1. Introduction
Emotions are defined as short-lived and intense affective reactions to specific events or stimuli important to the organism [
1]. They serve a crucial function, as they guide behavioral decisions in response to these triggering events or stimuli (e.g., approach or avoidance), including responses to social partners. They can be characterized by two key dimensions: valence (negative or positive; e.g., sad versus relaxed) and arousal (bodily activation or excitation; e.g., calm versus excited) [
2]. The arousal dimension can be regarded as the intensity of bipolar valence (“arousal as intensity” version [
3]). Negative emotions are part of the defensive motivational system and trigger avoidance behavior toward stimuli that threaten fitness, while positive emotions are part of the appetitive motivational system and trigger approach behavior toward stimuli that enhance fitness [
3,
4].
Although the existence of emotions in animals is now widely accepted [
4], ideal indicators (e.g., fast, reliable, and noninvasive) that could enable us to assess these affective states are still lacking. In particular, there is a clear need to discover indicators of valence, which could enable us to discriminate negative from positive situations of similar emotional arousal, in order to promote situations triggering positive emotions and enhance animal well-being (“positive welfare” [
5]). In many nonhuman mammals, vocalizations are assumed to be a direct expression of underlying emotions [
6], since nonhuman animals have relatively limited voluntary control over the structure of their vocalizations compared to humans. Acoustic expressions of emotion have been found across species [
7], even in those that can control vocal production, such as humans [
8,
9]. In most studied species, a change in emotional valence is associated with a change in call type (e.g., change from laughing to crying in humans, or from nickers to squeals in horses,
Equus caballus) [
7,
10]. The best studied example is the rat (
Rattus norvegicus), in which 22 kHz ultrasonic vocalizations (USVs) are produced in negative situations, while 50 kHz USVs are produced in positive situations [
11]. However, valence can also be communicated through acoustic variants of the same call type. For example, the range of the fundamental frequency (F0) of elephant (
Loxondota africana) rumbles diminishes in situations from negative to positive valence [
12], while the energy distribution of cat (
Felis catus) meows increases [
13]. In dog (
Canis familiaris) barks, the duration of calls decreases and F0 rises from negative to positive valence [
14]. This acoustic variation, which can occur between call types as well as within a given call type, might be perceived by conspecifics and modulate social interactions (retreat or approach [
15,
16]). Within- and/or between-call types of changes occurring with the valence experienced by the producer of the vocalizations might thus constitute ideal indicators of emotion, especially for wild species that cannot easily be approached.
Indicators of emotion might also have the advantage of being valid across species. This would allow us to use the same set of indicators to assess the emotions of any domestic or wild nonapproachable species, and to enable direct between-species comparisons of emotional reactions to certain stimuli. Vocal indicators of emotional valence and arousal that are shared between species, and particularly between closely related species, could occur if vocal expression of emotions, as suggested by Darwin [
17], has been conserved throughout evolution. There is now good evidence that this is the case for vocal expression of emotional arousal, suggesting that some vocal indicators of arousal could be shared across species [
7,
18]. However, whether this also applies to vocal indicators of valence remains unknown, since these indicators have been investigated in only a few species, and rarely in closely related species (but see [
19]). So far, it seems that across several species, calls associated with positive emotions tend to be shorter, with a lower and less variable fundamental frequency (F0), compared to those associated with negative emotions [
7,
20].
In this study, we investigated vocal indicators of emotional valence in wild boars, in order to compare those indicators to the ones found in other species and in closely related domestic pigs (
Sus scrofa domesticus, [
7,
21]), and to identify indicators that are shared across species. Wild boars live in large groups, including subadult males and adult females with their offspring, and are relatively vocal [
22]. According to some recent research, they produce four main types of calls: grunts, squeals, grunt-squeals, and trumpets [
23]. Yet, it is not known whether these call types can also vary with the emotion experienced by the producer, and whether the resulting indicators of emotion are similar to those found in domestic pigs [
21]. Wild boars are the principal genetic source of modern European domestic pig breeds, which were domesticated 9000 years ago, although there is evidence for a post-domestication gene flow from wild boars to pigs [
24]. The main genetic difference between the two species is that wild boars have 36 chromosomes, while domestic pigs have 38 [
25]. A comparison of vocal indicators of emotion between these two species could shed light on the impact of domestication on emotion expression, and highlight the potential of vocal indicators of valence to be applied across species [
19].
