Hypoxia and Oxidative Stress Are Associated with Reduced Fetal Growth in Twin and Undernourished Sheep Pregnancies
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animals and Experimental Procedure
2.3. Assessment of Maternal and Feto-Placental Oxygenation Status
2.4. Assessment of Oxidative Stress Biomarkers
2.5. Statistical Analysis
3. Results
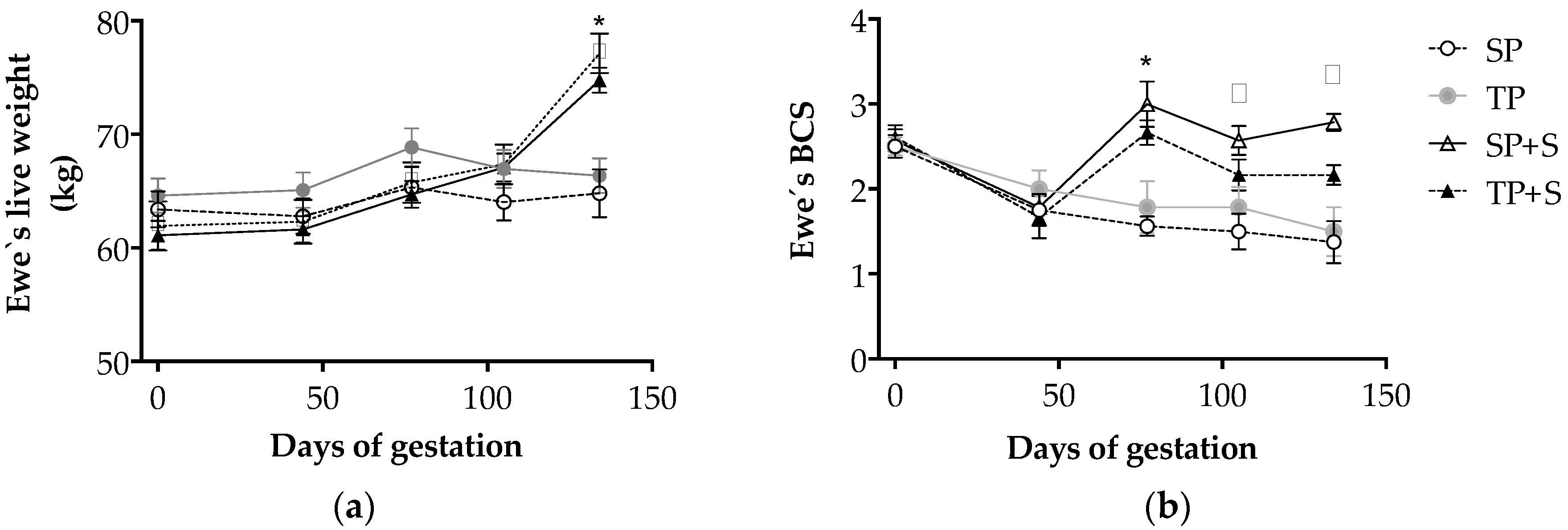
3.1. Effects of Nutrition and Twinning on Maternal Weight and Body Condition
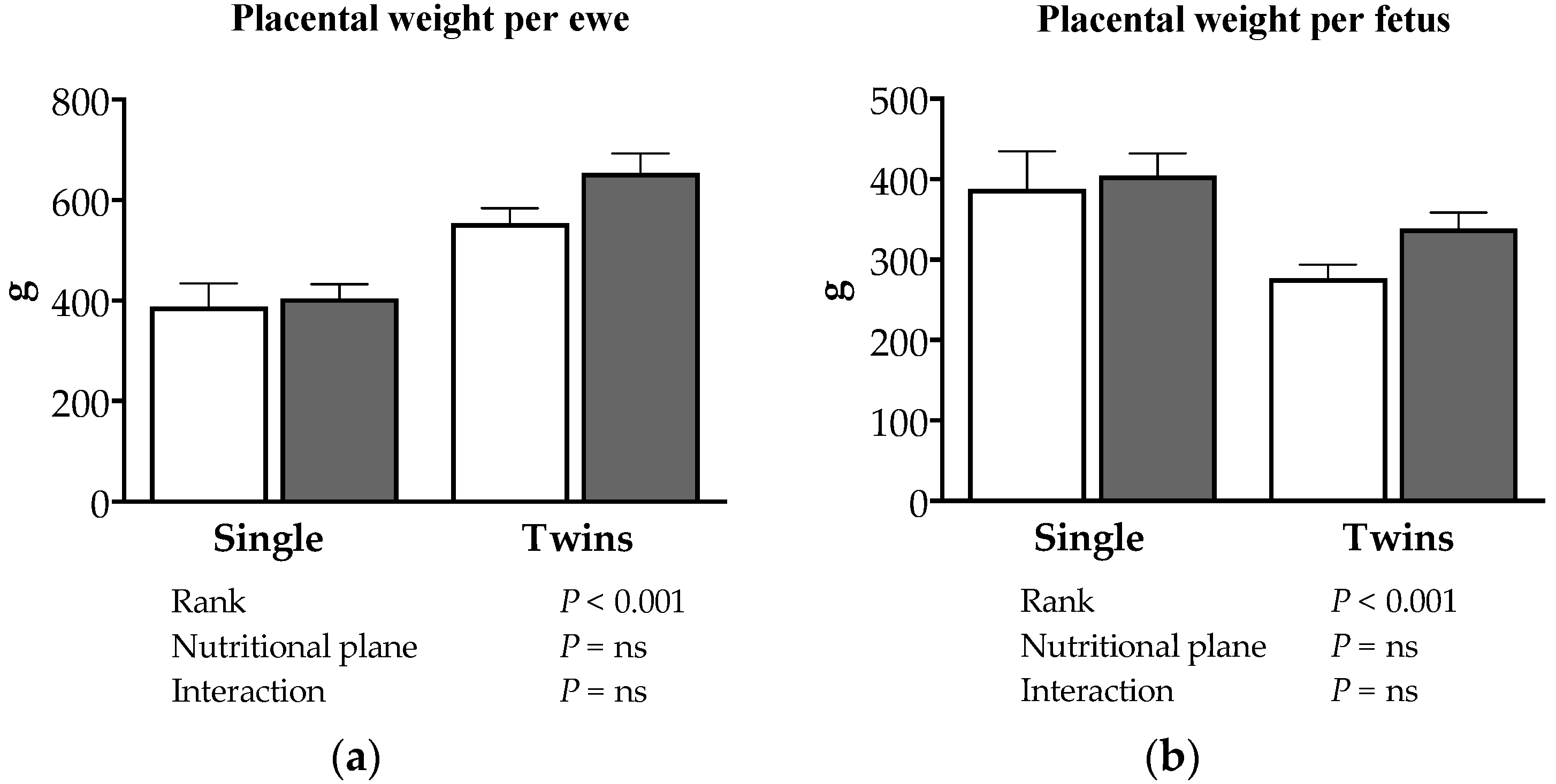
3.2. Effects of Nutrition and Twinning on Fetal and Maternal Traits
3.3. Effects of Nutrition and Twinning on Maternal and Feto-Placental Oxygenation Status
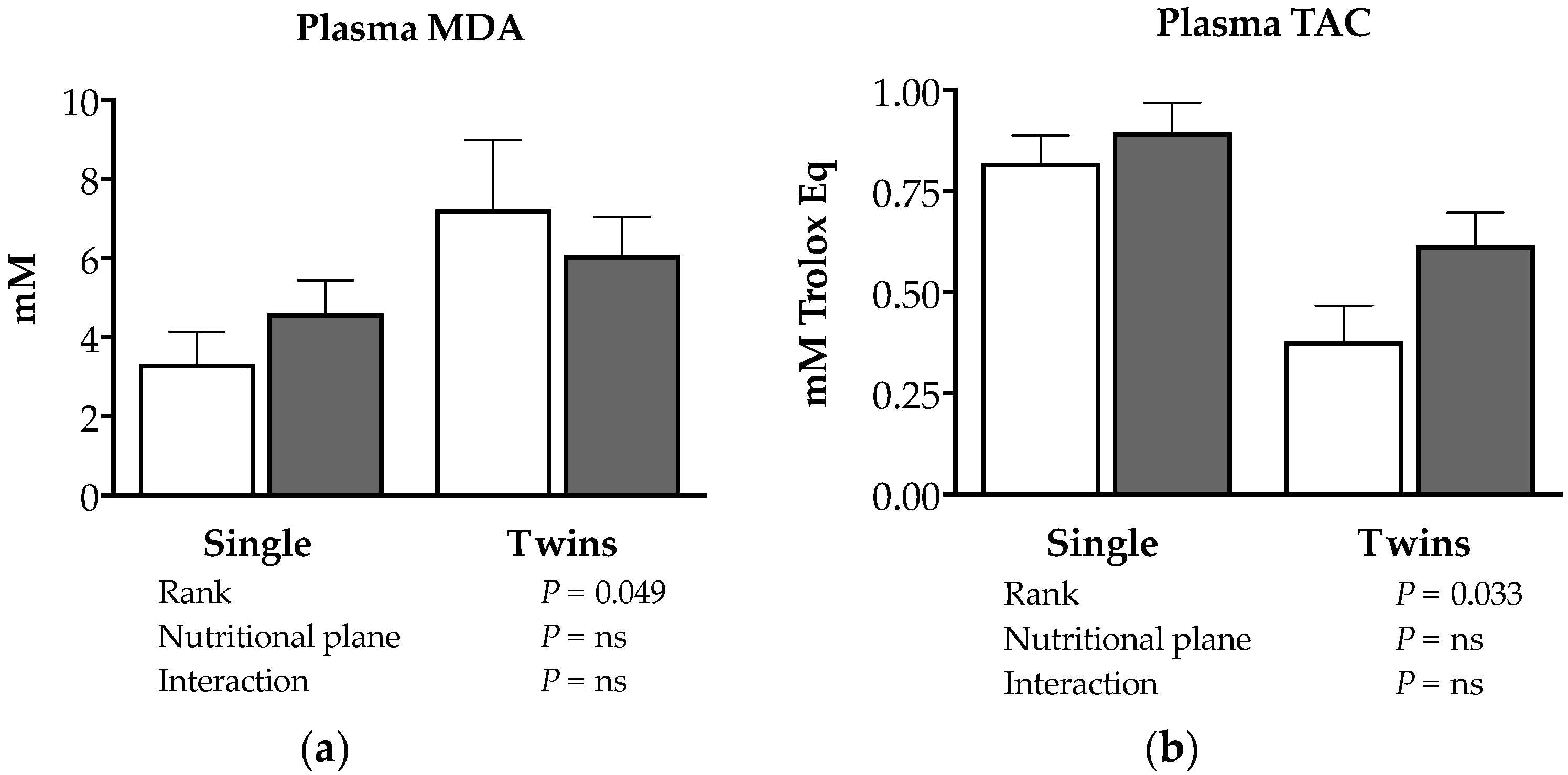
3.4. Effects of Nutrition and Twinning on Feto-Placental Stress Biomarkers
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- INE. Instituto Nacional de Estadísticas. VII Censo Nacional Agropecuario y Forestal. 2007. Available online: http://www.ine.cl/estadisticas/economicas/estad%C3%ADsticas-agropecuarias (accessed on 30 October 2018).
