Simple Summary
Given that goats are considered more climate resilient than other ruminant species, research efforts are therefore needed to understand goat productivity during exposure to high ambient temperatures. Heat stress can affect the digestion and rumen fermentation pattern of goats, which contributes to the reduction in production performance in goats. Diet composition, breed and environmental stresses are common factors which negatively influence rumen function and enteric methane (CH4) emission. There are three mechanisms by which enteric CH4 can be reduced: targeting end product of digestion to propionate, providing alternate hydrogen sink and selectively inactivating rumen methanogens. The various strategies that can be implemented to mitigate enteric CH4 include nutritional interventions, management strategies and application of advanced biotechnological tools.
Abstract
The ability of an animal to cope and adapt itself to the changing climate virtually depends on the function of rumen and rumen inhabitants such as bacteria, protozoa, fungi, virus and archaea. Elevated ambient temperature during the summer months can have a significant influence on the basic physiology of the rumen, thereby affecting the nutritional status of the animals. Rumen volatile fatty acid (VFA) production decreases under conditions of extreme heat. Growing recent evidence suggests there are genetic variations among breeds of goats in the impact of heat stress on rumen fermentation pattern and VFA production. Most of the effects of heat stress on rumen fermentation and enteric methane (CH4) emission are attributed to differences in the rumen microbial population. Heat stress-induced rumen function impairment is mainly associated with an increase in Streptococcus genus bacteria and with a decrease in the bacteria of Fibrobactor genus. Apart from its major role in global warming and greenhouse effect, enteric CH4 is also considered as a dietary energy loss in goats. These effects warrant mitigating against CH4 production to ensure optimum economic return from goat farming as well as to reduce the impact on global warming as CH4 is one of the more potent greenhouse gases (GHG). The various strategies that can be implemented to mitigate enteric CH4 emission include nutritional interventions, different management strategies and applying advanced biotechnological tools to find solution to reduce CH4 production. Through these advanced technologies, it is possible to identify genetically superior animals with less CH4 production per unit feed intake. These efforts can help the farming community to sustain goat production in the changing climate scenario.
1. Introduction
Morphologically versatile goat species with unique browsing potential adapt to a changing climate more readily than other ruminant species and consequently they continue to be an important source of income and nutrition to many poor and marginal farmers around the world [1]. Goats are also the major means of employment and income for women, children and aged people in tropical and subtropical regions [2]. The important sources of income from the sector include milk, meat, manure, wool and skin [3]. Small ruminant, and in particular goat, farming is very important because of the relatively low input requirements and the corresponding high expected output [4]. Furthermore, goats emit less enteric methane (CH4) than all other domestic ruminant animals per unit body weight [5].
A changing climate scenario for extensive grazing systems exposes the animals to various types of stressors that may affect their production, health and survival [6]. Among these, heat stress seems to be the major stressor which negatively influences the animal performance [7]. Furthermore, heat stress can also affect the digestion and rumen fermentation pattern of goats which contributes to the reduction in production performance [8]. The ability of an animal to cope and adapt itself to a changing climate depends on maintaining appropriate functioning of the rumen and ruminal microbes [9]. Elevated ambient temperature may prove detrimental to these processes and may ultimately result in influencing the level of CH4 production particularly with respect to the intensity of its production in goat and this will require appropriate mitigation strategies to curtail such emissions to sustain goat production in the changing climate scenario [8]. Given that goats are considered more climate resilient than other ruminant species, research efforts are therefore needed to understand goat productivity during exposure to high ambient temperatures. This review is therefore an attempt to collate and synthesize existing knowledge and recent research pertaining to the effects of heat stress on rumen fermentation, enteric CH4 emissions, and the various mechanisms associated with CH4 production and its mitigation in goats.
2. Goat as Ideal Climate Model Animal
Small ruminants, in particular goats, are considered an important source of income and nutrition for poor and marginal farmers around the world [5]. Low initial investment and high turnover rate for goat production are the primary reasons behind the promotion of the goat industry in developing countries [10]. Goats are often referred to as village banks in some rural areas where the villagers invest their money on purchasing and feeding goats and consider it as an appropriate way to save money for the future [11]. Globally, there are estimated to be over 860 million goats [12] and recent trends show an increased demand for dairy products from goats, particularly in developing countries where they act as a substitute for dairy products from large ruminants for human dietary needs [13].
Goats are versatile animals that adapt to a changing climate more readily than the other ruminant species and are well suited to small farming systems [1]. Much of the global goat population is concentrated in the arid and semi-arid agro-ecological zones that have frequent droughts and famines [14]. However, these species are reported to be less affected by the harsh climate compared with other ruminants that are highly sensitive to subtle changes in the surrounding environmental fluctuations [15]. Hence, goat rearing is a major source of human nutrition and also the means of economic stability for many small and marginal farmers, providing meat and manure as two major sources of income [14].
Because of their browsing habit and the anatomical advantage of the upper lips, goats can thrive well with limited feedstuffs, especially in arid and semi-arid regions [16]. In addition, goats also have a physiological advantage because they efficiently utilize poor quality feedstuffs and produce appreciably good output in terms of milk, meat and manure [17]. During feed scarcity, goats can reduce their metabolic processes to conserve energy resources [8]. Table 1 describes the advantageous characteristics in goats over other livestock species to survive harsh climatic conditions.

Table 1.
Advantageous characteristics associated with goats over other livestock species to survive in harsh climatic conditions.
3. Impact of Heat Stress on Rumen Function
Elevated ambient temperature during the summer months can have a significant influence on the basic physiology of rumen function, thereby affecting the nutritional status of the animals [23]. Rumen volatile fatty acid (VFA) production is altered during the conditions of extreme temperature, while feed digestibility is increased with increasing ambient temperature because of a reduction in feed intake and passage rate, which allows more time for the microbes and enzymes to digest feed [24]. Table 2 describes the various impacts of heat stress on rumen function.

Table 2.
Different impacts of heat stress on the rumen function in goats.
3.1. Rumen Fermentation Pattern
Environmental factors such as temperature and relative humidity (RH) can have significant role in the feed consumption of animals. An increase in temperature and RH decreases the dry matter intake of the animals and rumination as a result of increased amount of buffering agents entering the rumen and this could be attributed to the reduced chewing activity [7]. Additionally, blood flow is redirected from the gastrointestinal tract to the periphery for heat dissipation, which further decreases the digestibility [8]. Furthermore, an increased respiration rate during summer season increases expired CO2 output leading to decreased blood and rumen pH and acidosis [25]. Likewise, Castro-Costa et al. [26] reported a reduction in ruminal pH in heat exposed Murciano-Granadina dairy goats and attributed this to the reduced rumen fermentation during heat stress. Similarly, Yan-fen et al. [27] also reported a decreased rumen pH and NH3-N concentration in dairy goats exposed to heat stress.
3.2. Volatile Fatty Acid Production
There are reports showing a decrease in VFA production during the periods of heat stress [28,29]. Similarly, Tajima et al. [30] reported a decrease in acetate and acetate to propionate ratio and an increase in butyrate level in heat stressed animals, which they attributed to alterations in the number of rumen microbiota during the periods of heat stress. Likewise, Hirayama et al. [24] reported a reduction in plasma acetate and VFA concentrations in heat exposed (35 °C) Saanen goats compared to Saanen goats kept under thermoneutral conditions (20 °C). They attributed these changes to reduced feed intake and rumen microbial diversity. Further, in a study conducted in indigenous goat breeds, we [28] reported a reduced production of acetate concentrations in heat exposed Osmanabadi and Malabari goats, whereas the Salem goats did not exhibit any change. In the same experiment, we also observed an increase in propionate concentration in the Salem black goats and a decline in the propionate production in Malabari goats. These variations in the heat stress response could be explained by the differences in the adaptive capability among the breeds, suggesting Salem black as the superior adaptive breed in the climate change scenario. Further, Chaidanya et al. [29] reported a reduction in VFA concentrations in rumen of goats exposed to high ambient temperature coupled with high relative humidity. The reduction in the VFA concentration could be attributed to the increased rumen temperature during the heat stress periods.