2. Materials and Methods
2.1. Studied Animals
The observations and recordings of 19 wild boars (12 females and 7 males) took place between February and April 2014 in 4 wildlife parks in Switzerland (Tierpark Dählhölzli Bern, Parc d’acceuil Pierre Challandes, Wildpark Bruderhaus, and Wildnispark Zürich Langenberg). All animals were housed in groups (6 groups of 2 to 7 animals). Their enclosures were 90–150 m2 and included a shelter. One group was kept in a larger enclosure, including a forest patch where they could forage in the soil ad libitum. All adult individuals had been in their group for at least 1 year. Fourteen individuals were categorized as adults (animals older than 3 years) and 5 were categorized as young (animals younger than 1 year). The animals were taken care of by park employees or volunteers. They were fed 2 times per day (at 8:30 in the morning and at 3:30 in the afternoon) with corn and various leftovers.
2.2. Observations and Recordings
We observed each group during 3 h each day, for as many days as there were individuals in the group (e.g., we observed a group of 3 animals for 3 days). Half of the observations were conducted from 7:00 a.m. to 10:00 a.m. (i.e., around the morning feeding time) and the other half from 2:00 p.m. to 5:00 p.m. (i.e., around the afternoon feeding time). The parks’ policies did not allow us to manipulate the animals. Therefore, recording of individual animals was performed opportunistically during presumed positive and negative expressions of emotions in naturally occurring situations [
12,
19,
26].
2.3. Emotional Valence of the Situations
Three different situations were observed: anticipation of a food reward (considered as positive), affiliative interactions (considered as positive), and agonistic interactions (considered as negative;
Table 1).
Since established behavioral indicators of emotion in wild boars are lacking, we used knowledge of the function of emotions and of wild boar behavior to assess the valence of the recording situations [
27,
28]. Encounters with rewarding stimuli that enhance fitness and trigger approach behavior toward the reward lead to positive emotions. In contrast, encounters with punishing stimuli that threaten fitness result in avoidance behavior and negative emotions [
4]. We thus attributed a positive valence to the anticipation for food and affiliative interactions (
Table 1). Conversely, we attributed a negative valence to agonistic interactions (
Table 1) [
4].
2.4. Emotional Arousal of the Situations
The emotional arousal that the animals were experiencing during vocal production was evaluated using body movements. This parameter was used as a control factor in our statistical model. Body movements have been revealed to be good indicators of arousal across species [
29], including domestic pigs [
21], and might also affect vocal parameters through changes in breathing patterns.
2.5. Data Collected
We recorded the calls from outside of the enclosures (between 5 and 45 m from the animals) with a directional microphone (Sennheiser MKH 70) connected to a digital recorder (Marantz PMD 661 MK II). We identified each animal using individual characteristics (e.g., body size, tail size, coat color, sex). Recordings were then uploaded to a computer at a sampling rate of 44.1 kHz and saved in WAV format at 16-bit amplitude resolution. All the acoustic analyses were performed using Praat v.5.3.61 DSP Package [
30]. Calls were visualized on spectrograms with the following settings: FFT method, window length = 0.01 s, time steps = 1000, frequency steps = 250, Gaussian window shape, dynamic range = 60 dB. Calls with high levels of background noise and/or saturation present (as visualized on the spectrogram) were not selected for acoustic analysis. In addition, the situations in which vocalizations were produced were filmed whenever possible (when the camera was oriented toward the individuals that were vocalizing), using a Canon Legria FS2000 camcorder.