- Covacevich, C.N.; Ruz, J.E. Praderas en la zona austral: XII Región (Magallanes). Capítulo 39. In Praderas para Chile, 2nd ed.; Ruiz, N.I., Ed.; Ministerio de Agricultura, Instituto de Investigaciones Agropecuarias INIA: Santiago, Chile, 1996; pp. 639–655. [Google Scholar]
- Lira, R. Suplementación estratégica. In Bases Para La Producción ovina en Magallanes; Strauch, O., Lira, R., Eds.; INIA Kampenaike: Punta Arenas, Chile, 2012; pp. 92–103. [Google Scholar]
- Covacevich, N. El coironal y las necesidades de los ovinos. In Manejo Sustentable de las Praderas Naturales de Magallanes; Tierra Adentro: Edición Especial; INIA: Santiago, Chile, 2006; pp. 24–27. [Google Scholar]
- Crempien, C. Control de la mortalidad neonatal de corderos. In Cursos Avances en Producción Ovina 2001; González, M., Ed.; Serie Actas INIA N° 10; INIA: Santiago, Chile, 2001; pp. 51–67. [Google Scholar]
- Rumball, C.W.H.; Harding, J.E.; Oliver, M.H.; Bloomfield, F.H. Effects of twin pregnancy and periconceptional undernutrition on maternal metabolism, fetal growth and glucose-insulin axis function in ovine pregnancy. J. Physiol. 2008, 586, 1399–1411. [Google Scholar] [CrossRef] [PubMed]
- Van der Linden, D.S.; Sciascia, Q.; Sales, F.; McCoard, S.A. Placental nutrient transport is affected by pregnancy rank in sheep. J. Anim. Sci. 2014, 91, 644–653. [Google Scholar] [CrossRef] [PubMed]
- Freetly, H.C.; Leymaster, K.A. Relationship between litter birth weight and litter size in six breeds of sheep. J. Anim. Sci. 2004, 82, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Gootwine, E.; Spencer, T.E.; Bazer, F.W. Litter-size-dependent intrauterine growth restriction in sheep. Animal 2007, 1, 547–564. [Google Scholar] [CrossRef] [PubMed]
- Gardner, D.S.; Buttery, P.J.; Daniel, Z.; Symonds, M.E. Factors affecting birth weight in sheep: Maternal environment. Reproduction 2007, 133, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, M.L.; Reed, S.A.; Pillai, S.M.; Jones, A.K.; McFadden, K.K.; Zinn, S.A.; Govoni, K.E. Physiology and endocrinology symposium: The effects of poor maternal nutrition during gestation on offspring postnatal growth and metabolism. J. Anim. Sci. 2017, 95, 2222–2232. [Google Scholar] [CrossRef] [PubMed]
- Bloomfield, F.H.; Oliver, M.H.; Harding, J.E. Effects of twinning, birth size, and postnatal growth on glucose tolerance and hypothalamic-pituitary-adrenal function in postpubertal sheep. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E231–E237. [Google Scholar] [CrossRef] [PubMed]
- Gluckman, P.D.; Hanson, M.A. Living with the past: Evolution, development, and patterns of disease. Science 2004, 305, 1733–1736. [Google Scholar] [CrossRef] [PubMed]
- Zambrano, E.; Guzmán, C.; Rodríguez-González, G.L.; Durand-Carvajal, M.; Nathanielsz, P.W. Fetal programming of sexual development and reproductive function. Mol. Cell. Endocrinol. 2014, 382, 538–549. [Google Scholar] [CrossRef] [PubMed]
- McCoard, S.; Koolaard, J.; Charteris, A.; Luo, D. Brief communication: Effect of twinning and sex on carcass weight and composition in lambs. In Proceedings of the New Zealand Society of Animal Production, Leipzig, Germany, 1–6 August 2010; pp. 133–136. [Google Scholar]