3.3. Rumen Microbial Population
Heat stress induced rumen function impairment is mainly associated with an increase of Streptococcus genus bacteria and a decrease in the bacteria of Fibrobactor genus [31]. Further, Tajima et al. [30] also reported these changes along with altered rumen bacterial diversity with a decrease in uncultivated Cluster E group sequences during heat stress. Similarly, Uyeno et al. [32] observed a decrease in the Streptococcus genus and an increase in both Streptococcus spp. and Clostridium coccoides–Eubacterium genus in the rumen. Changes in the rumen microbial ecosystem due to heat exposure can influence feed digestibility and composition of the end products by altering the rumen fermentation pattern [32]. Further, Bernabucci et al. [9] observed a decline in the concentrations of amylolytic and cellulolytic bacteria in animals exposed to ambient conditions having a temperature humidity index (THI) 85. The decreased dry matter intake and passage rate in heat stressed animals could reduce the bacterial diversity ultimately culminating in decreased diet digestibility [9]. There are few research reports available on how high ambient temperature selectively affects microbial population. However, this impact could be attributed to the sensitivity of certain rumen microbes to increased temperature exposure.
3.4. Enteric Methane Emission
Environmental temperature is a key factor that determines CH4 production, since feed intake and digestibility differ with ambient temperature. Mbanzamihigo et al. [33] reported an increase in enteric CH4 emissions during late summer (August–September) compared to early summer (June–July) in the Northern Hemisphere. Similarly, in another experiment conducted in young wethers grazing a moist hilly island pasture, a perennial rye grass/white clover dominant pasture and a late summer season pasture showed CH4 yields of 4.1%, 3.9% and 5.3%, respectively. Increased CH4 yield in wethers grazing late summer season pastures is attributed to the quality deterioration (poor dry matter digestibility, lower protein and soluble carbohydrate content and increased cell wall content) of the pastures during the summer season [34]. This study revealed the indirect effect of elevated ambient temperature on the CH4 production through altered pasture characteristics. Further, Ulyatt et al. [35] reported an increase in CH4 emission during grazing of summer grassland compared to Kikuyu grassland. Figure 1 shows the impact of heat stress on various rumen functions in goat.
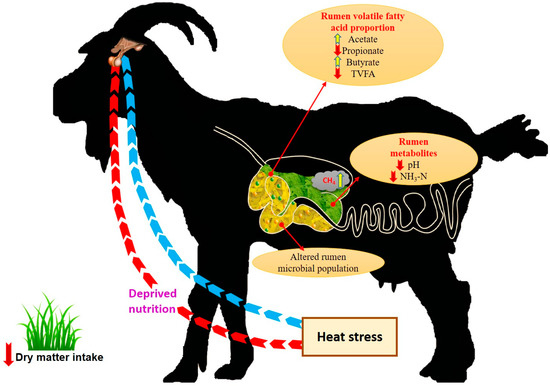
Figure 1.
Impact of heat stress on various rumen functions in goat (these concepts were adopted from References [28,29]). TVFA: Total Volatile Fatty Acid.
3.5. Factors Influencing Enteric Methane Emission in Goats
Various factors affect the enteric methane production in goats and these are broadly classified as weather associated factors such season and increased ambient temperature; feed associated factors such as diet composition, time after feeding, and feed additives; and animal associated factors that include inflow of saliva, types of microbial population, and breed [36].
Composition of feed is the primary factor that determines the rumen fermentation pattern and enteric methane emissions [37]. Further, the propionate to acetate ratio also influences the rumen fermentation pattern and is determined by the concentrate to forage content of the diets [38]. In comparison with roughage feed, concentrates contain less structural carbohydrates, so the intake of concentrates may increase the production of propionate and decrease the production of acetate, ultimately resulting in reduced CH4 production. An increase in concentrate intake is associated with increased propionate production and this may reduce the number of H2 atoms available to the methanogenic bacteria, again resulting in reduced methane production. However, the higher level of concentrate feeding can cause sub-acute acidosis, both sub-clinical and clinical, which may adversely impact normal ruminal fermentation processes through both alteration of the functions of essential rumen microbes and impaired VFA absorption due to low ruminal pH [39].
In recent years, the usage of microbial feed additives has increased to improve growth performance of meat animals. In addition, some microbial feed additives have been used to reduce CH4 production in ruminant animals. Malik et al. [40] used acetogens as a feed additive to replace prominent CH4-producing methanogenic bacteria to reduce enteric methane production by acting as alternate hydrogen. The prominent CH4-producing methanogenic bacteria have a low H2 threshold level, thus do not allow the naturally resident acetogens to utilize hydrogen. Other feed additives such as fat and oil supplements have also been reported to have an effect on the rumen fermentation profile, thereby reducing rumen protozoan population and CH4 reduction [41]. However, high fat diets can alter the rumen microbial population and ultimately it can hamper the fibre digestibility by specifically inactivating the rumen microbes that are associated with fibre digestion [41]. Plant bioactives, including saponins and tannins, can reduce CH4 production in ruminants [42].
Breed is another important factor that determines enteric CH4 production [43]. These breed-to-breed differences in enteric CH4 production could be attributed to their variation in body size, adaptation, rumen volume and the variation in the feed intake [43]. Rumen associated factors such as rumen pH, type of volatile fatty acids fermented, type of substrates fermented, rate of fermentation, absorption capacity of rumen wall, and rumen protozoa concentration determine the level of CH4 production [44]. Rumen methanogens remove H2 molecules that are synthesized during the organic matter fermentation produced during fermentation of organic matter in the hind gut and rumen and produce CH4 [45]. Further, the increased production of propionate decreases the CH4 production by consuming H2 molecules [46].
Geographic location and climate are known to be the most crucial factors significantly affecting CH4 production and this could be due to ambient temperature differences as well as difference in feed resources available [44]. Animals reared in arid and semi-arid regions have been reported to produce less CH4 production compared with animals in temperate regions, and this could be due to the differences in the type or amount of feed consumed in different locations [44]. Among the climate variables temperature, humidity, solar radiation and wind velocity are the important variables that influences CH4 production. Increased ambient temperature coupled with high relative humidity (RH) directly affects CH4 production by altering the rumen fermentation profile and indirectly by altering the quality of pasture or forage [46]. Although heat stress may reduce the feed intake, the increased methane emission could still be attributed to the heat stress associated negative impact on feed digestibility by inhibiting the rumen microbial populations that are essential for the normal digestion process. The various factors influencing enteric methane production from goats are summarized in Figure 2.
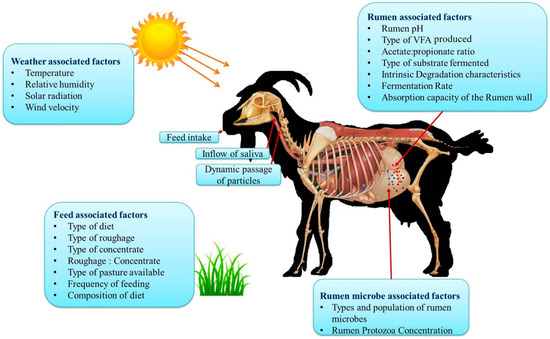
Figure 2.
Various factors influencing enteric methane emission in goats (these concepts were adopted from References [8,28,29]).
3.6. Enteric Methane Mitigation Strategies in Goats
Apart from its major role in global warming and the greenhouse effect, enteric CH4 is also considered as a dietary energy loss of around 2–12% in ruminants. Consequently, the global scientific community is targeting the development of suitable CH4 mitigation strategies to reduce both global warming and dietary energy loss. The various strategies that can be implemented to mitigate enteric CH4 include feeding feed sources containing plant secondary metabolites, ration manipulation, fat and oil supplementation, bacteriocin supplementation, rumen modification, etc. [29,45,46,47]. Various mechanisms to reduce enteric methane production in goats are summarised in Figure 3.