2.6. Data Analysis
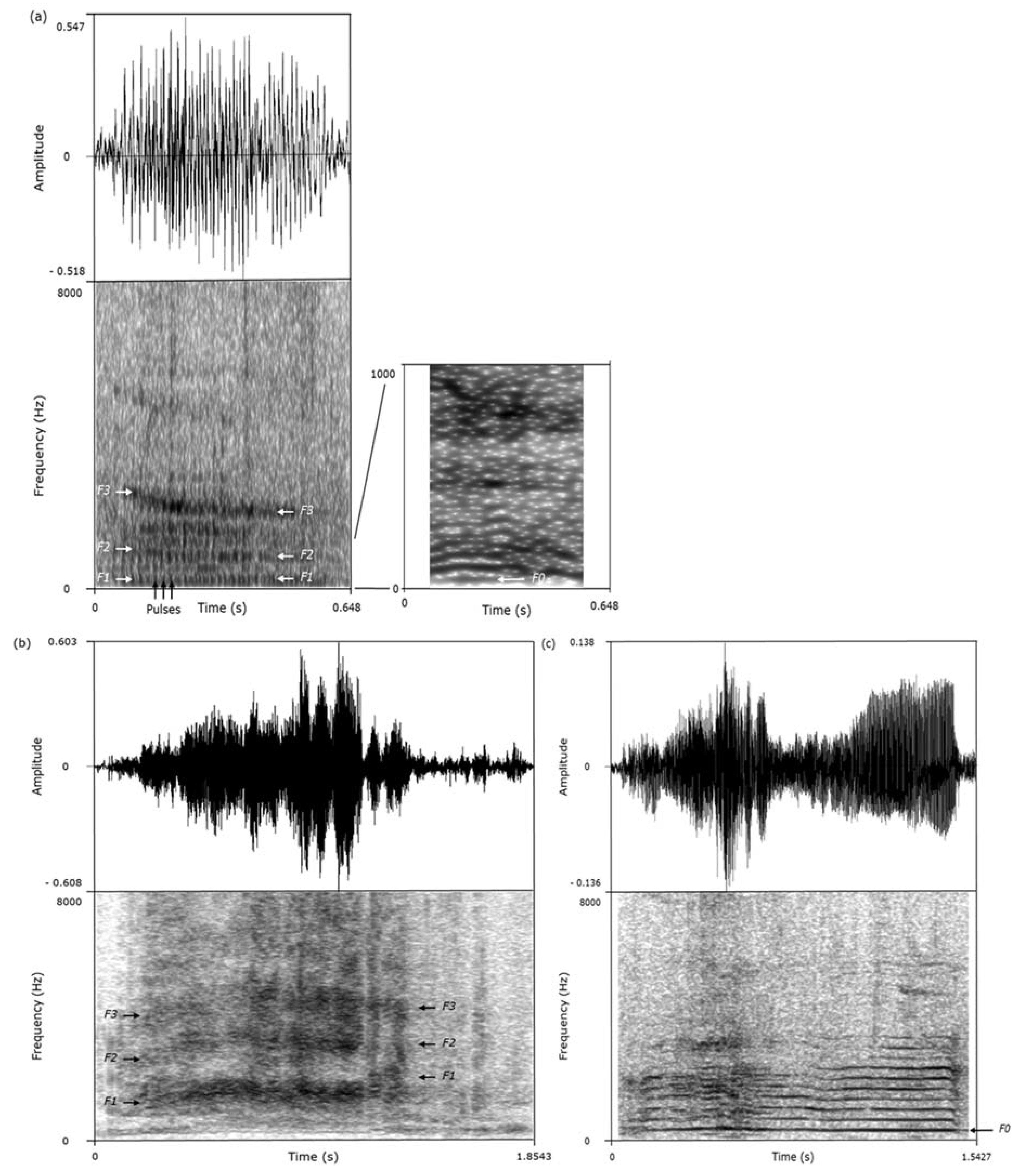
We used the acoustic features of the calls to classify them as grunts, screams, or squeals (
Figure 1,
Table 2) [
23,
31]. This classification was performed while blind to the valence of the situations in which the calls had been recorded. As calls produced consecutively are more likely to be homogeneous, only the calls separated by at least 10 s intervals and of sufficient quality were considered for analysis (
Table 3).