- Nash, M.L.; Hungerford, L.L.; Nash, T.G.; Zinn, G.M. Risk factors for perinatal and postnatal mortality in lambs. Vet. Rec. 1996, 139, 64–67. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, P.L.; Cafe, L.M.; Hearnshaw, H.; Hennessy, D.W.; Thompson, J.M.; Morris, S.G. Long-term consequences of birth weight and growth to weaning on carcass, yield and beef quality characteristics of Piedmontese- and Wagyu-sired cattle. Aust. J. Exp. Agric. 2006, 46, 257–269. [Google Scholar] [CrossRef]
- Schinckel, A.P.; Einstein, M.E.; Jungst, S.; Booher, C.; Newman, S. Evaluation of the impact of pig birth weight on grow-finish performance, backfat depth, and loin depth. Prof. Anim. Sci. 2010, 26, 51–69. [Google Scholar] [CrossRef]
- Morley, R.; Moore, V.M.; Dwyer, T.; Owens, J.A.; Umstad, M.P.; Carlin, J.B. Association between erythropoietin in cord blood of twins and size at birth: Does it relate to gestational factors during labor or delivery. Pediatr. Res. 2005, 5, 680–684. [Google Scholar] [CrossRef] [PubMed]
- Westgate, J.A.; Wassink, G.; Bennet, L.; Gunn, A.J. Spontaneous hypoxia in multiple pregnancies is associated with early fetal decompensation and enhanced T-wave elevation during brief repeated cord occlusion in near-term fetal sheep. Am. J. Obstet. Gynecol. 2005, 193, 1526–1533. [Google Scholar] [CrossRef] [PubMed]
- Rurak, D.; Bessette, N.W. Changes in fetal lamb arterial blood gas and acid-base status with advancing gestation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 304, R908–R916. [Google Scholar] [CrossRef] [PubMed]
- Parraguez, V.H.; Atlagich, M.; Araneda, O.; García, C.; Muñoz, A.; De los Reyes, M.; Urquieta, B. Effects of antioxidant vitamins on newborn and placental traits in gestations at high altitude: Comparative study in high and low altitude native sheep. Reprod. Fertil. Dev. 2011, 23, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Jefferies, B.C. Body condition scoring and its use in management. Tasm. J. Agric. 1961, 32, 19–21. [Google Scholar]
- Van der Linden, D.S.; Kenyon, P.R.; Jenkinson, C.M.C.; Peterson, S.W.; Blair, H.T. Carry-over effects of ewe nutrition and birth rank during the previous pregnancy on the milking performance during the subsequent lactation of Romney ewes. Anim. Prod. Sci. 2011, 51, 102–110. [Google Scholar] [CrossRef]
- Gootwine, E. Meta-analysis of morphometric parameters of late-gestation fetal sheep developed under natural and artificial constraints. J. Anim. Sci. 2013, 91, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Merck Veterinary Manual. 2018. Available online: http://www.merckvetmanual.com (accessed on 19 January 2018).
- Danielson, L.; McMillen, I.C.; Dyer, J.L.; Morrison, J.L. Restriction of placental growth results in greater hypotensive response to α-adrenergic blockade in sheep during late gestation. J. Physiol. 2005, 563, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Cofré, E.; Peralta, O.A.; Raggi, A.; De los Reyes, M.; Sales, F.; González-Bulnes, A.; Parraguez, V.H. Ram semen deterioration by short-term exposure to high altitude is prevented by improvement of antioxidant status. Animal 2017, 12, 1007–1014. [Google Scholar] [CrossRef] [PubMed]
