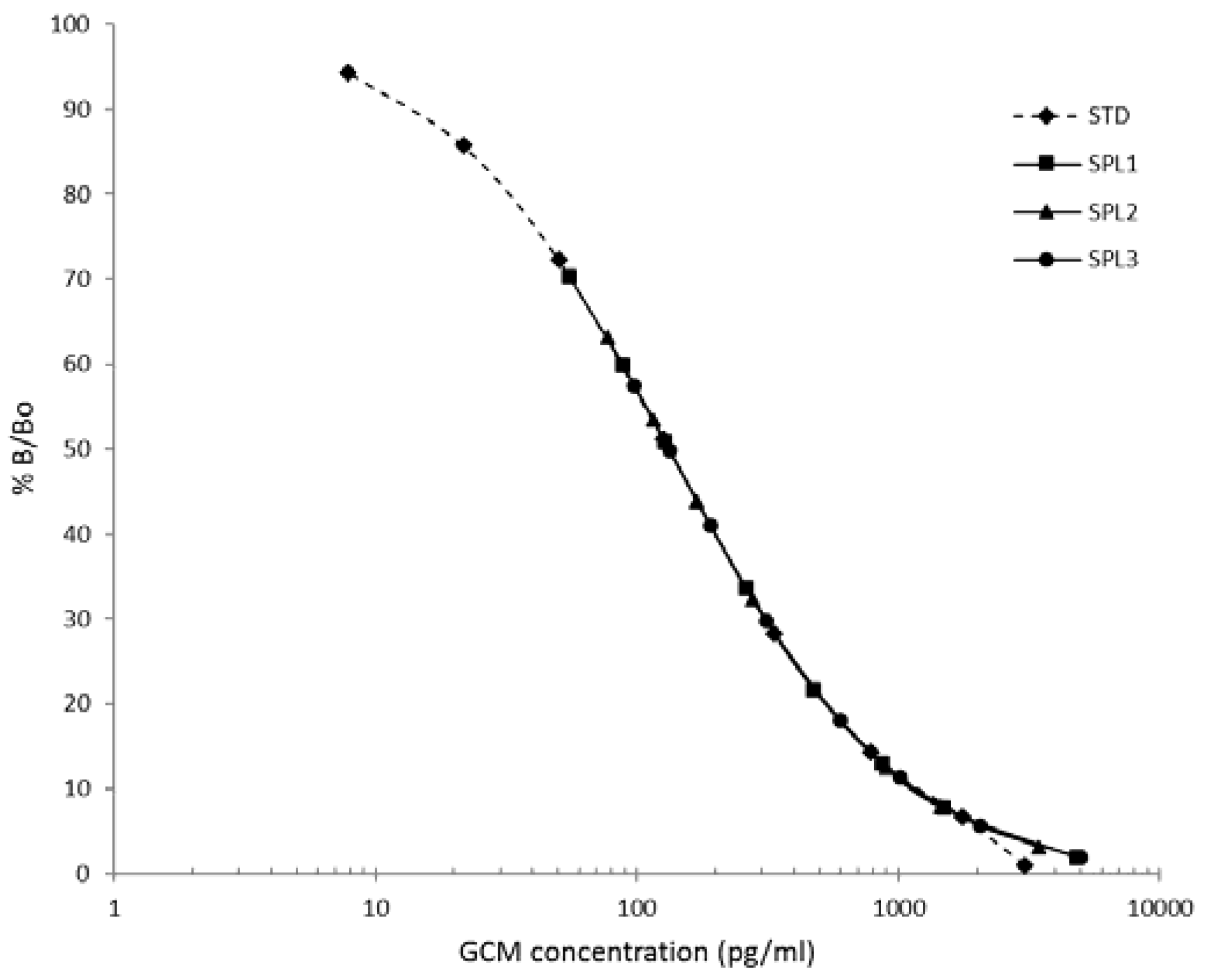
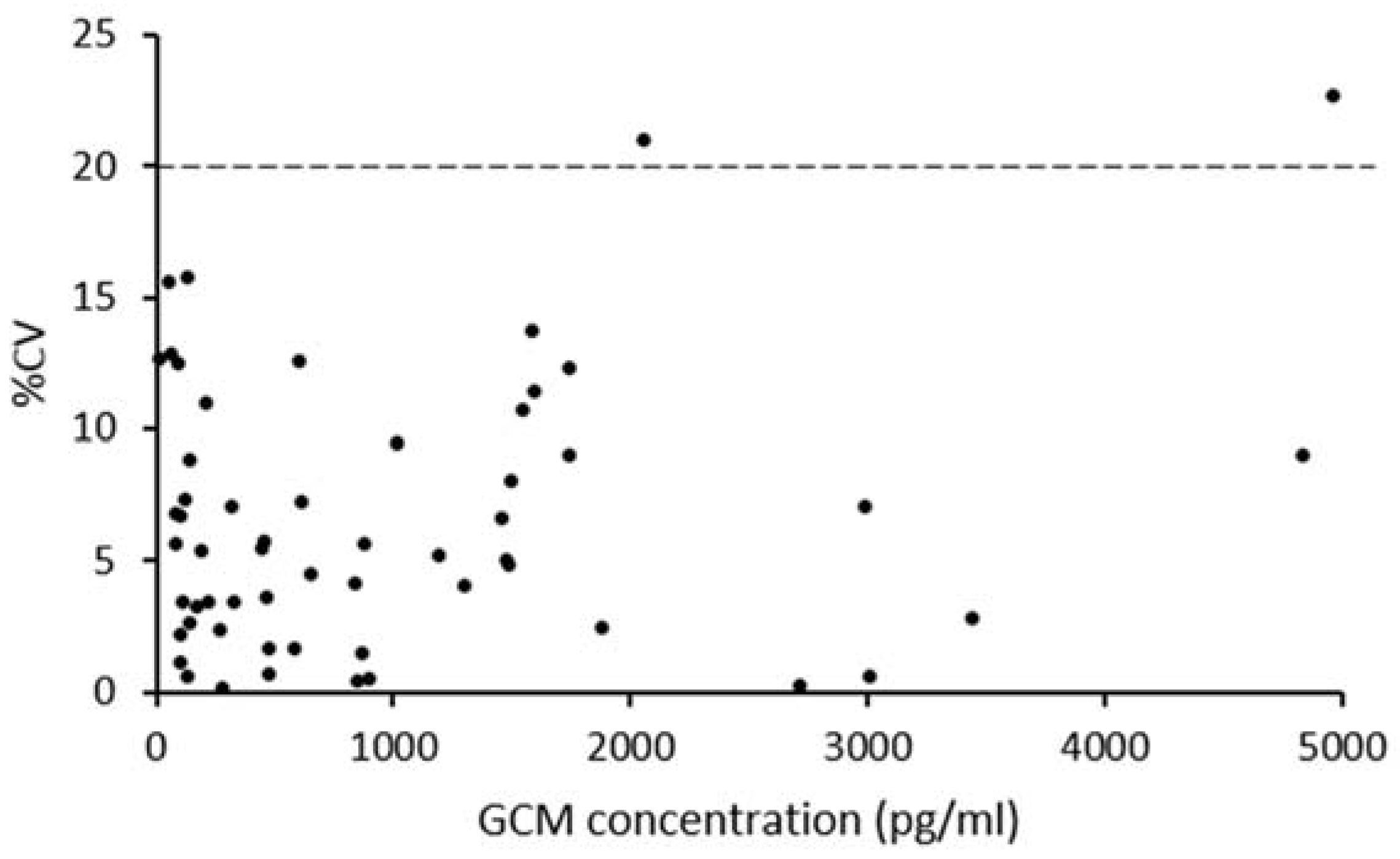
3.2. Procedure Selection and Analytical Validation
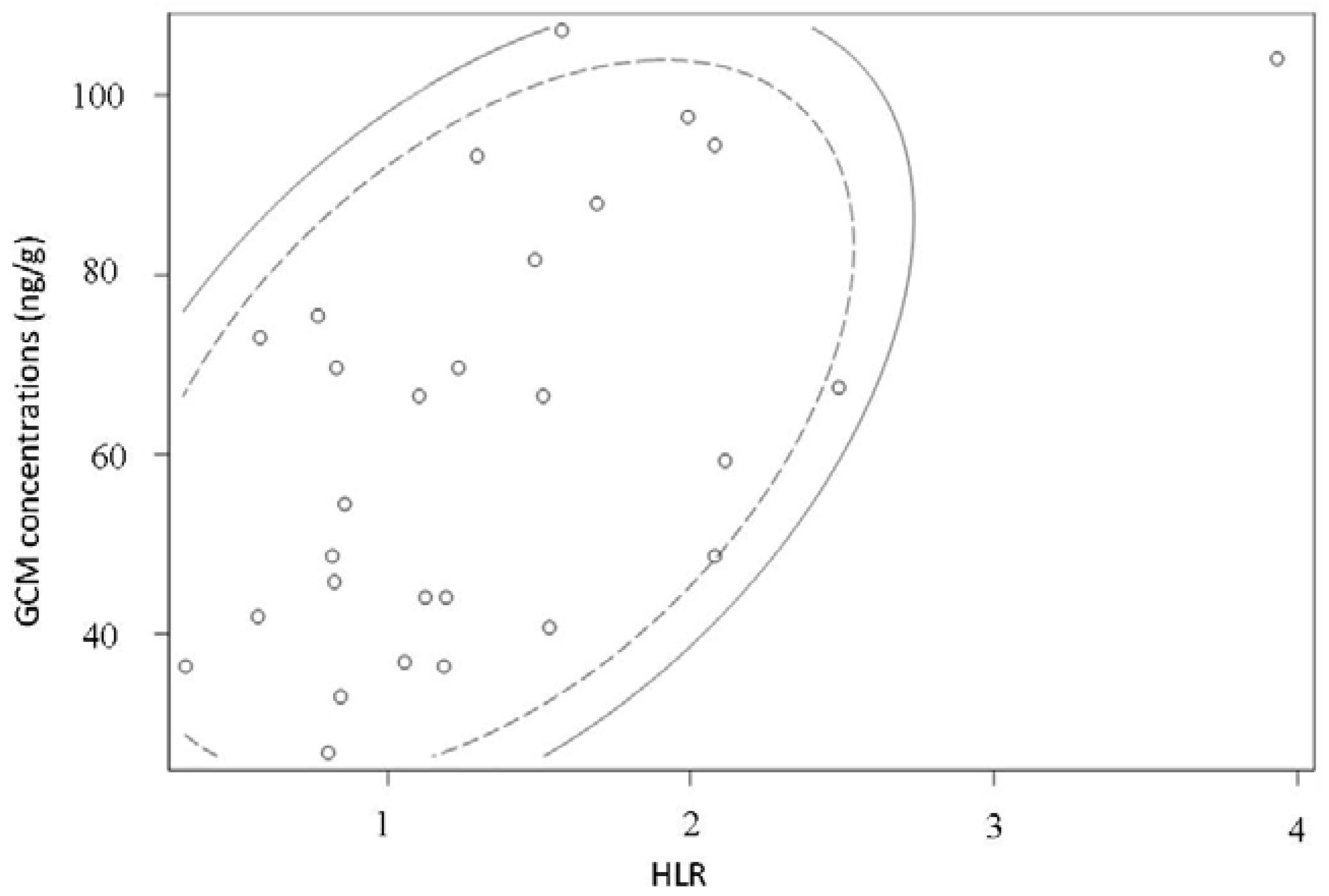
3.3. Biological Assessment of the Selected Method
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Forshaw, J.M. Parrots of the World; Princeton University Press: Princeton, NJ, USA, 2010; ISBN 1400836204. [Google Scholar]
- BirdLife International Psittacus Erithacus (Amended Version of 2016 Assessment). The IUCN Red List of Threatened Species 2017. Available online: http://dx.doi.org/10.2305/IUCN.UK.2017-1.RLTS.T22724813A111471911.en (accessed on 30 May 2018).