The vocal parameters were extracted using a custom-built program in Praat [
33], which batch-processed the analyses and the exporting of output data. To prevent biases related to the settings that we used for the analyses, the best settings to extract the vocal parameters of each individual were input in the script. Then, both negative and positive vocalizations of each animal were analyzed using the same settings. In total, we included 16 parameters in our analyses. The measured parameters are listed in
Table 2 (further details can be found in the
Supplementary Methods).
The videos of the recording situations were observed to score the body movements, using Interact software v. 9.0.7 (Mangold International GmbH, Arnstorf, Germany). Observations were made for 20 s, starting 10 s before each call. We then used the frequency of movement (proportion of time spent walking or running during these 20 s) as an indicator of emotional arousal for our analyses. The arousal scores were available for 154/256 calls, because it was not always possible to film every instance of vocal production.
2.7. Statistical Analysis
Linear mixed effects models (LMMs) were conducted in order to evaluate the effect of emotional valence on the vocal parameters. R (version 3.3.1, R Development Core Team, 2015) was used to perform the statistical analyses using the lmer function from the lme4 library [
34]. The vocal parameters were used as response variables (one model per parameter). The fixed factors were the sex (female or male), age (young or adult), and number of individuals in the group (2 to 7) in order to control for the effects of the parameters. The factors of interest were the type of call (grunt, scream, or squeal), the valence (positive or negative), and the interaction between these two parameters. Finally, the random structure of the models was as follows: the situation nested within the identity of the animals, itself nested within their group, crossed with the date of the observations. This allowed us to control for repeated measurements of the same individuals, and for differences between groups and days. We removed any nonsignificant interactions from the models [
35]. When an interaction was significant, we performed further post hoc tests by comparing the changes due to the emotional valence in each type of call separately using Tukey’s honest significant difference (HSD) test including the same control and fixed and random effects.
Because body movement (arousal score) was available for only 154/256 calls, we ran a second series of models on the reduced sample. To this aim, we used the same LMMs as for the first series of models described above, and added the body movements (proportion of time spent in movement, an indicator of arousal level) as an additional fixed factor.
The residuals of every model were checked graphically for normal distribution and homoscedasticity. We used a log transformation on
F0mean,
AMextent,
Q25,
Q50,
Q75,
Duration,
F0AbsSlope, and
F2range (see
Table 2 for abbreviations) and a square root transformation on
Harmonicity to satisfy these assumptions. The transformed parameters were then input into models fitted with a Gaussian family distribution and identity link function. We used the anova function of the lmerTest package in R to calculate
p-values based on Satterthwaite’s approximations, and all the models were fitted with restricted maximum likelihood (REML) estimation. Additionally, we used Chi-square tests to compare the call types’ distribution across valences and situations to a set of expected values. We set the significance level at α = 0.05.
We present the results as the residuals of the models carried out, after controlling for all fixed factors with the exception of the factor of interest (e.g., without the factor “valence” when testing the difference between negative and positive situations, which corresponds to the response variable after removing the variance related to the control factors; raw values are available in
Supplementary Tables S1–S3). All means are given with standard deviations (SDs).
2.8. Ethics
All observations of the animals were carried out in accordance with the “Guidelines for the treatment of animals in behavioural research and teaching” of the Association for the Study of Animal Behaviour (ASAB, 2012) and the current laws of Switzerland. The wildlife parks where the animals were studied are all open to the public, and the animals are well accustomed to the presence of surrounding visitors. This enabled us to be close enough to the animals to conduct observations from outside of the enclosures. Therefore, the animals were never manipulated during the experiment and all our data were collected by observation only.