- Herrera, E. Metabolic adaptations in pregnancy and their implications for the availability of substrates to the fetus. Eur. J. Clin. Nutr. 2000, 54, S47–S51. [Google Scholar] [CrossRef] [PubMed]
- Quigley, S.P.; Kleemann, D.O.; Walker, S.K.; Speck, P.A.; Rudiger, S.R.; Nattrass, G.S.; DeBlasio, M.J.; Owens, J.A. Effect of variable long-term maternal feed allowance on the development of the ovine placenta and fetus. Placenta 2008, 29, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Cranston, L.M.; Kenyon, P.R.; Corner-Thomas, R.A.; Morris, T. The potential interaction between ewe body condition score and nutrition during very late pregnancy and lactation on the performance of twin-bearing ewes and their lambs. Asian-Australas. J. Anim. Sci. 2017, 30, 1270–1277. [Google Scholar] [CrossRef] [PubMed]
- Hancock, S.N.; Oliver, M.H.; McLean, C.; Jaquiery, A.L.; Bloomfield, F.H. Size at birth and adult fat mass in twin sheep are determined in early gestation. J. Physiol. 2012, 590, 1273–1285. [Google Scholar] [CrossRef] [PubMed]
- McCoard, S.A.; Sales, F.A.; Sciascia, Q.L. Invited review: Impact of specific nutrient interventions during mid-to-late gestation on physiological traits important for survival of multiple-born lambs. Animal 2017, 11, 1727–1736. [Google Scholar] [CrossRef] [PubMed]
- Zywicki, M.; Blohowiak, S.E.; Magness, R.R.; Segar, J.L.; Kling, P.J. Increasing fetal ovine number per gestation alters fetal plasma clinical chemistry values. Physiol. Rep. 2016, 4, 1–12. [Google Scholar] [CrossRef] [PubMed]
- McMillen, I.C.; Adams, M.B.; Ross, J.T.; Coulter, C.L.; Simonetta, G.; Owens, J.A.; Robinson, J.S.; Edwards, L.J. Fetal growth restriction: Adaptations and consequences. Reproduction 2001, 122, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, P.L.; Hunt, A.S.; Harmanson, J.W.; Bell, A.W. Effects of birth weight and postnatal nutrition on neonatal sheep: I. Body growth and composition, and some aspects of energetic efficiency. J. Anim. Sci. 1998, 76, 2354–2367. [Google Scholar] [CrossRef] [PubMed]
- Bhutta, Z.A.; Das, J.K.; Rizvi, A.; Gaffey, M.F.; Walker, N.; Horton, S.; Webb, P.; Lartey, A.; Black, R.E. Lancet Nutrition Interventions Review Group; Maternal and Child Nutrition Study Group. Evidence-based interventions for improvement of maternal and child nutrition: What can be done and at what cost? Lancet 2013, 382, 452–477. [Google Scholar] [CrossRef]
- Blickstein, I. Growth aberration in multiple pregnancy. Obstet. Gynecol. Clin. N. Am. 2005, 32, 39–54. [Google Scholar] [CrossRef] [PubMed]
- Ghidini, A. Idiopathic fetal growth restriction: A pathophysiologic approach. Obstet. Gynecol. Surv. 1996, 51, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Murphy, V.E.; Smith, R.; Giles, W.B.; Clifton, V.L. Endocrine regulation of human fetal growth: The role of the mother, placenta, and fetus. Endocr. Rev. 2006, 27, 141–169. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Barker, P.; Botting, K.J.; Roberts, C.T.; McMillan, C.M.; McMillen, I.C.; Morrison, J.L. Early restriction of placental growth results in placental structural and gene expression changes in late gestation independent of fetal hypoxemia. Physiol. Rep. 2016, 4, e13049. [Google Scholar] [CrossRef] [PubMed]