- Greenwell, P.J.; Montrose, V.T. The gray matter: Prevention and reduction of abnormal behavior in companion gray parrots (Psittacus erithacus). J. Vet. Behav. Clin. Appl. Res. 2017, 20, 44–51. [Google Scholar] [CrossRef]
- Pepperberg, I.M. The Alex Studies: Cognitive and Communicative Abilities of Grey Parrots; Harvard University Press: Cambridge, MA, USA, 1999. [Google Scholar]
- Pepperberg, I.M. Ordinality and inferential abilities of a grey parrot (Psittacus erithacus). J. Comp. Psychol. 2006, 120, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Engebretson, M. The welfare and suitability of parrots as companion animals: A review. Anim. Welf. 2006, 15, 263–276. [Google Scholar]
- Grant, R.A.; Montrose, V.T.; Wills, A.P. ExNOTic: Should we be keeping exotic pets? Animals 2017, 7, 47. [Google Scholar] [CrossRef] [PubMed]
- Fox, R. Hand-rearing: Behavioral Impacts and Implications for Captive Parrot Welfare. In Manual of Parrot Behavior; Luescher, A.U., Ed.; Blackwell Publishing: Hoboken, NJ, USA, 2006; pp. 83–91. [Google Scholar]
- Williams, I.; Hoppitt, W.; Grant, R. The effect of auditory enrichment, rearing method and social environment on the behavior of zoo-housed psittacines (Aves: Psittaciformes); implications for welfare. Appl. Anim. Behav. Sci. 2017, 186, 85–92. [Google Scholar] [CrossRef]
- Schmid, R.; Doherr, M.G.; Steiger, A. The influence of the breeding method on the behaviour of adult African grey parrots (Psittacus erithacus). Appl. Anim. Behav. Sci. 2006, 98, 293–307. [Google Scholar] [CrossRef]
- Anderson, P.K. A bird in the house: An anthropological perspective on companion parrots. Soc. Anim. 2003, 11, 393–418. [Google Scholar] [CrossRef]
- Rose, P.E.; Nash, S.M.; Riley, L.M. To pace or not to pace? A review of what abnormal repetitive behavior tells us about zoo animal management. J. Vet. Behav. Clin. Appl. Res. 2017, 20, 11–21. [Google Scholar] [CrossRef]
- Orosz, S.E.; Bradshaw, G.A. Avian Neuroanatomy Revisited: From Clinical Principles to Avian Cognition. Vet. Clin. N. Am. Exot. Anim. Pract. 2007, 10, 775–802. [Google Scholar] [CrossRef] [PubMed]
- Rosén, C.; Hansson, O.; Blennow, K.; Zetterberg, H. Fluid biomarkers in Alzheimer’s disease—Current concepts. Mol. Neurodegener. 2013, 8, 20. [Google Scholar] [CrossRef] [PubMed]
- Lightfoot, T.; Nacewicz, C.L. Psittacine behavior. In Exotic Pet Behavior: Birds, Reptiles and Small Mammals; Bradley-Bays, T., Lightfoot, T., Mayer, J., Eds.; Elsevier: New York, NY, USA, 2006; pp. 51–102. [Google Scholar]
- Garner, J.P.; Meehan, C.L.; Mench, J.A. Stereotypies in caged parrots, schizophrenia and autism: Evidence for a common mechanism. Behav. Brain Res. 2003, 145, 125–134. [Google Scholar] [CrossRef]
- Van Zeeland, Y.R.A.; Spruit, B.M.; Rodenburg, T.B.; Riedstra, B.; van Hierden, Y.M.; Buitenhuis, B.; Korte, S.M.; Lumeij, J.T. Feather damaging behaviour in parrots: A review with consideration of comparative aspects. Appl. Anim. Behav. Sci. 2009, 121, 75–95. [Google Scholar] [CrossRef]
- Jayson, S.L.; Williams, D.L.; Wood, J.L.N. Prevalence and risk factors of feather plucking in african grey parrots (psittacus erithacus erithacus and psittacus erithacus timneh) and cockatoos (Cacatua spp.). J. Exot. Pet Med. 2014, 23, 250–257. [Google Scholar] [CrossRef]
- Welle, K.R.; Wilson, L. Clinical Evaluation of Psittacine Behavioral Disorders. In Manual of Parrot Behavior; Luescher, A.U., Ed.; Blackwell Publishing: Hoboken, NJ, USA, 2006; pp. 175–193. [Google Scholar]
- Clubb, S.L.; Cray, C.; Arheart, K.L.; Goodman, M. Comparison of Selected Diagnostic Parameters in African Grey Parrots (Psittacus erithacus) with Normal Plumage and Those Exhibiting Feather Damaging Behavior. J. Avian Med. Surg. 2007, 21, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Smith, T. Zoo Research Guidelines—Monitoring Stress in Zoo Animals; The Federation of Zoological Gardens of Great Britain and Ireland: London, UK, 2004. [Google Scholar]
- Sheriff, M.J.; Dantzer, B.; Delehanty, B.; Palme, R.; Boonstra, R. Measuring stress in wildlife: Techniques for quantifying glucocorticoids. Oecologia 2011, 166, 869–887. [Google Scholar] [CrossRef] [PubMed]