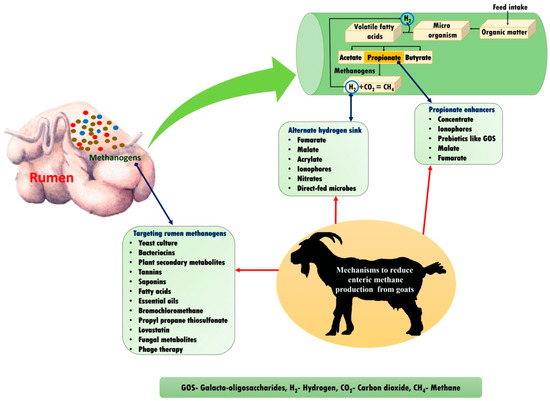
Figure 3.
Various mechanisms to reduce enteric methane emission in goats.
3.6.1. Nutritional Intervention to Reduce Enteric Methane Production in Goats
Among the various CH4 mitigation strategies, nutritional intervention or dietary manipulation is the most effective and commonly used strategy to mitigate enteric CH4 emission in ruminant livestock [48,49]. It is well known that increasing the ratio of concentrate to forage in the diet can reduce the amount of energy loss as enteric CH4 and this is mainly due to change of fermented substrate from fibre to starch [48]. In an experiment conducted on Murciano-Granadina goats in late lactation, Ibáñez et al. [50] observed a lower CH4 production in goats fed with concentrate (ground corn) diet than beet pulp fed goats. However, concentrate feeding beyond a certain limit is not appreciable as it can cause severe damage to the animal itself and to its production performance. In addition, grains that may be used for concentrates are more valuable for human feeds in arid and semi-arid regions where much of the global goat production is located.
Supplementing the feed with more lipids and fatty acids was reported to reduce the dietary energy loss in goats [51]. However, the effectiveness of lipid supplementation relies on the source, inclusion rate, fatty acid profile and the composition of the rest of the diet [48,52]. Reduction in enteric CH4 emission to the tune of around 40% is possible using high quality lipid feed supplements [46]. By differing the mode of action, lipid feed additives may reduce the methanogen and ciliated protozoan population in the rumen. Further, lipid supplementation reduces fibre and organic matter degradability and decreases the fermentable substrate availability and thereby minimising CH4 production [53]. Abubakr et al. [54] conducted an experiment in Boer X Catcang crossbred goats where they found that adding decanter cake and palm kernel cake at up to 80% inclusion decreases methanogenesis by reducing rumen protozoa in goats. Further, Zhou et al. [55] reported the ability of lauric acid to reduce CH4 production by reducing the viability of Methanobrevibacter ruminantium. Likewise, Kong et al. [56] reported a significant reduction in the methanogenesis without affecting the quantity of rumen methanogenic archaea after flaxseed supplementation.
Ionophore supplementation is another extensively researched CH4 abatement strategy. Ionophores cause a shift in the rumen fermentation pattern from acetate and butyrate production to propionate by increasing the gram-positive bacteria population, resulting in decreasing the production of CH4 [57]. Monensin is the most studied ionophore and routinely used as an animal nutrition supplement [58]. Saanen goats supplemented with oils with sodium bicarbonate and monensin showed a shift in the production of molar concentrations of acetate to propionate, thereby reducing the production of CH4 [59]. Furthermore, up to a 75% reduction in CH4 production was observed on addition of 10% encapsulated fumarate to the diet without any negative effect on animal growth [58].
Although several anti-methonogenic compounds are well proved in terms of their CH4 reduction potential, certain individual components have antinutritional properties that inhibit their commercial usage. However, data obtained from anti-methonogenic supplementation studies are good models and they can pave a way towards effective CH4 mitigation strategies [60,61]. Abecia et al. [45] conducted an experiment in Murciano-Granadina lactating goats to evaluate the potential of bromochloromethane (BCM) complex to reduce enteric CH4 production and they observed 32% reduction in BCM fed goats as compared to the control group. In another experiment conducted in Murciano-Granadina goats, Martínez-Fernández et al. [62] observed 33% and 64% methane reduction per kg of dry matter intake with propyl propane thiosulfinate (PTS) and BCM supplementation, respectively. Further, Murciano–Granadina goats supplemented with PTS and BCM decreased CH4 production by 48% and 98%, respectively, which was attributed to the redirection of H2 from CH4 production to propionate metabolic pathways [63]. Similarly, Mitsumori et al. [64] also reported 71% and 91% reductions in CH4 production in Shiba Japanese goats supplemented with 2 g/100 kg Live Weight and 5 g/100 kg LW of BCM, respectively. Candyrine et al. [65] conducted a study on Saanen goats with three levels of lovastatin (naturally produced from fermentation of palm kernel with Aspergillus terreus) supplementation and the authors observed 7.8%, 20% and 21% CH4 reduction for low (2 mg lovastatin/kg BW/day), medium (4 mg lovastatin/kg BW/day) and high (6 mg lovastatin/kg BW/day) treatment groups, respectively. Further, Azlan et al. [66] reported 32% reduction in enteric CH4 production when supplementing Boer crossbred goats with 14 mg/kg BW of lovastatin produced from rice straw treated with Aspergillus terreus.
Microbial feed additives are another important nutritional intervention in the CH4 mitigation studies. Apart from the effects on CH4 mitigation, probiotic feeding can improve the growth performance of meat animals and it can also reduce the incidence of diarrhoea [67]. However, studies proving the efficiency of direct fed probiotics to reduce the production of enteric CH4 are few [68]. The same authors also reported that nitrate as feed additive can reduce rumen methanogenesis in different ruminant species and production conditions [68]. Chaucheyras-Durand et al. [69] showed that yeast cells can reduce the production of enteric CH4 by deviating hydrogen atoms from methanogens to acetogenic strains of ruminal bacteria to enhance the production of acetate. Yeasts such as S. cerevisiae and the lactic acid utilizing bacteria Propionibacterium spp. and Megasphaera elsdenii can decrease rumen methanogenesis when included in the diet as supplements [60]. Wang et al. [61] found that replacing ordinary rice feed with red yeast rice, which is a traditional Chinese culinary and medicinal product, resulted in a 13% reduction in CH4/DM intake in Boer crossbred goats.
Organic acids such as malic acid and fumerate have the potential to reduce CH4 production in the ruminant by serving as an alternative hydrogen sink. Organic acid administration has been proven to reduce methane production in a dose-dependent manner in several in vitro studies [70]. In an experiment conducted in Xinong Saanen dairy goats, Li et al. [71] reported a significant reduction in CH4 production in goats supplemented with fumaric acid. Further, in the same study, along with fumeric acid supplementation, the authors also altered the particle size of concentrate and forage feed and observed 32% and 18% CH4 reduction in low forage and concentrate particle size diet and high forage and concentrate particle size diet, respectively [71].
Phenolic monomers, condensed tannins and other plant secondary metabolites in dose-dependent manner can reduce enteric CH4 emission from the ruminants because of their ability to reduce methanogenesis. Puchala et al. [72] reported 57% reduction in CH4 in terms of g/kg DMI in condensed tannin containing Lespedeza cuneata fed Angora goats compared to Angora goats fed a combination of Festucaarundinacea and Digitariaischaemum. Dietary tannins can directly hinder CH4 production as well as indirectly limit methanogenesis through reducing the availability of hydrogen atoms. In a meta-analysis using 30 experiments comprising 171 treatments to evaluate the extent of dietary tannins to reduce the CH4 emission, Jayanegara et al. [73] found a negative correlation between enteric CH4 production and tannin supplementation. Furthermore, Wina et al. [74] reported a reduction in methanogens in methanol extract saponin containing Sapindus rarak fed animals. Similarly, Mao et al. [75] reported a 27% reduction in enteric CH4 production with tea saponin supplementation. Further, in an experiment conducted in goats fed with natural tannin containing Mimosa spp., Bhatta et al. [46] reported a CH4 reduction after Mimosa spp. supplementation even at low concentrations (2–8 g/kg DM of the diet). In a study conducted in Nanjiang Yellow goats, Dong et al. [76] reported a reduction in enteric methane production on Artemisiae annuae extract and herbal medicines mixture supplementation to different diets. Further, under in vitro condition, Denman et al. [77] reported 91% reduction in methane production using bromochloromethane at 5 g/100 kg LW in Japanese native goats.
