Evidence for Right-Sided Horses Being More Optimistic than Left-Sided Horses
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Location
2.2. Experimental Procedure
2.3. Cognitive Bias Test
2.3.1. Apparatus and Test Arena for Cognitive Bias
2.3.2. Habituation, Habituation Area, and Training
2.3.3. Test
2.3.4. Analysis of the Horses’ Performance
2.4. Laterality
2.4.1. Motor Laterality
2.4.2. Sensory Laterality
2.5. Inter-Observer Reliability
2.6. Statistical Analysis
3. Results
3.1. Correlation between the Three Measurements of Motor Laterality
3.2. Training Criterion
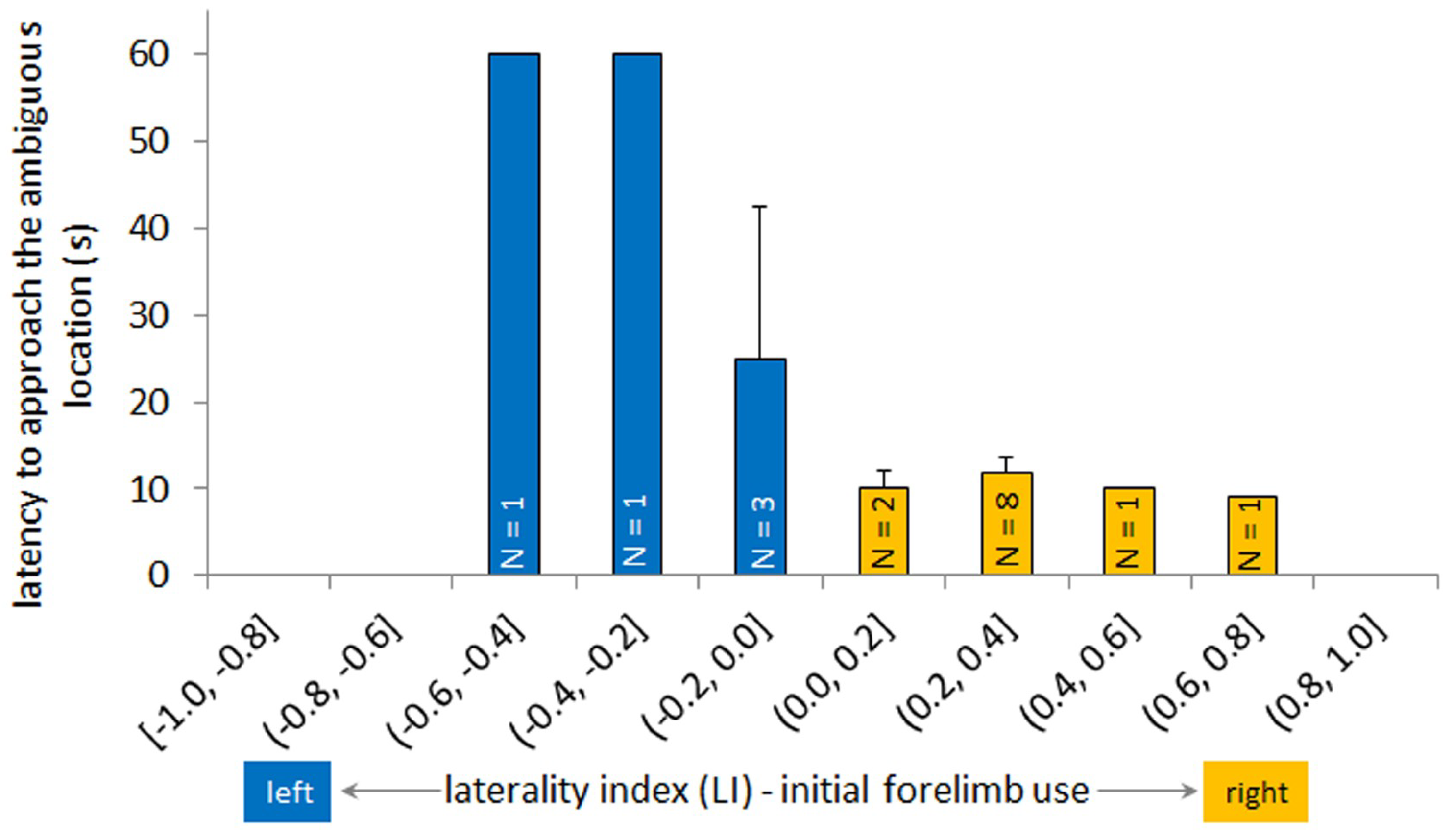
3.3. Latency to Approach Ambiguous Box and Laterality
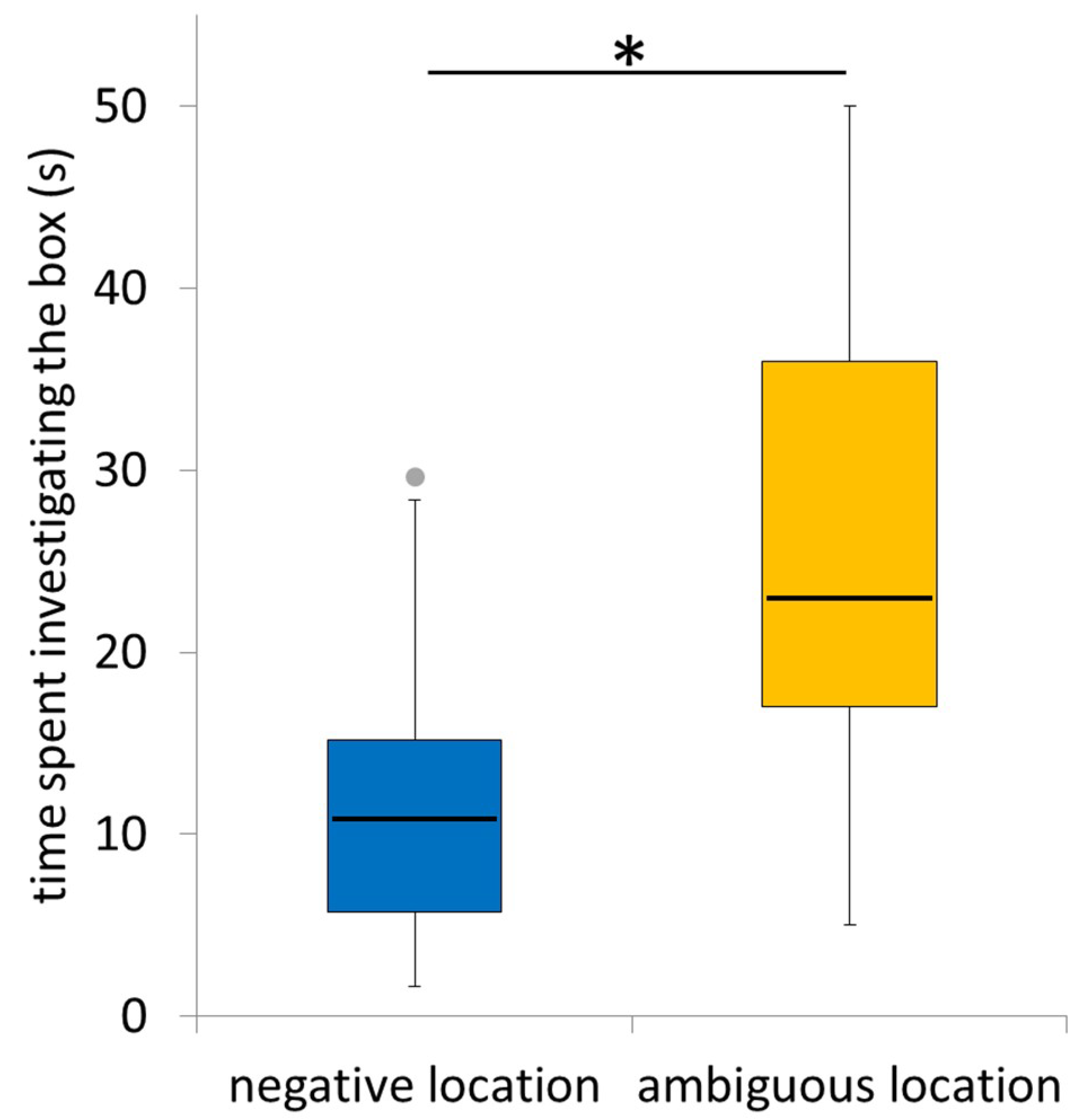
3.4. Time Spent Investigating the Ambiguous Box
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Ethics Statement
References
- Roelofs, S.; Boleij, H.; Nordquist, R.E.; van der Staay, F.J. Making decisions under ambiguity: Judgment bias tasks for assessing emotional state in animals. Front. Behav. Neurosci. 2016, 10, 119. [Google Scholar] [CrossRef] [PubMed]
- Boissy, A.; Manteuffel, G.; Jensen, M.B.; Moe, R.O.; Spruijt, B.; Keeling, L.J.; Winckler, C.; Forkman, B.; Dimitrov, I.; Langbein, J.; et al. Assessment of positive emotions in animals to improve their welfare. Physiol. Behav. 2007, 92, 375–397. [Google Scholar] [CrossRef] [PubMed]
- Paul, E.S.; Harding, E.J.; Mendl, M. Measuring emotional processes in animals: The utility of a cognitive approach. Neurosci. Biobehav. Rev. 2005, 29, 469–491. [Google Scholar] [CrossRef] [PubMed]
- Harding, E.J.; Paul, E.S.; Mendl, M. Cognitive bias and affective state: Animal behaviour. Nature 2004, 427, 312. [Google Scholar] [CrossRef] [PubMed]
- Burman, O.H.P.; Parker, R.; Paul, E.S.; Mendl, M. A spatial judgement task to determine background emotional state in laboratory rats, Rattus norvegicus. Anim. Behav. 2008, 76, 801–809. [Google Scholar] [CrossRef]
- Bateson, M.; Matheson, S. Performance on a categorisation task suggests that removal of environmental enrichment induces ‘pessimism’ in captive European starlings (Sturnus vulgaris). Anim. Welf. 2007, 16, 33–36. [Google Scholar]