- Shepherdson, D.; Carlstead, K.; Wielebnowski, N. Cross-institutional assessment of stress responses in zoo animals using longitudinal monitoring of faecal corticoids and behaviour. Anim. Welf. 2004, 13, S105–S114. [Google Scholar]
- Palme, R.; Rettenbacher, S.; Touma, C.; El-Bahr, S.M.; Möstl, E. Stress hormones in mammals and birds: Comparative aspects regarding metabolism, excretion, and noninvasive measurement in fecal samples. Ann. N. Y. Acad. Sci. 2005, 1040, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Wielebnowski, N.C.; Fletchall, N.; Carlstead, K.; Busso, J.M.; Brown, J.L. Noninvasive assessment of adrenal activity associated with husbandry and behavioral factors in the North American clouded leopard population. Zoo Biol. 2002, 21, 77–98. [Google Scholar] [CrossRef]
- Millspaugh, J.J.; Washburn, B.E. Use of fecal glucocorticoid metabolite measures in conservation biology research: Considerations for application and interpretation. Gen. Comp. Endocrinol. 2004, 138, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Hulsman, A.; Dalerum, F.; Ganswindt, A.; Muenscher, S.; Bertschinger, H.J.; Paris, M. Non-invasive monitoring of glucocorticoid metabolites in brown hyaena (Hyaena brunnea) feces. Zoo Biol. 2011, 30, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Stead, S.K.; Meltzer, D.G.; Palme, R. The measurement of glucocorticoid concentrations in the serum and faeces of captive African elephants (Loxodonta africana) after ACTH stimulation. J. S. Afr. Vet. Assoc. 2000, 71, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Dehnhard, M.; Schreer, A.; Krone, O.; Jewgenow, K.; Krause, M.; Grossmann, R. Measurement of plasma corticosterone and fecal glucocorticoid metabolites in the chicken (Gallus domesticus), the great cormorant (Phalacrocorax carbo), and the goshawk (Accipiter gentilis). Gen. Comp. Endocrinol. 2003, 131, 345–352. [Google Scholar] [CrossRef]
- Bortolotti, G.R.; Marchant, T.A.; Blas, J.; German, T. Corticosterone in feathers is a long-term, integrated measure of avian stress physiology. Funct. Ecol. 2008, 22, 494–500. [Google Scholar] [CrossRef]
- Fairhurst, G.D.; Frey, M.D.; Reichert, J.F.; Szelest, I.; Kelly, D.M.; Bortolotti, G.R. Does environmental enrichment reduce stress? An integrated measure of corticosterone from feathers provides a novel perspective. PLoS ONE 2011, 6, e17663. [Google Scholar] [CrossRef]
- Kouwenberg, A.L.; Mckay, D.W.; Fitzsimmons, M.G.; Storey, A.E. Measuring corticosterone in feathers using an acetonitrile/hexane extraction and enzyme immunoassay: Feather corticosterone levels of food-supplemented Atlantic Puffin chicks. J. Field Ornithol. 2015, 86, 73–83. [Google Scholar] [CrossRef]
- Will, A.P.; Suzuki, Y.; Elliott, K.H.; Hatch, S.A.; Watanuki, Y.; Kitaysky, A.S. Feather corticosterone reveals developmental stress in seabirds. J. Exp. Biol. 2014, 217, 2371–2376. [Google Scholar] [CrossRef] [PubMed]
- Bortolotti, G.R. Flaws and pitfalls in the chemical analysis of feathers: Bad news-good news for avian chemoecology and toxicology. Ecol. Appl. 2010, 20, 1766–1774. [Google Scholar] [CrossRef] [PubMed]
- McDonald, P.G.; Griffith, S.C. To pluck or not to pluck: The hidden ethical and scientific costs of relying on feathers as a primary source of DNA. J. Avian Biol. 2011, 42, 197–203. [Google Scholar] [CrossRef]
- Touma, C.; Palme, R. Measuring fecal glucocorticoid metabolites in mammals and birds: The importance of validation. Ann. N. Y. Acad. Sci. 2005, 1046, 54–74. [Google Scholar] [CrossRef] [PubMed]
- Goymann, W. On the use of non-invasive hormone research in uncontrolled, natural environments: The problem with sex, diet, metabolic rate and the individual. Methods Ecol. Evol. 2012, 3, 757–765. [Google Scholar] [CrossRef]
- Costa, P.; Macchi, E.; Valle, E.; De Marco, M.; Nucera, D.M.; Gasco, L.; Schiavone, A. An association between feather damaging behavior and corticosterone metabolite excretion in captive African grey parrots (Psittacus erithacus). PeerJ 2016, 4, e2462. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.K.; Maney, D.L.; Maerz, J.C. The use of leukocyte profiles to measure stress in vertebrates: A review for ecologists. Funct. Ecol. 2008, 22, 760–772. [Google Scholar] [CrossRef]