3.6.2. Management Strategies to Reduce CH4 Production from Goats
Improving management strategies not only reduces enteric methane emission but also helps to improve animal productivity [78]. Reduction or culling of unproductive animals from the herd has the potential to simultaneously improve the productivity and to reduce CH4 emission [79,80]. In subsistence production systems, reduction in the herd size allows distribution of adequate amount of feed and proper veterinary care to all animals. Additionally, selective culling can reduce CH4 production both per unit of animal product and for the total herd [81]. However, in some subsistence farming systems, there may be insufficient high breeding value animals to allow selective culling. Slaughter weight of goats can be advanced at a young age through early finishing approaches. This can potentially reduce the lifetime net CH4 emissions, thus making available proportionally few CH4 producing animals [79].
Reductions in enteric CH4 production can be achieved through efficient pasture management practices in goats. Feeding animals high quality fodder can reduce the wastage of dietary energy. Improving quality of the forage also increases feed intake and reduces the retention time of digesta in the rumen, thereby stimulating energetically more efficient post-ruminal digestion and decreases the percentage of energy transformed to CH4 [79]. Sejian et al. [82] reported a reduced CH4 emission in animals fed with high quality fodder as compared to animals consuming low quality fodder. Reductions in enteric CH4 production can also be achieved through feeding high quality fodder with higher soluble carbohydrates and lower fibre or through grazing on less-matured pastures [48,79]. Harvesting or grazing of forage at early stages of maturity also reduces the plant cell wall lignification, thereby increasing digestibility and reducing the CH4 emission per unit of digestible dry matter [83]. Similarly, Pinares-Patiño et al. [84] conducted a grazing experiment in timothy pasture at four different vegetative phases, namely, early vegetative stage, heading, flowering and senescence, and they observed lower CH4 production only at heading stage, which confirms the significance of growth stages of forage in CH4 production. Waghorn and Hegarty [85] calculated that animals grazing on high quality pasture (20% higher ME value) may show a reduction in enteric CH4 production of approximately 50%. Likewise, animals consuming certain high quality tropical and temperate legumes show reduced enteric CH4 production, as the legumes contain condensed tannins that are toxic to methanogenic archaea, ciliate protozoa, and fibre degrading bacteria [86]. Further, the grasses with high concentrations of water-soluble carbohydrates have been investigated as suitable tool to reduce enteric CH4 emission from the ruminant livestock [87]. De Ramus et al. [88] reported 22% reduction in enteric CH4 production annually through the efficient use of grazed forage crops through management-intensive grazing. Furthermore, around 5% reduction in enteric CH4 production is possible through improving total tract NDF digestibility [53]. Archimede et al. [89] reported 17% more CH4 production from animals fed with C4 grasses than the animals fed with C3 grasses.
In the majority of regions around the globe, goats are raised under continuous grazing systems, where animals have ad libitum access to pasture. However, unrestricted access to the pasture can result in the elimination of edible pasture and the domination of less edible pasture due to the uncontrolled selective grazing [31]. Hence, adoption of controlled grazing is a reliable strategy to reduce enteric CH4 and to improve productivity. In these systems, grazing land is divided into different paddocks that are alternatively grazed and rested until the pasture restores its quality. A continuous supply of uniform quality feed throughout the year enables animals to increase their production and to decrease CH4 production per kilogram of weight gain [20].
Size of the forage has profound effect on the CH4 emissions. Animals deviate considerable amount of their energy to the chewing process [90]. Particle size reduction of fodder by mechanical means helps to enhance digestibility through bringing more microbial access to the substrate, decreasing energy expenses, CH4 production and increasing the passage rate of digesta and animal productivity [91].
Selection of genetically superior animals with less CH4 production per unit feed intake is another management strategy that can be employed to reduce CH4 production from the ruminants [92]. The direct selection of low CH4 producing animals is practically impossible because of high cost for measuring CH4. However, selection is possible through the indirect means such as rumen digesta retention time and feed intake [80]. Genetic selection of goats with higher feed conversion efficiency generates a reduced amount of CH4. Further, genetic selection for the less CH4 production indirectly helps the farmer to increase their profits without any extra carbon credits by increasing the feed conversion efficiency and growth rate per animal [92]. A 3–10% reduction in CH4 production can be achieved through improving the feed use efficiency by 10% [93].
3.6.3. Advanced Biotechnological Tools for Methane Mitigation
The inhibition of enteric CH4 emission in ruminant animals is possible though biotechnological interventions. One of the possible future strategies to reduce enteric CH4 production is to immunize the animals against their own methanogens. In an experiment conducted in Australia using vaccines against three selected methanogens, Wright et al. [94] reported 8% reduction in CH4 production. However, another experiment conducted in different geographical zone with vaccines prepared using different set of bacteria could not elicit any positive response [94]. The reasons for the immunization failures could be due to the variation in rumen methanogenic diversity present in the animals raised in different conditions and the replacement of the biological niche left by the targeted species by another methanogen [95]. CH4 inhibition was also attempted through oral supplementation IgY as a feed additive [96]. Zhang et al. [97] conducted an experiment in Boer goats to evaluate the efficiency of a candidate vaccine protein (EhaF) on the rumen methanogens and microbes but did not find any changes in CH4 production among control and vaccinated goats. However, vaccination influenced the composition of rumen bacteria.
Use of bacteriocins offers another possible strategy to reduce CH4 emission from ruminant animals. Bacteriocins are the proteins produced by bacteria that can obstruct certain microbial species in the rumen [98]. An in vitro study conducted by Lee et al. [99] using bovicin HC5 (a bacteriocin produced by Streptococcus spp.) showed 50% reduction in CH4 production without inducing methanogen adaptation. Likewise, Santoso et al. [100] reported a 10% reduction in CH4 emission in an in vivo study that used nisin, a bacteriocin produced by Lactobacillus lactis subsp.
The lytic potential and genes of the bacteriophages makes them potential tools to mitigate enteric CH4 emission [101]. Certain bacteriophages may inhabit the rumen wall to maintain the homeostasis of the rumen micro fauna. Due to their host specific nature, they lyse certain microbes such as methanogens and Streptococcus bovis or pathogens such as salmonella and E. coli O157:H7 [47]. McAllister and Newbold [70] reported that siphophages (Siphoviridae phage) can infect methanogens such as Methano brevibacter, Methanobacterium and Methanococcus spp.; however, siphophages have yet to be isolated from the rumen. However, there are few available data relating to the genetic functionality and blueprint of the archaeal methanogenic phages and, to date, no bacteriophages from rumen have been isolated [47].
Another plausible method of biological control of methanogens is the use of CH4 oxidizers. The CH4 oxidizing bacteria have already been isolated from the rumen [102]. However, in vitro studies conducted using carbon isotopes reveal that only 0.3–8% of CH4 oxidation to CO2 happens in the rumen [103]. Valdez et al. [104] reported a reduction in CH4 production by adding CH4 oxidizing bacterium isolated from the gut of young pigs. However, detailed in vivo studies are needed to establish the level of CH4 reduction. Another novel approach for enteric CH4 reduction is through the genetic modification of fermentation characteristics of rumen bacteria. However, research is still in the preliminary stages and very little progress has been made pertaining to applying the molecular techniques to characterize and quantify the microbial populations [86].
4. Conclusions and Future Perspectives
Goats undoubtedly need to be the priority focus for livestock industries due to their advantages over other ruminant animals from a climate resilience point of view. Elevated ambient temperature during the summer months can have a significant influence on the basic physiology of rumen, thereby affecting the rumen fermentation pattern, VFA and other rumen metabolites production. Furthermore, growing evidence suggests that heat stress influences the rumen microbial population, resulting in alterations in ruminal digestion process in goats. In addition, heat stress has also been shown to increase the production of enteric CH4 emission resulting in dietary energy loss. Thus, the productive performances of the animals are compromised. Nutritional interventions and other management strategies are traditional ways by which enteric CH4 emission is reduced in goats. More recently, several researchers have targeted reducing enteric CH4 through advanced biotechnological tools such immunization therapy, using bacteriocins, etc. but without much success. Further refinements in these technologies are essential before these technologies are implemented at field level. In the near future, these technologies offer scope for identifying genetically superior animals with less CH4 production per unit feed intake. However, further research efforts are needed to elucidate the mechanisms associated with enteric CH4 emission during heat stress exposure by establishing the relationships among the rumen microbes through metagenomics approaches in goats in the changing climate scenario. Such efforts may help to develop more focussed mitigation strategies for reducing enteric CH4 emission in goats. This may help to sustain goat production in the changing climate scenario by preventing the dietary energy loss incurred during the process of enteric CH4 emission.