- Bethell, E.; Holmes, A.; Maclarnon, A.; Semple, S. Cognitive bias in a non-human primate: Husbandry procedures influence cognitive indicators of psychological well-being in captive rhesus macaques. Anim. Welf. 2012, 21, 185–195. [Google Scholar] [CrossRef]
- Brydges, N.M.; Leach, M.; Nicol, K.; Wright, R.; Bateson, M. Environmental enrichment induces optimistic cognitive bias in rats. Anim. Behav. 2011, 81, 169–175. [Google Scholar] [CrossRef]
- Doyle, R.E.; Fisher, A.D.; Hinch, G.N.; Boissy, A.; Lee, C. Release from restraint generates a positive judgement bias in sheep. Appl. Anim. Behav. Sci. 2010, 122, 28–34. [Google Scholar] [CrossRef]
- Douglas, C.; Bateson, M.; Walsh, C.; Bédué, A.; Edwards, S.A. Environmental enrichment induces optimistic cognitive biases in pigs. Appl. Anim. Behav. Sci. 2012, 139, 65–73. [Google Scholar] [CrossRef]
- Briefer Freymond, S.; Briefer, E.F.; Zollinger, A.; Gindrat-von Allmen, Y.; Wyss, C.; Bachmann, I. Behaviour of horses in a judgment bias test associated with positive or negative reinforcement. Appl. Anim. Behav. Sci. 2014, 158, 34–45. [Google Scholar] [CrossRef]
- Löckener, S.; Reese, S.; Erhard, M.; Wöhr, A.-C. Pasturing in herds after housing in horseboxes induces a positive cognitive bias in horses. J. Vet. Behav. 2016, 11, 50–55. [Google Scholar] [CrossRef]
- Brilot, B.O.; Asher, L.; Bateson, M. Stereotyping starlings are more ‘pessimistic’. Anim. Cogn. 2010, 13, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Novak, J.; Bailoo, J.D.; Melotti, L.; Rommen, J.; Würbel, H. An exploration based cognitive bias test for mice: effects of handling method and stereotypic behaviour. PLoS ONE 2015, 10, e0130718. [Google Scholar] [CrossRef] [PubMed]
- Doyle, R.E.; Vidal, S.; Hinch, G.N.; Fisher, A.D.; Boissy, A.; Lee, C. The effect of repeated testing on judgement biases in sheep. Behav. Process. 2010, 83, 349–352. [Google Scholar] [CrossRef] [PubMed]
- Gordon, D.J.; Rogers, L.J. Cognitive bias, hand preference and welfare of common marmosets. Behav. Brain Res. 2015, 287, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Brooks, D.E.; Komaromy, A.M.; Källberg, M.E. Comparative retinal ganglion cell and optic nerve morphology. Vet. Ophthalmol. 1999, 2, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Rogers, L.J. Relevance of brain and behavioural lateralization to animal welfare. Appl. Anim. Behav. Sci. 2010, 127, 1–11. [Google Scholar] [CrossRef]