- Goessling, J.M.; Kennedy, H.; Mendonça, M.T.; Wilson, A.E. A meta-analysis of plasma corticosterone and heterophil: Lymphocyte ratios—Is there conservation of physiological stress responses over time? Funct. Ecol. 2015, 29, 1189–1196. [Google Scholar] [CrossRef]
- Palme, R. Measuring fecal steroids: Guidelines for practical application. Ann. N. Y. Acad. Sci. 2005, 1046, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Young, A.M.; Hallford, D.M. Validation of a fecal glucocorticoid metabolite assay to assess stress in the budgerigar (Melopsittacus undulatus). Zoo Biol. 2013, 32, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Campbell, T.; Ellis, C. Avian and Exotic Animal Haematology and Cytology, 3rd ed.; Blackwell Publishing: Hoboken, NJ, USA, 2007. [Google Scholar]
- Möstl, E.; Rettenbacher, S.; Palme, R. Measurement of corticosterone metabolites in birds’ droppings: An analytical approach. Ann. N. Y. Acad. Sci. 2005, 1046, 17–34. [Google Scholar] [CrossRef] [PubMed]
- Anfossi, L.; Ozella, L.; Di Nardo, F.; Giovannoli, C.; Passini, C.; Favaro, L.; Pessani, D.; Möstl, E.; Baggiani, C. A broad-selective enzyme immunoassay for non-invasive stress assessment in African penguins (Spheniscus demersus) held in captivity. Anal. Methods 2014, 6, 8222–8231. [Google Scholar] [CrossRef]
- Lobato, E.; Merino, S.; Moreno, J.; Morales, J.; Tomàs, G.; Martinez-de la Puente, J.; Osorno, J.L.; Kuchar, A.; Möstl, E. Corticosterone metabolites in blue tit and pied flycatcher droppings: Effects of brood size, ectoparasites and temperature. Horm. Behav. 2008, 53, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Palme, R.; Touma, C.; Arias, N.; Dominchin, M.F.; Lepschy, M. Steroid extraction: Get the best out of faecal samples. Wien Tierarztl Monatsschr 2013, 100, 238–246. [Google Scholar]
- Martin, P.; Bateson, P. Measuring Behaviour: An Introductory Guide, 3rd ed.; Cambridge University Press: Cambridge, UK, 2007; ISBN 9780521828680. [Google Scholar]
- Andreasson, U.; Perret-Liaudet, A.; van Waalwijk van Doorn, L.J.C.; Blennow, K.; Chiasserini, D.; Engelborghs, S.; Fladby, T.; Genc, S.; Kruse, N.; Kuiperij, H.B.; et al. A practical guide to immunoassay method validation. Front. Neurol. 2015, 6, 179. [Google Scholar] [CrossRef] [PubMed]
- Wasser, S.K.; Hunt, K.E.; Brown, J.L.; Cooper, K.; Crockett, C.M.; Bechert, U.; Millspaugh, J.J.; Larson, S.; Monfort, S.L. A generalized fecal glucocorticoid assay for use in a diverse array of nondomestic mammalian and avian species. Gen. Comp. Endocrinol. 2000, 120, 260–275. [Google Scholar] [CrossRef] [PubMed]
- Quillfeldt, P.; Möstl, E. Resource allocation in Wilson’s storm-petrels Oceanites oceanicus determined by measurement of glucocorticoid excretion. Acta Ethol. 2003, 5, 115–122. [Google Scholar] [CrossRef]
- Blickley, J.L.; Word, K.R.; Krakauer, A.H.; Phillips, J.L.; Sells, S.N.; Taff, C.C.; Wingfield, J.C.; Patricelli, G.L. Experimental Chronic Noise Is Related to Elevated Fecal Corticosteroid Metabolites in Lekking Male Greater Sage-Grouse (Centrocercus urophasianus). PLoS ONE 2012, 7, e50462. [Google Scholar] [CrossRef] [PubMed]
- Fanson, K.V.; Wielebnowski, N.C.; Shenk, T.M.; Lucas, J.R. Comparative patterns of adrenal activity in captive and wild Canada lynx (Lynx canadensis). J. Comp. Physiol. B 2012, 182, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Montiglio, P.-O.; Pelletier, F.; Palme, R.; Garant, D.; Réale, D.; Boonstra, R. Noninvasive monitoring of fecal cortisol metabolites in the eastern chipmunk (Tamias striatus): Validation and comparison of two enzyme immunoassays. Physiol. Biochem. Zool. 2012, 85, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Stöwe, M.; Bugnyar, T.; Schloegl, C.; Heinrich, B.; Kotrschal, K.; Möstl, E. Corticosterone excretion patterns and affiliative behavior over development in ravens (Corvus corax). Horm. Behav. 2008, 53, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Goymann, W. Noninvasive monitoring of hormones in bird droppings: Physiological validation, sampling, extraction, sex differences, and the influence of diet on hormone metabolite levels. Ann. N. Y. Acad. Sci. 2005, 1046, 35–53. [Google Scholar] [CrossRef] [PubMed]