Author Contributions
Conceptualization, P.P. and V.S.; Preparation of Manuscript, P.P., V.S., S.S.C., B.J.L. and F.R.D.; Figure Preparation, P.P., V.S. and S.S.C.; Table Preparation, P.P., S.S.C. and V.S.; and Scientific Editing, F.R.D., B.J.L. and S.S.C.
Funding
This research received no external funding.
Acknowledgments
The authors profusely thank Frank R. Dunshea for supporting this manuscript by bearing the publication charge.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Feleke, F.B.; Berhe, M.; Gebru, G.; Hoag, D. Determinants of adaptation choices to climate change by sheep and goat farmers in Northern Ethiopia: The case of Southern and Central Tigray, Ethiopia. SpringerPlus 2016, 5, 1692. [Google Scholar] [CrossRef] [PubMed]
- Bezabih, M.Y.; Berhane, G. Livestock Production Systems Analysis. Am. Int. J. Contemp. Sci. Res. 2014, 1, 16–51. [Google Scholar]
- Thornton, P.K. Livestock production: Recent trends, future prospects. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2010, 365, 2853–2867. [Google Scholar] [CrossRef] [PubMed]
- Brahmi, A.; Khaldi, R.; Jaouad, M.; Hicheri, A.; Touati, I.; Rkhissi, A.; Ifaoui, H.; Khaldi, G. Impacts of climate change on the small ruminants farming systems in north western Tunisia and adaptation tools. In New Approaches for Grassland Research in a Context of Climate and Socio-Economic Changes; Acar, Z., López-Francos, A., Porqueddu, C., Eds.; Options Méditerranéennessérie; CIHEAM: Zaragoza, Spain, 2012; pp. 427–431. [Google Scholar]
- Koluman, N.; Silanikove, N.; Koluman, A. Climate Change and Goat Agriculture Interactions in the Mediterranean Region. In Sustainable Goat Production in Adverse Environments; Simões, J., Gutiérrez, C., Eds.; Springer: Cham, Switzerland, 2017; pp. 393–405. [Google Scholar]
- Sejian, V.; Bhatta, R.; Gaughan, J.B.; Dunshea, F.R.; Lacetera, N. Review: Adaptation of animals to heat stress. Animal 2018. [Google Scholar] [CrossRef] [PubMed]
- Bernabucci, U.; Lacetera, N.; Baumgard, L.H.; Rhoads, R.P.; Ronchi, B.; Nardone, A. Metabolic and hormonal acclimation to heat stress in domesticated ruminants. Animal 2010, 4, 1167–1183. [Google Scholar] [CrossRef] [PubMed]
- Yadav, B.; Gynendra, S.; Verma, A.K.; Dutta, N.; Sejian, V. Impact of heat stress on rumen functions. Vet. World 2013, 6, 992–996. [Google Scholar] [CrossRef]
- Bernabucci, U.; Lacetera, N.; Danieli, P.P.; Bani, P.; Nardone, A.; Ronchi, B. Influence of different periods of exposure to hot environment on rumen function and diet digestibility in sheep. Int. J. Biometeorol. 2009, 53, 387–395. [Google Scholar] [CrossRef]
- Pollott, G.; Wilson, R.T. Sheep and Goats for Diverse Products and Profits; FAO Diversification Booklet No. 9; FAO Library: Rome, Italy, 2009; 42p. [Google Scholar]
- Oluwatayo, I.B.; Oluwatayo, T.B. Small Ruminants as a Source of Financial Security, a Case Study of Women in Rural Southwest Nigeria; Working Paper 1; Institute for Money, Technology and Financial Inclusion (IMTFI): Irvine, CA, USA, 2012; 21p. [Google Scholar]
- Aziz, M.A. Present status of the world goat populations and their productivity. World 2010, 861, 1. [Google Scholar]
- Lérias, J.R.; Hernández-Castellano, L.E.; Suárez-Trujillo, A.; Castro, N.; Pourlis, A.; Almeida, A.M. The mammary gland in small ruminants: Major morphological and functional events underlying milk production—A review. J. Dairy Res. 2014, 81, 304–318. [Google Scholar] [CrossRef]
- Kumar, S.; Roy, M.M. Small Ruminant’s Role in Sustaining Rural Livelihoods in Arid and Semiarid Regions and their Potential for Commercialization. In New Paradigms in Livestock Production from Traditional to Commercial Farming and Beyond; Agrotech Publishing Academy: Udaipur, India, 2013; pp. 57–80. [Google Scholar]
- Agossou, D.J.; Dougba, T.D.; Koluman, N. Recent Developments in Goat Farming and Perspectives for a Sustainable Production in Western Africa. Int. J. Environ. Agric. Biotechnol. 2017, 2, 2047–2051. [Google Scholar] [CrossRef]
- Yami, A.; Merkel, R.C. Sheep and Goat Production Handbook for Ethiopia. Ethiopia Sheep and Goat Productivity Improvement Program. 2008. Available online: https://archive.org/details/SheepGoatHandbook (accessed on 15 October 2018).
- Kosgey, I.S.; Rowlands, G.J.; Van Arendonk, J.A.; Baker, R.L. Small ruminant production in smallholder and pastoral/extensive farming systems in Kenya. Small Rumin. Res. 2008, 77, 11–24. [Google Scholar] [CrossRef]
- Amankwah, K.; Klerkx, L.; Oosting, S.J.; Sakyi-Dawson, O.; Van der Zijpp, A.J.; Millar, D. Diagnosing constraints to market participation of small ruminant producers in northern Ghana: An innovation systems analysis. NJAS-Wageningen J. Life Sci. 2012, 60, 37–47. [Google Scholar] [CrossRef]
- Silanikove, N. The physiological basis of adaptation in goats to harsh environments. Small Rumin. Res. 2000, 35, 181–193. [Google Scholar] [CrossRef]
- Soren, N.M.; Sejian, V.; Terhuja, M.; Dominic, G. Enteric methane emission in sheep: Process description and factors influencing production. In Sheep Production Adapting to Climate Change; Sejian, V., Bhatta, R., Gaughan, J., Malik, P.K., Naqvi, S.M.K., Lal, R., Eds.; Springer: Singapore, 2017; pp. 209–233. [Google Scholar]
- Archana, P.R.; Sejian, V.; Ruban, W.; Bagath, M.; Krishnan, G.; Aleena, J.; Manjunathareddy, G.B.; Beena, V.; Bhatta, R. Comparative assessment of heat stress induced changes in carcass traits, plasma leptin profile and skeletal muscle myostatin and HSP70 gene expression patterns between indigenous Osmanabadi and Salem Black goat breeds. Meat Sci. 2018, 141, 66–80. [Google Scholar] [CrossRef] [PubMed]
- Getaneh, G.; Mebrat, A.; Wubie, A.; Kendie, H. Review on Goat Milk Composition and its Nutritive Value. J. Nutr. Health Sci. 2016, 3, 1–10. [Google Scholar]
- Baumgard, L.H.; Rhoads, R.P. Ruminant Nutrition Symposium: Ruminant production and metabolic responses to heat stress. J. Anim. Sci. 2012, 90, 1855–1865. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, T.; Katoh, K.; Obara, Y. Effects of heat exposure on nutrient digestibility, rumen contraction and hormone secretion in goats. Anim. Sci. J. 2004, 75, 237–243. [Google Scholar] [CrossRef]
- Choubey, M.; Kumar, A. Nutritional interventions to combat heat stress in dairy animals. Vetscan 2012, 7, 19–27. [Google Scholar]
- Castro-Costa, A.; Salama, A.A.; Moll, X.; Aguiló, J.; Caja, G. Using wireless rumen sensors for evaluating the effects of diet and ambient temperature in non-lactating dairy goats. J. Dairy Sci. 2015, 98, 4646–4658. [Google Scholar] [CrossRef]