- Schultheiss, O.C.; Riebel, K.; Jones, N.M. Activity inhibition: A predictor of lateralized brain function during stress? Neuropsychology 2009, 23, 392–404. [Google Scholar] [CrossRef] [PubMed]
- McGreevy, P.D.; Thomson, P.C. Differences in motor laterality between breeds of performance horse. Appl. Anim. Behav. Sci. 2006, 99, 183–190. [Google Scholar] [CrossRef]
- Austin, N.P.; Rogers, L.J. Limb preferences and lateralization of aggression, reactivity and vigilance in feral horses, Equus caballus. Anim. Behav. 2012, 83, 239–247. [Google Scholar] [CrossRef]
- Siniscalchi, M.; Padalino, B.; Lusito, R.; Quaranta, A. Is the left forelimb preference indicative of a stressful situation in horses? Behav. Process. 2014, 107, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Farmer, K.; Krüger, K.; Byrne, R.W.; Marr, I. Sensory laterality in affiliative interactions in domestic horses and ponies (Equus caballus). Anim. Cogn. 2018, 21, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Hook-Costigan, M.A.; Rogers, L.J. Eye preferences in common marmosets (Callithrix jacchus): Influence of age, stimulus, and hand preference. Laterality Asymmetries Body Brain Cogn. 1998, 3, 109–130. [Google Scholar] [CrossRef] [PubMed]
- Marr, I.; Preissler, V.; Farmer, K.; Stefanski, V.; Krueger, K. Does stress cause changes in sensory laterality and faecal immunoglobulin a in horses (Equus caballus)? Anim. Welf. 2018. under review. [Google Scholar]
- McGreevy, P.D.; Rogers, L.J. Motor and sensory laterality in thoroughbred horses. Appl. Anim. Behav. Sci. 2005, 92, 337–352. [Google Scholar] [CrossRef]
- Farmer, K.; Krueger, K.; Byrne, R.W. Visual laterality in the domestic horse (Equus caballus) interacting with humans. Anim. Cogn. 2010, 13, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Larose, C.; Richard-Yris, M.-A.; Hausberger, M.; Rogers, L.J. Laterality of horses associated with emotionality in novel situations. Laterality Asymmetries Body Brain Cogn. 2006, 11, 355–367. [Google Scholar] [CrossRef] [PubMed]
- De Boyer Des Roches, A.; Richard-Yris, M.-A.; Henry, S.; Ezzaouïa, M.; Hausberger, M. Laterality and emotions: Visual laterality in the domestic horse (Equus caballus) differs with objects’ emotional value. Physiol. Behav. 2008, 94, 487–490. [Google Scholar] [CrossRef] [PubMed]