- Seferovic, M.D.; Gupta, M.B. Increased umbilical cord PAI-1 levels in placental insufficiency are associated with fetal hypoxia and angiogenesis. Dis. Markers 2016, 2016, 7124186. [Google Scholar] [CrossRef] [PubMed]
- Parraguez, V.H.; Atlagich, M.; Díaz, R.; Bruzzone, M.E.; Behn, C.; Raggi, L.A. Effect of hypobaric hypoxia on lamb intrauterine growth: Comparison between high and low altitude native ewes. Reprod. Fertil. Dev. 2005, 17, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Parraguez, V.H.; Atlagich, M.; Díaz, R.; Cepeda, R.; González, C.; De los Reyes, M.; Bruzzone, M.E.; Behn, C.; Raggi, L.A. Ovine placenta at high altitudes: Comparison of animals with different times of adaptation to hypoxic environment. Anim. Reprod. Sci. 2006, 95, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Parraguez, V.H.; Urquieta, B.; De los Reyes, M.; González-Bulnes, A.; Astiz, S.; Muñoz, A. Steroidogenesis in sheep pregnancy with intrauterine growth retardation by high-altitude hypoxia: Effects of maternal altitudinal status and antioxidant treatment. Reprod. Fertil. Dev. 2013, 25, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Morrison, J.L. Sheep models of intrauterine growth restriction: Fetal adaptations and consequences. Clin. Exp. Pharmacol. Physiol. 2008, 35, 730–743. [Google Scholar] [CrossRef] [PubMed]
- Herrera, E.A.; Krause, B.; Ebensperger, G.; Reyes, R.V.; Casanello, P.; Parra-Cordero, M.; Llanos, A.J. The placental pursuit for an adequate oxidant balance between the mother and the fetus. Front. Pharmacol. 2014, 5, 149. [Google Scholar] [CrossRef] [PubMed]
- Gür, S.; Türk, G.; Demirci, E.; Yüce, A.; Sönmez, M.; ÖZer, S.; Aksu, E.H. Effect of pregnancy and foetal number on diameter of corpus luteum, maternal progesterone concentration and oxidant/antioxidant balance in ewes. Reprod. Domest. Anim. 2011, 46, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Minghetti, L.; Suppiel, A.; Greco, A.; Franzoi, M.; Pascoli, I.; Zanardo, V. Oxidative stress in twin neonates is influenced by birth weight and weight discordance. Clin. Biochem. 2011, 44, 654–658. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.L.; He, Z.M.; Shi, X.M.; Gou, C.Y.; Gao, Y.; Fang, Q. Discordant HIF 1A mRNA levels and oxidative stress in placental shares of monochorionic twins with selective intra-uterine growth restriction. Placenta 2015, 36, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Narang, M.; Banerjee, B.D.; Basu, S. Oxidative stress in term small for gestational age neonates born to undernourished mothers: A case control study. BMC Pediatr. 2004, 4, 14. [Google Scholar] [CrossRef] [PubMed]
- Kamath, U.; Rao, G.; Kamath, S.U.; Rai, L. Maternal and fetal indicators of oxidative stress during intrauterine growth retardation (IUGR). Indian J. Clin. Biochem. 2006, 21, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.L.; Mark, P.J.; Waddell, B.J. Maternal dietary omega-3 fatty acids and placental function. Reproduction 2014, 147, R143–R152. [Google Scholar] [CrossRef] [PubMed]