- Ozella, L.; Anfossi, L.; Di Nardo, F.; Pessani, D. Non-invasive monitoring of adrenocortical activity in captive African Penguin (Spheniscus demersus) by measuring faecal glucocorticoid metabolites. Gen. Comp. Endocrinol. 2015, 224, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Popp, L.G.; Serafini, P.P.; Reghelin, A.L.S.; Spercoski, K.M.; Roper, J.J.; Morais, R.N. Annual pattern of fecal corticoid excretion in captive Red-tailed parrots (Amazona brasiliensis). J. Comp. Physiol. B 2008, 178, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Collette, J.C.; Millam, J.R.; Klasing, K.C.; Wakenell, P.S. Neonatal handling of Amazon parrots alters the stress response and immune function. Appl. Anim. Behav. Sci. 2000, 66, 335–349. [Google Scholar] [CrossRef]
- Ewenson, E.L.; Zann, R.A.; Flannery, G.R. Body condition and immune response in wild zebra finches: Effects of capture, confinement and captive-rearing. Naturwissenschaften 2001, 88, 391–394. [Google Scholar] [CrossRef] [PubMed]
- Moreno, J.; Merino, S.; MartÍnez, J.; Sanz, J.; Arriero, E. Heterophil/lymphocyte ratios and heat-shock protein levels are related to growth in nestling birds. Ecoscience 2002, 9, 434–439. [Google Scholar] [CrossRef]
- Vleck, C.; Vertalino, N.; Vleck, D.; Bucher, T. Stress, corticosterone, and heterophil to lymphocyte ratios in free-living Adélie Penguins. Condor 2000, 102, 392–400. [Google Scholar] [CrossRef]
- Masello, J.F.; Choconi, R.G.; Helmer, M.; Kremberg, T.; Lubjuhn, T.; Quillfeldt, P. Do leucocytes reflect condition in nestling burrowing parrots Cyanoliseus patagonus in the wild? Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2009, 152, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Alnot-Perronin, M.; Cozzi, A.; Lafont-Lecuelle, C.; Pageat, P. A physiological indicator of stress in African Grey parrots. In Proceedings of the 9th International Veterinary Behaviour Meeting; Mills, D.S., Da Graça Pereira, G., Jacinto, D., Eds.; PsiAnimal: Pontinha, Portugal, 2013; p. 161. [Google Scholar]
- Ferreira, J.C.P.; Fujihara, C.J.; Fruhvald, E.; Trevisol, E.; Destro, F.C.; Teixeira, C.R.; Pantoja, J.C.F.; Schmidt, E.M.S.; Palme, R. Non-invasive measurement of adrenocortical activity in Blue-fronted parrots (Amazona aestiva, Linnaeus, 1758). PLoS ONE 2015, 10, e145909. [Google Scholar] [CrossRef] [PubMed]
- Coppes, J.; Kämmerle, J.-L.; Willert, M.; Kohnen, A.; Palme, R.; Braunisch, V. The importance of individual heterogeneity for interpreting faecal glucocorticoid metabolite levels in wildlife studies. J. Appl. Ecol. 2018. [Google Scholar] [CrossRef]
- Koolhaas, J.M.; Korte, S.M.; De Boer, S.F.; Van Der Vegt, B.J.; Van Reenen, C.G.; Hopster, H.; De Jong, I.C.; Ruis, M.A.W.; Blokhuis, H.J. Coping styles in animals: Current status in behavior and stress-physiology. Neurosci. Biobehav. Rev. 1999, 23. [Google Scholar] [CrossRef]
- Staley, A.M.; Blanco, J.M.; Dufty, A.M.; Wildt, D.E.; Monfort, S.L. Fecal steroid monitoring for assessing gonadal and adrenal activity in the golden eagle and peregrine falcon. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 2007, 177, 609–622. [Google Scholar] [CrossRef] [PubMed]
- Thiel, D.; Jenni-Eiermann, S.; Braunisch, V.; Palme, R.; Jenni, L. Ski tourism affects habitat use and evokes a physiological stress response in capercaillie Tetrao urogallus: A new methodological approach. J. Appl. Ecol. 2008, 45, 845–853. [Google Scholar] [CrossRef]
- Dantzer, B.; Fletcher, Q.E.; Boonstra, R.; Sheriff, M.J. Themed Issue Article: Stress in Vertebrates Measures of physiological stress: A transparent or opaque window into the status, management and conservation of species? Conserv. Physiol. 2014, 2, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Dantzer, B.; McAdam, A.G.; Palme, R.; Boutin, S.; Boonstra, R. How does diet affect fecal steroid hormone metabolite concentrations? An experimental examination in red squirrels. Gen. Comp. Endocrinol. 2011, 174, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Tempel, D.J.; Guitierrez, R.J. Factors related to fecal corticosterone levels in California spotted owls: Implications for asssessing chronic stress. Conserv. Biol. 2004, 18, 538–547. [Google Scholar] [CrossRef]
| Kit Provider | Enzo Life Sciences | Cayman Chemical | ImmunoDiagnostic Systems |
|---|---|---|---|