- Van Heel, M.C.V.; Kroekenstoel, A.M.; van Dierendonck, M.C.; van Weeren, P.R.; Back, W. Uneven feet in a foal may develop as a consequence of lateral grazing behaviour induced by conformational traits. Equine Vet. J. 2006, 38, 646–651. [Google Scholar] [CrossRef] [PubMed]
- Mendl, M.; Burman, O.H.P.; Parker, R.M.A.; Paul, E.S. Cognitive bias as an indicator of animal emotion and welfare: Emerging evidence and underlying mechanisms. Appl. Anim. Behav. Sci. 2009, 118, 161–181. [Google Scholar] [CrossRef]
- Mal, M.E.; McCall, C.A.; Newland, C.; Cummins, K.A. Evaluation of a one-trial learning apparatus to test learning ability in weanling horses. Appl. Anim. Behav. Sci. 1993, 35, 305–311. [Google Scholar] [CrossRef]
- Grzimek, B. Rechts- und Linkshändigkeit bei Pferden, Papageien und Affen. Ethology 1949, 6, 406–443. [Google Scholar] [CrossRef]
- Austin, N.P.; Rogers, L.J. Lateralization of agonistic and vigilance responses in Przewalski horses (Equus przewalskii). Appl. Anim. Behav. Sci. 2014, 151, 43–50. [Google Scholar] [CrossRef]
- Tomkins, L.M.; McGreevy, P.D.; Branson, N.J. Lack of standardization in reporting motor laterality in the domestic dog (Canis familiaris). J. Vet. Behav. 2010, 5, 235–239. [Google Scholar] [CrossRef]
- Rygula, R.; Papciak, J.; Popik, P. Trait pessimism predicts vulnerability to stress-induced anhedonia in rats. Neuropsychopharmacology 2013, 38, 2188–2196. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marr, I.; Farmer, K.; Krüger, K. Evidence for Right-Sided Horses Being More Optimistic than Left-Sided Horses. Animals 2018, 8, 219. https://doi.org/10.3390/ani8120219
Marr I, Farmer K, Krüger K. Evidence for Right-Sided Horses Being More Optimistic than Left-Sided Horses. Animals. 2018; 8(12):219. https://doi.org/10.3390/ani8120219
Chicago/Turabian StyleMarr, Isabell, Kate Farmer, and Konstanze Krüger. 2018. "Evidence for Right-Sided Horses Being More Optimistic than Left-Sided Horses" Animals 8, no. 12: 219. https://doi.org/10.3390/ani8120219
APA StyleMarr, I., Farmer, K., & Krüger, K. (2018). Evidence for Right-Sided Horses Being More Optimistic than Left-Sided Horses. Animals, 8(12), 219. https://doi.org/10.3390/ani8120219