- Ma, Y.-F.; Du, R.-P.; Gao, M. Effect of Heat Stress on Dairy Goat Performance and Rumen Epithelial Cell Morphology. Sci. Agric. Sin. 2013, 46, 4486–4495. [Google Scholar]
- Pragna, P.; Sejian, V.; Soren, N.M.; Bagath, M.; Krishnan, G.; Beena, V.; Devi, P.I.; Bhatta, R. Summer season induced rhythmic alterations in metabolic activities to adapt to heat stress in three indigenous (Osmanabadi, Malabari and Salem Black) goat breeds. Biol. Rhythm Res. 2018, 49, 551–565. [Google Scholar] [CrossRef]
- Chaidanya, K.; Soren, N.M.; Sejian, V.; Bagath, M.; Manjunathareddy, G.B.; Kurien, K.E.; Varma, G.; Bhatta, R. Impact of heat stress, nutritional stress and combined (heat and nutritional) stresses on rumen associated fermentation characteristics, histopathology and HSP70 gene expression in goats. J. Anim. Behav. Biometeorol. 2017, 5, 36–48. [Google Scholar] [CrossRef]
- Tajima, K.; Nonaka, I.; Higuchi, K.; Takusari, N.; Kurihara, M.; Takenaka, A.; Mitsumori, M.; Kajikawa, H.; Aminov, R.I. Influence of high temperature and humidity on rumen bacterial diversity in Holstein heifers. Anaerobe 2007, 13, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Freestone, P.; Lyte, M. Stress and microbial endocrinology: Prospects for ruminant nutrition. Animal 2010, 4, 1248–1257. [Google Scholar] [CrossRef] [PubMed]
- Uyeno, Y.; Sekiguchi, Y.; Tajima, K.; Takenaka, A.; Kurihara, M.; Kamagata, Y. An rRNA-based analysis for evaluating the effect of heat stress on the rumen microbial composition of Holstein heifers. Anaerobe 2010, 16, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Mbanzamihigo, L.; Fievez, V.; da Costa Gomez, C.; Piattoni, F.; Carlier, L.; Demeyer, D. Methane emissions from the rumen of sheep fed a mixed grass-clover pasture at two fertilisation rates in early and late season. Can. J. Anim. Sci. 2002, 82, 69–77. [Google Scholar] [CrossRef]
- Ulyatt, M.J.; Lassey, K.R.; Shelton, I.D.; Walker, C.F. Methane emission from sheep grazing four pastures in late summer in New Zealand. N. Z. J. Agric. Res. 2005, 48, 385–390. [Google Scholar] [CrossRef]
- Ulyatt, M.J.; Lassey, K.R.; Shelton, I.D.; Walker, C.F. Methane emission from dairy cows and wether sheep fed subtropical grass-dominant pastures in midsummer in New Zealand. N. Z. J. Agric. Res. 2002, 45, 227–234. [Google Scholar] [CrossRef]
- Aluwong, T.; Wuyep, P.A.; Allam, L. Livestock-environment interactions: Methane emissions from ruminants. Afr. J. Biotechnol. 2011, 10, 1265–1269. [Google Scholar]
- Brouček, J. Methane yield from cattle, sheep, and goats housing with emphasis on emission factors: A review. Slovak J. Anim. Sci. 2015, 48, 122–139. [Google Scholar]
- Bhatta, R.; Malik, P.K.; Sejian, V. Enteric methane emission and reduction strategies in sheep. In Sheep Production Adapting to Climate Change; Sejian, V., Bhatta, R., Gaughan, J., Malik, P.K., Naqvi, S.M.K., Lal, R., Eds.; Springer: Singapore, 2017; pp. 291–305. [Google Scholar]
- Castillo-González, A.R.; Burrola-Barraza, M.E.; Domínguez-Viveros, J.; Chávez-Martínez, A. Rumen microorganisms and fermentation. Arch. Med. Vet. 2014, 46, 349–361. [Google Scholar] [CrossRef]
- Malik, P.K.; Thulasi, A.; Soren, N.M.; Jose, L.; Prasad, K.S.; Prasad, C.S. Phylogenic diversity analysis of rumen acetogens in adult sheep fed on conventional roughage diet. Indian J. Anim. Sci. 2015, 85, 1104–1107. [Google Scholar]
- Patra, A.K. A meta-analysis of the effect of dietary fat on enteric methane production, digestibility and rumen fermentation in sheep, and a comparison of these responses between cattle and sheep. Livest. Sci. 2014, 162, 97–103. [Google Scholar] [CrossRef]
- Rochfort, S.; Parker, A.; Dunshea, F.R. Plant bioactives for ruminant health and productivity—A review. Phytochemistry 2008, 69, 299–322. [Google Scholar] [CrossRef] [PubMed]
- Fraser, M.D.; Fleming, H.R.; Moorby, J.M. Traditional vs Modern: Role of Breed Type in Determining Enteric Methane Emissions from Cattle Grazing as Part of Contrasting Grassland-Based Systems. PLoS ONE 2014, 9, e107861. [Google Scholar] [CrossRef] [PubMed]
- Cottle, D.J.; Nolan, J.V.; Wiedemann, S.G. Ruminant enteric methane mitigation: A review. Anim. Prod. Sci. 2011, 51, 491–514. [Google Scholar] [CrossRef]
- Pragna, P.; Sejian, V.; Bagath, M.; Krishnan, G.; Devaraj, C.; Afsal, A.; Aleena, J.; Archana, P.R.; Bhatta, R. Metabolic adaptation of livestock in heat stress challenges. In National Initiative on Climate Resilient Agriculture Workshop Compendium on “Livestock and Climate Change”; Hemanth, G.K., Sejian, V., Giridhar, K., Naveen, K.G.S., Eds.; Veterinary College, Karnataka Veterinary Animal and Fishery Sciences University: Hassan, Karnataka, India; National Initiatives on Climate Resilient Agriculture, Indian Council of Agriculture Research and Hyderabad: India, 2018; pp. 33–36. [Google Scholar]
- Chaidanya, K.; Soren, N.M.; Sejian, V.; Bagath, M.; Kurien, E.K.; Varma, G.; Bhatta, R. Impact of heat stress, nutritional stress and combined (heat and Nutritional) stresses on rumen enzymes and fermentation metabolites in Osmanabadi bucks. In Proceedings of the Interenational Conference Compendium on Climate Change Adaptation and Biodiversity: Ecological Sustainability and Resource Management for Livelihood Security, Portblair, Andaman and Nicobar Island, 8–10 December 2016; Andaman Science Association at ICAR-Central Island Agricultural Research Institute: Portblair, Andaman and Nicobar Island. [Google Scholar]
- Baumgard, L.H.; Rhoads, R.P.; Rhoads, M.L.; Gabler, N.K.; Ross, J.W.; Keating, A.F.; Boddicker, R.L.; Lenka, S.; Sejian, V. Impact of climate change on livestock production. In Environmental Stress and Amelioration in Livestock Production; Sejian, V., Naqvi, S.M.K., Ezeji, T., Lakritz, J., Lal, R., Eds.; Springer-Verlag GMbH Publisher: Germany, 2012; pp. 413–468. [Google Scholar]
- Beauchemin, K.A.; Kreuzer, M.; O’mara, F.; McAllister, T.A. Nutritional management for enteric methane abatement: A review. Aust. J. Exp. Agric. 2008, 48, 21–27. [Google Scholar] [CrossRef]
- Sejian, V.; Lal, R.; Lakritz, J.; Ezeji, T. Measurement and prediction of enteric methane emission. Int. J. Biometeorol. 2011, 55, 1–6. [Google Scholar] [CrossRef]
- Ibáñez, C.; López, M.C.; Criscioni, P.; Fernández, C. Effect of replacing dietary corn with beet pulp on energy partitioning, substrate oxidation and methane production in lactating dairy goats. Anim. Prod. Sci. 2015, 55, 56–63. [Google Scholar] [CrossRef]