| Antibody | Sheep polyclonal corticosterone antibody | Corticosterone specific sheep antiserum | Polyclonal Corticosterone antibody |
| Detection level | 26.99 pg/mL | 30 pg/mL | 0.55 ng/mL |
| Compounds and % of cross-reactivity | Deoxycorticosterone 28.6% Progesterone 1.7% Testosterone 0.13% Tetrahydrocorticosterone 0.28% Aldosterone 0.18% Cortisol 0.046% Pregnenolone < 0.03% Estradiol < 0.03% Cortisone < 0.03% 11-dehydrocorticosterone acetate < 0.03% | 11-Dehydrocorticosterone 11% Progesterone 7% Cortisol 0.31% Aldosterone 0.17% Testosterone 0.06% Pregnenolone 0.3% 5α-DHT 0.02% Androstenedione 0.1% Cortisone < 0.1% DHEA < 0.1% DHEA-S < 0.1% | 11-Dehydrocorticosterone 6.60% 11-Deoxycorticosterone 5.93% Progesterone 1.39% Cortiso l 0.85% Prednisolone 0.60% 21-Deoxycortisol 0.34% 5α-Pregnan-3, 20-dione 0.21% Tetrahydrocortisone < 0.07% Dexamethasone 0.07% DHEA < 0.07% Prednisone < 0.07% Pregnantriol < 0.07% 20β-Hydroxyprogesterone < 0.07% 4-Pregnen-20α-ol-3-one < 0.06% Oestriol < 0.06% Oestradiol < 0.06% Oestrone < 0.06% Pregnenolone < 0.06% 17α-Hydroxypregnolone < 0.05% Cortisone 0.05% Testosterone 0.02% 11-Desoxycortisol 0.02% Aldosterone 0.02% 17α-Hydroxyprogesterone 0.01% Tetrahydrocortisol 0.01% |
| Initial Sample (Mass in g) a | Sub-Sample Codes | Pre-Extraction Treatment | Dropping Mass (Fresh or Dry) before Extraction (g) | Extraction Buffer (Volume in mL) |
|---|---|---|---|---|
| S1 (13.78) | S1DE | Dry | 0.84 | 60% ethanol (8.4) |
| S1DM | 0.82 | 60% methanol (8.2) | ||
| S1FE | Fresh | 3.44 | 60% ethanol (34.4) | |
| S1FM | 3.44 | 60% methanol (34.4) | ||
| S2 (7.03) | S2DE | Dry | 0.45 | 60% ethanol (4.5) |
| S2DM | 0.43 | 60% methanol (4.3) | ||
| S2FE | Fresh | 1.74 | 60% ethanol (17.4) | |
| S2FM | 1.74 | 60% methanol (17.4) | ||
| S3 b (7.03) | S3DE | Dry | 0.42 | 60% ethanol (4.2) |
| S3DM | 0.41 | 60% methanol (4.1) | ||
| S3FE | Fresh | 1.74 | 60% ethanol (17.4) | |
| S3FM | 1.74 | 60% methanol (17.4) |
| Kit Provider | Enzo Life Sciences | Cayman Chemical | ImmunoDiagnostic Systems | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OD of normal a Bo | 0.880 | 1.391 | 1.149 | |||||||||
| OD of normal a Blk | 0.170 | 0.122 | 0.063 | |||||||||
| Extraction buffer | 60% ethanol | 60% methanol | 60% ethanol | 60% methanol | 60% ethanol | 60% methanol | ||||||
| Working dilution | 1:2 | 1:4 | 1:2 | 1:4 | 1:2 | 1:4 | 1:2 | 1:4 | 1:2 | 1:4 | 1:2 | 1:4 |
| OD of Bo measured in extraction buffer | 0.799 | 0.936 | 0.982 | 0.978 | 0.747 | 0.923 | 1.054 | 1.178 | 0.888 | 0.873 | 1.052 | 1.046 |
| OD of Blk measured in extraction buffer | 0.174 | 0.176 | 0.173 | 0.173 | 0.120 | 0.120 | 0.118 | 0.120 | 0.062 | 0.060 | 0.060 | 0.060 |
| EIA Kit | Extraction Buffer | Working Dilution | Expected a Corticosterone Concentration (ng/mL) | Measured Corticosterone Concentration (ng/mL) | Recovery (%) |
|---|---|---|---|---|---|
| ELS | 60% ethanol | 1:2 | 0.80 | 0.09 | 11 |
| 1:4 | 0.10 | 13 | |||
| 60% methanol | 1:2 | <0.03 | ND | ||
| 1:4 | 0.11 | 13 | |||
| CCC | 60% ethanol | 1:2 | 0.13 | 0.32 | 258 |
| 1:4 | 0.21 | 170 | |||
| 60% methanol | 1:2 | 0.13 | 101 | ||
| 1:4 | 0.11 | 88 | |||
| IDS | 60% ethanol | 1:2 | 16.40 | 8.21 | 50 |
| 1:4 | 14.71 | 89 | |||
| 60% methanol | 1:2 | 4.16 | 25 | ||
| 1:4 | 11.68 | 71 |
| EIA Kit | Sub-Sample Code (n = 2 or 3) | Working Dilution | Measured GCM Concentration in S2 (ng/g Droppings) | Expected GCM Concentration in S3 (ng/g Droppings) | Measured GCM Concentration in S3 (ng/g Droppings) | Recovery (%) |
|---|---|---|---|---|---|---|
| ELS | SnDE | 1:2 | 34.00 | 34.62 | 29.15 | 84 |
| 1:4 | 56.14 | 56.76 | 47.70 | 84 | ||
| SnFE | 1:2 | 30.48 | 31.10 | 29.79 | 96 | |
| 1:4 | 47.93 | 48.55 | 47.13 | 97 | ||
| SnDM | 1:2 | 47.17 | 47.79 | 58.73 | 123 a | |
| 1:4 | 79.53 | 80.15 | 99.59 | 124 a | ||
| SnFM | 1:2 | 29.02 | 29.64 | 25.65 | 87 | |
| 1:4 | 64.04 | 64.66 | 50.55 | 78 a | ||
| CCC | SnDE | 1:2 | ND | ND | 104.61 | ND |
| 1:4 | 76.67 | 77.29 | 64.9 | 84 | ||
| SnFE | 1:2 | 143.55 | 144.17 | 138.77 | 96 | |
| 1:4 | 99.14 | 99.76 | 97.69 | 98 | ||
| SnDM | 1:2 | 59.62 | 60.24 | 66.00 | 110 | |
| 1:4 | 52.25 | 52.87 | 65.12 | 123 a | ||
| SnFM | 1:2 | 66.59 | 67.21 | 76.56 | 114 | |
| 1:4 | 71.78 | 72.40 | 72.65 | 100 | ||