- Lascano, C.E.; Carulla, J.E.; Vargas, J.J. Strategies for reducing methane emissions from ruminants. Rev. Bras. Geogr. Fís. 2011, 6, 1315–1335. [Google Scholar]
- Knapp, J.R.; Laur, G.L.; Vadas, P.A.; Weiss, W.P.; Tricarico, J.M. Invited review: Enteric methane in dairy cattle production: Quantifying the opportunities and impact of reducing emissions. J. Dairy Sci. 2014, 97, 3231–3261. [Google Scholar] [CrossRef] [PubMed]
- McGinn, S.M.; Beauchemin, K.A.; Coates, T.; Colombatto, D. Methane emissions from beef cattle: Effects of monensin, sunflower oil, enzymes, yeast, and fumaric acid. J. Anim. Sci. 2004, 82, 3346–3356. [Google Scholar] [CrossRef] [PubMed]
- Abubakr, A.R.; Alimon, A.R.; Yaakub, H.; Abdullah, N.; Ivan, M. Digestibility, rumen protozoa, and ruminal fermentation in goats receiving dietary palm oil by-products. J. Saudi Soc. Agric. Sci. 2013, 12, 147–154. [Google Scholar] [CrossRef]
- Zhou, X.; Meile, L.; Kreuzer, M.; Zeitz, J.O. The effect of saturated fatty acids on methanogenesis and cell viability of Methanobrevibacter ruminantium. Archaea 2013, 2013, 106916. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; He, M.; McAlister, T.; Seviour, R.; Forster, R. Quantitative fluorescence in situ hybridization of microbial communities in the rumens of cattle fed different diets. Appl. Environ. Microbiol. 2010, 76, 6933–6938. [Google Scholar] [CrossRef] [PubMed]
- Kataria, R.P. Use of feed additives for reducing greenhouse gas emissions from dairy farms. Microbiol. Res. 2016, 6, 19–25. [Google Scholar] [CrossRef]
- Wallace, R.J.; Wood, T.A.; Rowe, A.; Price, J.; Yanez, D.R.; Williams, S.P.; Newbold, C.J. Encapsulated fumaric acid as a means of decreasing ruminal methane emissions. Int. Congr. Ser. 2006, 1293, 148–151. [Google Scholar] [CrossRef]
- Li, X.Z.; Yan, C.G.; Long, R.J.; Jin, G.L.; Khuu, J.S.; Ji, B.J.; Choi, S.H.; Lee, H.G.; Song, M.K. Conjugated linoleic acid in rumen fluid and milk fat, and methane emission of lactating goats fed a soybean oil-based diet supplemented with sodium bicarbonate and monensin. Asian-Aust. J. Anim. Sci. 2009, 22, 1521–1530. [Google Scholar] [CrossRef]
- Seo, J.K.; Kim, S.W.; Kim, M.H.; Upadhaya, S.D.; Kam, D.K.; Ha, J.K. Direct-fed microbials for ruminant animals. Asian-Australas. J. Anim. Sci. 2010, 23, 1657–1667. [Google Scholar] [CrossRef]
- Wang, L.Z.; Zhou, M.L.; Wang, J.W.; Wu, D.; Yan, T. The effect of dietary replacement of ordinary rice with red yeast rice on nutrient utilization, enteric methane emission and rumen archaeal diversity in goats. PLoS ONE 2016, 11, e0160198. [Google Scholar] [CrossRef]
- Martínez-Fernández, G.; Abecia, L.; Martín-García, A.I.; Ramos-Morales, E.; Hervás, G.; Molina-Alcaide, E.; Yáñez-Ruiz, D.R. In vitro–in vivo study on the effects of plant compounds on rumen fermentation, microbial abundances and methane emissions in goats. Animal 2013, 7, 1925–1934. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Fernández, G.; Abecia, L.; Martín-García, A.I.; Ramos-Morales, E.; Denman, S.E.; Newbold, C.J.; Molina-Alcaide, E.; Yáñez-Ruiz, D.R. Response of the rumen archaeal and bacterial populations to anti-methanogenic organosulphur compounds in continuous-culture fermenters. FEMS Microbiol. Ecol. 2015, 91. [Google Scholar] [CrossRef] [PubMed]
- Mitsumori, M.; Shinkai, T.; Takenaka, A.; Enishi, O.; Higuchi, K.; Kobayashi, Y.; Nonaka, I.; Asanuma, N.; Denman, S.E.; McSweeney, C.S. Responses in digestion, rumen fermentation and microbial populations to inhibition of methane formation by a halogenated methane analogue. Br. J. Nutr. 2012, 108, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Candyrine, S.C.; Mahadzir, M.F.; Garba, S.; Jahromi, M.F.; Ebrahimi, M.; Goh, Y.M.; Samsudin, A.A.; Sazili, A.Q.; Chen, W.L.; Ganesh, S.; et al. Effects of naturally-produced lovastatin on feed digestibility, rumen fermentation, microbiota and methane emissions in goats over a 12-week treatment period. PLoS ONE 2018, 13, e0199840. [Google Scholar] [CrossRef] [PubMed]
- Azlan, P.M.; Jahromi, M.F.; Ariff, M.O.; Ebrahimi, M.; Candyrine, S.C.; Liang, J.B. Aspergillus terreus treated rice straw suppresses methane production and enhances feed digestibility in goats. Trop. Anim. Health Prod. 2018, 50, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Soren, N.M.; Tripathi, M.K.; Bhatt, R.S.; Karim, S.A. Effect of yeast supplementation on the growth performance of Malpura lambs. Trop. Anim. Health Prod. 2013, 45, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Jeyanathan, J.; Martin, C.; Morgavi, D.P. The use of direct-fed microbials for mitigation of ruminant methane emissions: A review. Animal 2014, 8, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Chaucheyras-Durand, F.; Walker, N.D.; Bach, A. Effects of active dry yeasts on the rumen microbial ecosystem: Past, present and future. Anim. Feed Sci. Technol. 2008, 145, 5–26. [Google Scholar] [CrossRef]
- McAllister, T.A.; Newbold, C.J. Redirecting rumen fermentation to reduce methanogenesis. Aust. J. Exp. Agric. 2008, 48, 7–13. [Google Scholar] [CrossRef]
- Li, Z.; Liu, N.; Cao, Y.; Jin, C.; Li, F.; Cai, C.; Yao, J. Effects of fumaric acid supplementation on methane production and rumen fermentation in goats fed diets varying in forage and concentrate particle size. J. Anim. Sci. Biotechnol. 2018, 9, 21. [Google Scholar] [CrossRef] [PubMed]
- Puchala, R.; Min, B.R.; Goetsch, A.L.; Sahlu, T. The effect of a condensed tannin-containing forage on methane emission by goats. J. Anim. Sci. 2005, 83, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Jayanegara, A.; Leiber, F.; Kreuzer, M. Meta-analysis of the relationship between dietary tannin level and methane formation in ruminants from in vivo and in vitro experiments. J. Anim. Physiol. Anim. Nutr. 2012, 96, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Wina, E.; Muetzel, S.; Hoffmann, E.; Makkar, H.P.; Becker, K. Saponins containing methanol extract of Sapindus rarak affect microbial fermentation, microbial activity and microbial community structure in vitro. Anim. Feed Sci. Technol. 2005, 121, 159–174. [Google Scholar] [CrossRef]
- Mao, H.L.; Wang, J.K.; Zhou, Y.Y.; Liu, J.X. Effects of addition of tea saponins and soybean oil on methane production, fermentation and microbial population in the rumen of growing lambs. Livest. Sci. 2010, 129, 56–62. [Google Scholar] [CrossRef]
- Dong, G.Z.; Wang, X.J.; Liu, Z.B.; Wang, F. Effects of phytogenic products on in vitro rumen fermentation and methane emission in goats. J. Anim. Feed Sci. 2010, 19, 218–229. [Google Scholar] [CrossRef]