| IDS | SnDE | 1:2 | 582.23 | 582.85 | 488.73 | 84 |
| 1:4 | 485.71 | 486.33 | 485.30 | 100 | ||
| SnFE | 1:2 | 898.81 | 899.43 | 1063.46 | 118 | |
| 1:4 | 684.06 | 684.68 | 702.36 | 103 | ||
| SnDM | 1:2 | 368.91 | 369.53 | 408.95 | 111 | |
| 1:4 | 337.61 | 338.23 | 360.84 | 107 | ||
| SnFM | 1:2 | 454.38 | 455.00 | 440.72 | 97 | |
| 1:4 | 185.02 | 185.64 | 219.13 | 118 |
| EIA Kit | Sub-Sample Code (n = 1 or 2) | Working Dilution | Measured GCM Concentration in S1 (ng/g Droppings) | Measured GCM Concentration in S2 (ng/g Droppings) | Recovery (%) |
|---|---|---|---|---|---|
| ELS | SnDE | 1:2 | 34.76 | 34.00 | 102 |
| 1:4 | 52.83 | 56.14 | 94 | ||
| SnFE | 1:2 | 28.77 | 30.48 | 94 | |
| 1:4 | 52.41 | 47.93 | 109 | ||
| SnDM | 1:2 | 47.98 | 47.17 | 102 | |
| 1:4 | 79.71 | 79.53 | 100 | ||
| SnFM | 1:2 | 34.96 | 29.02 | 120 | |
| 1:4 | 65.15 | 64.04 | 102 | ||
| CCC | SnDE | 1:2 | ND | ND | ND |
| 1:4 | 67.76 | 76.67 | 88 | ||
| SnFE | 1:2 | 125.74 | 143.55 | 88 | |
| 1:4 | 90.66 | 99.14 | 91 | ||
| SnDM | 1:2 | 65.67 | 59.62 | 110 | |
| 1:4 | 57.09 | 52.25 | 109 | ||
| SnFM | 1:2 | 69.35 | 66.59 | 104 | |
| 1:4 | 70.56 | 71.78 | 98 | ||
| IDS | SnDE | 1:2 | 569.91 | 582.23 | 98 |
| 1:4 | 470.51 | 485.71 | 97 | ||
| SnFE | 1:2 | 1048.88 | 898.81 | 117 | |
| 1:4 | 601.18 | 684.06 | 88 | ||
| SnDM | 1:2 | 376.34 | 368.91 | 102 | |
| 1:4 | 334.55 | 337.61 | 99 | ||
| SnFM | 1:2 | 430.53 | 454.38 | 95 | |
| 1:4 | 170.15 | 185.02 | 92 |
| Parrot Code | Sex | HLR | Mass of Dry Droppings (g) | GCM Concentration (ng/g) |
|---|---|---|---|---|
| G1 | M | 0.99 | 0.22 | ND |
| G2 | M | 1.12 | 0.27 | 44.25 |
| G3 | M | 1.22 | NC | ND |
| G4 | M | 1.53 | 0.11 | 40.80 |
| G5 | M | 2.08 | 0.18 | 94.50 |
| G6 | M | 0.82 | 0.12 | 48.79 |
| G7 | M | 0.58 | 0.16 | 73.00 |
| G8 | F | 1.49 | 0.38 | 81.63 |
| G9 | M | 1.19 | 0.15 | 44.05 |
| G10 | M | 1.19 | 0.40 | 36.53 |
| G11 | F | 1.30 | 0.18 | 93.38 |
| G12 | F | 1.11 | 0.03 | 66.43 |
| G13 | M | 2.49 | 0.11 | 67.43 |
| G14 | F | 1.23 | 0.20 | 69.64 |
| G15 | M | 0.34 | 0.11 | 36.41 |
| G16 | M | 0.86 | 0.12 | 54.37 |
| G17 | M | 2.19 | 0.03 | ND |
| G18 | M | 1.51 | 0.06 | 66.43 |
| G19 | F | 1.06 | 0.33 | 36.78 |
| G20 | F | 2.03 | NC | ND |
| G21 | F | 0.85 | 0.19 | 33.08 |
| G22 | M | 3.93 | 0.06 | 104.21 |
| G23 | M | 0.90 | 0.22 | 104.29 |
| G24 | F | 2.08 | 0.26 | 48.67 |
| G25 | F | 0.81 | 0.24 | 26.73 |
| G26 | F | 0.83 | 0.14 | 45.91 |
| G27 | F | 1.58 | 0.07 | 107.27 |
| G28 | F | 0.83 | 0.19 | 69.58 |
| G29 | F | 1.69 | 0.14 | 88.09 |
| G30 | M | 0.57 | 0.14 | 41.93 |
| G31 | F | 1.99 | 0.09 | 97.64 |
| G32 | F | 2.12 | 0.29 | 59.19 |
| G33 | M | 0.77 | 0.15 | 75.33 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bienboire-Frosini, C.; Alnot-Perronin, M.; Chabaud, C.; Asproni, P.; Lafont-Lecuelle, C.; Cozzi, A.; Pageat, P. Assessment of Commercially Available Immunoassays to Measure Glucocorticoid Metabolites in African Grey Parrot (Psittacus Erithacus) Droppings: A Ready Tool for Non-Invasive Monitoring of Stress. Animals 2018, 8, 105. https://doi.org/10.3390/ani8070105
Bienboire-Frosini C, Alnot-Perronin M, Chabaud C, Asproni P, Lafont-Lecuelle C, Cozzi A, Pageat P. Assessment of Commercially Available Immunoassays to Measure Glucocorticoid Metabolites in African Grey Parrot (Psittacus Erithacus) Droppings: A Ready Tool for Non-Invasive Monitoring of Stress. Animals. 2018; 8(7):105. https://doi.org/10.3390/ani8070105
Chicago/Turabian StyleBienboire-Frosini, Cécile, Muriel Alnot-Perronin, Camille Chabaud, Pietro Asproni, Céline Lafont-Lecuelle, Alessandro Cozzi, and Patrick Pageat. 2018. "Assessment of Commercially Available Immunoassays to Measure Glucocorticoid Metabolites in African Grey Parrot (Psittacus Erithacus) Droppings: A Ready Tool for Non-Invasive Monitoring of Stress" Animals 8, no. 7: 105. https://doi.org/10.3390/ani8070105
APA StyleBienboire-Frosini, C., Alnot-Perronin, M., Chabaud, C., Asproni, P., Lafont-Lecuelle, C., Cozzi, A., & Pageat, P. (2018). Assessment of Commercially Available Immunoassays to Measure Glucocorticoid Metabolites in African Grey Parrot (Psittacus Erithacus) Droppings: A Ready Tool for Non-Invasive Monitoring of Stress. Animals, 8(7), 105. https://doi.org/10.3390/ani8070105