- Denman, S.E.; Martinez-Fernandez, G.; Shinkai, T.; Mitsumori, M.; McSweeney, C.S. Metagenomic analysis of the rumen microbial community following inhibition of methane formation by a halogenated methane analog. Front. Microbiol. 2015, 6, 1087. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, S.M.; Sejian, V. Global climate change: Role of livestock. Asian J. Agric. Sci. 2011, 3, 19–25. [Google Scholar]
- Eckard, R.J.; Grainger, C.; De Klein, C.A. Options for the abatement of methane and nitrous oxide from ruminant production: A review. Livest. Sci. 2010, 130, 47–56. [Google Scholar] [CrossRef]
- Hegarty, R.S.; McEwan, J.C. Genetic opportunities to reduce enteric methane emissions from ruminant livestock. In Proceedings of the 9th World Congress on Genetics Applied to Livestock Production, Leipzig, Germany, 1–6 August 2010; pp. 1–6. [Google Scholar]
- Tarawali, S.; Herrero, M.; Descheemaeker, K.; Grings, E.; Blümmel, M. Pathways for sustainable development of mixed crop livestock systems: Taking a livestock and pro-poor approach. Livest. Sci. 2011, 139, 11–21. [Google Scholar] [CrossRef]
- Sejian, V.; Lakritz, J.; Ezeji, T.; Lal, R. Forage and Flax seed impact on enteric methane emission in dairy cows. Res. J. Vet. Sci. 2011, 4, 1–8. [Google Scholar] [CrossRef]
- Boadi, D.A.; Wittenberg, K.M. Methane production from dairy and beef heifers fed forages differing in nutrient density using the sulphur hexafluoride (SF6) tracer gas technique. Can. J. Anim. Sci. 2002, 82, 201–206. [Google Scholar] [CrossRef]
- Pinares-Patiño, C.S.; Baumont, R.; Martin, C. Methane emissions by Charolais cows grazing a monospecific pasture of timothy at four stages of maturity. Can. J. Anim. Sci. 2003, 83, 769–777. [Google Scholar] [CrossRef]
- Waghorn, G.C.; Hegarty, R.S. Lowering ruminant methane emissions through improved feed conversion efficiency. Anim. Feed Sci. Technol. 2011, 166, 291–301. [Google Scholar] [CrossRef]
- Lascano, C.E.; Cárdenas, E. Alternatives for methane emission mitigation in livestock systems. R. Bras. Zootec. 2010, 39, 175–182. [Google Scholar] [CrossRef]
- Ellis, J.L.; Dijkstra, J.; France, J.; Parsons, A.J.; Edwards, G.R.; Rasmussen, S.; Kebreab, E.; Bannink, A. Effect of high-sugar grasses on methane emissions simulated using a dynamic model. J. Dairy Sci. 2012, 95, 272–285. [Google Scholar] [CrossRef] [PubMed]
- De Ramus, H.A.; Clement, T.C.; Giampola, D.D.; Dickison, P.C. Methane emissions of beef cattle on forages. J. Environ. Qual. 2003, 32, 269–277. [Google Scholar] [CrossRef]
- Archimède, H.; Eugène, M.; Magdeleine, C.M.; Boval, M.; Martin, C.; Morgavi, D.P.; Lecomte, P.; Doreau, M. Comparison of methane production between C3 and C4 grasses and legumes. Anim. Feed Sci. Technol. 2011, 166, 59–64. [Google Scholar] [CrossRef]
- Gerber, P.J.; Hristov, A.N.; Henderson, B.; Makkar, H.; Oh, J.; Lee, C.; Meinen, R.; Montes, F.; Ott, T.; Firkins, J.; et al. Technical options for the mitigation of direct methane and nitrous oxide emissions from livestock: A review. Animal 2013, 7, 220–234. [Google Scholar] [CrossRef]
- Hristov, A.N.; Oh, J.; Firkins, J.L.; Dijkstra, J.; Kebreab, E.; Waghorn, G.; Makkar, H.P.; Adesogan, A.T.; Yang, W.; Lee, C.; et al. Special topics—Mitigation of methane and nitrous oxide emissions from animal operations: I. A review of enteric methane mitigation options 1. J. Anim. Sci. 2013, 91, 5045–5069. [Google Scholar] [CrossRef]
- Pinares-Patiño, C.S.; Hickey, S.M.; Young, E.A.; Dodds, K.G.; MacLean, S.; Molano, G.; Sandoval, E.; Kjestrup, H.; Harland, R.; Hunt, C.; et al. Heritability estimates of methane emissions from sheep. Animal 2013, 7, 316–321. [Google Scholar] [CrossRef]
- Sudmeyer, R. Carbon Farming: Selective Breeding of Sheep for Reduced Methane Emissions, Climate Change, Agriculture and Food, Department of Primary Industries and Regional Development, Government of Western Australia. 2018. Available online: https://www.agric.wa.gov.au/climate-change/carbon-farming-selective-breeding-sheep-reduced-methane-emissions (accessed on 15 October 2018).
- Wright, A.D.; Kennedy, P.; O’Neill, C.J.; Toovey, A.F.; Popovski, S.; Rea, S.M.; Pimm, C.L.; Klein, L. Reducing methane emissions in sheep by immunization against rumen methanogens. Vaccine 2004, 22, 3976–3985. [Google Scholar] [CrossRef]
- Williams, Y.J.; Popovski, S.; Rea, S.M.; Skillman, L.C.; Toovey, A.F.; Northwood, K.S.; Wright, A.D. A vaccine against rumen methanogens can alter the composition of archaeal populations. Appl. Environ. Microbiol. 2009, 75, 1860–1866. [Google Scholar] [CrossRef]
- Cook, S.R.; Maiti, P.K.; Chaves, A.V.; Benchaar, C.; Beauchemin, K.A.; McAllister, T.A. Avian (IgY) anti-methanogen antibodies for reducing ruminal methane production: In vitro assessment of their effects. Aust. J. Exp. Agric. 2008, 48, 260–264. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, X.; Xue, B.; Peng, Q.; Wang, Z.; Yan, T.; Wang, L. Immunization against rumen methanogenesis by vaccination with a new recombinant protein. PLoS ONE 2015, 10, e0140086. [Google Scholar] [CrossRef]
- Patra, A.; Park, T.; Kim, M.; Yu, Z. Rumen methanogens and mitigation of methane emission by anti-methanogenic compounds and substances. J. Anim. Sci. Biotechnol. 2017, 8, 13. [Google Scholar] [CrossRef]
- Lee, S.S.; Hsu, J.T.; Mantovani, H.C.; Russell, J.B. The effect of bovicin hc5, a bacteriocin from Streptococcus bovis hc5, on ruminal methane production in vitro. FEMS Microbiol. Lett. 2002, 217, 51–55. [Google Scholar] [CrossRef]
- Santoso, B.; Mwenya, B.; Sar, C.; Gamo, Y.; Kobayashi, T.; Morikawa, R.; Kimura, K.; Mizukoshi, H.; Takahashi, J. Effects of supplementing galacto-oligosaccharides, Yucca schidigera or nisin on rumen methanogenesis, nitrogen and energy metabolism in sheep. Livest. Prod. Sci. 2004, 91, 209–217. [Google Scholar] [CrossRef]
- Ackermann, H.W. 5500 Phages examined in the electron microscope. Arch. Virol. 2007, 152, 227–243. [Google Scholar] [CrossRef]
- Moss, A.R.; Jouany, J.P.; Newbold, J. Methane production by ruminants: Its contribution to global warming. Ann. Zootech. 2000, 49, 231–253. [Google Scholar] [CrossRef]
- Boadi, D.; Benchaar, C.; Chiquette, J.; Massé, D. Mitigation strategies to reduce enteric methane emissions from dairy cows: Update review. Can. J. Anim. Sci. 2004, 84, 319–335. [Google Scholar] [CrossRef]
- Valdez, C.; Newbold, C.J.; Hillman, K.; Wallace, R.J. Evidence for methane oxidation in rumen fluid in vitro. Ann. Zootech. 1996, 45, 351. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).