- Biri, A.; Bozkurt, N.; Turp, A.; Kavutcu, M.; Himmetoglu, O.; Durak, I. Role of oxidative stress in intrauterine growth restriction. Gynecol. Obstet. Investig. 2007, 64, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Herrera, E.; Ortega-Senovilla, H. Lipid metabolism during pregnancy and its implications for fetal growth. Curr. Pharm. Biotechnol. 2014, 15, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Bobiński, R.; Mikulska, M. The ins and outs of maternal-fetal fatty acid metabolism. Acta Biochim. Pol. 2015, 62, 499–507. [Google Scholar] [CrossRef] [PubMed]
- McCoard, S.; Sales, F.; Sciascia, Q. Amino acids in sheep production. Front. Biosci. 2016, 1, 264–288. [Google Scholar] [CrossRef]
- McCoard, S. Issues and opportunities to capitalize on increased litter size in hill country sheep farming systems—A New Zealand perspective. Anim. Front. 2017, 7, 32–37. [Google Scholar] [CrossRef]
| p-Value | |||||||
|---|---|---|---|---|---|---|---|
| SP | SP+S | TP | TP+S | R | NP | R × NP | |
| Fetal morphometry | |||||||
| Body weight (kg) | 4.3 ± 0.2 | 4.5 ± 0.2 | 3.4 ± 0.1 | 4.0 ± 0.1 | <0.001 | 0.025 | ns |
| Thorax perimeter (cm) | 34.5 ± 0.4 | 36.1 ± 0.7 | 32.3 ± 0.6 | 34.0 ± 0.5 | 0.001 | 0.016 | ns |
| CRL (cm) | 44.8 ± 0.7 | 45.8 ± 0.6 | 40.9 ± 0.5 | 43.9 ± 0.6 | <0.001 | 0.006 | ns |
| Forelimb length (cm) | 31.5 ± 0.5 | 31.9 ± 0.4 | 28.9 ± 0.4 | 30.8 ± 0.4 | <0.001 | 0.020 | ns |
| Hindlimb length (cm) | 37.0 ± 0.5 | 37.3 ± 0.5 | 33.9 ± 0.5 | 35.2 ± 0.5 | <0.001 | ns | ns |
| Brain weight (g) | 52.9 ± 1.1 | 52.4 ± 1.5 | 52.8 ± 1.4 | 52.0 ± 1.3 | ns | ns | ns |
| Liver weight (g) | 113.5 ± 6.3 | 118.9 ± 11.4 | 74.3 ± 3.6 | 90.1 ± 3.8 | <0.001 | 0.073 | ns |
| Brain/liver weight | 0.47 ± 0.03 | 0.46 ± 0.03 | 0.73 ± 0.04 | 0.59 ± 0.02 | <0.001 | 0.017 | 0.052 |
| Fetal growth indexes | |||||||
| GI | 14.3 ± 0.6 | 14.5 ± 0.5 | 12.8 ± 0.4 | 13.5 ± 0.4 | 0.005 | ns | ns |
| BMI | 21.3 ± 0.9 | 21.4 ± 0.7 | 20.1 ± 0.6 | 20.4 ± 0.4 | 0.056 | ns | ns |
| p-Value | |||||||
|---|---|---|---|---|---|---|---|
| SP | SP+S | TP | TP+S | R | NP | R × NP | |
| Maternal blood | |||||||
| PaO2 (mm Hg) | 93.5 ± 2.5 | 93.1 ± 7.2 | 90.8 ± 7.8 | 91.4 ± 5.1 | ns | ns | ns |
| PaCO2 (mm Hg) | 43.3 ± 2.3 | 38.9 ± 1.7 | 44.2 ± 8.2 | 41.8 ± 2.3 | ns | ns | ns |
| Hb (g/dL) | 10.7 ± 0.3 | 11.0 ± 0.4 | 10.9 ± 0.3 | 10.8 ± 0.2 | ns | ns | ns |
| SatHb (%) | 91.8 ± 0.3 | 91.1 ± 1.4 | 92.3 ± 0.4 | 91.8 ± 0.3 | ns | ns | ns |
| pH | 7.44 ± 0.02 | 7.46 ± 0.02 | 7.45 ± 0.04 | 7.46 ± 0.02 | ns | ns | ns |
| Umbilical cord blood | |||||||
| PO2 (mm Hg) | 27.1 ± 1.8 | 34.1 ± 2.6 | 22.2 ± 1.7 | 26.8 ± 1.7 | 0.004 | 0.006 | ns |
| PCO2 (mm Hg) | 43.2 ± 0.8 | 39.5 ± 1.5 | 45.5 ± 1.2 | 43.2 ± 2.0 | ns | ns | ns |
| Hb (g/dL) | 9.6 ± 0.2 | 10.0 ± 0.5 | 10.9 ± 0.4 | 10.6 ± 0.3 | 0.023 | ns | ns |
| SatHb (%) | 43.9 ± 4.3 | 61.0 ± 6.0 | 36.4 ± 4.1 | 43.4 ± 4.2 | 0.025 | 0.019 | ns |
| pH | 7.44 ± 0.01 | 7.45 ± 0.02 | 7.43 ± 0.01 | 7.43 ± 0.01 | ns | ns | ns |
| O2 cont. (mL/dL) | 6.0 ± 0.4 | 8.3 ± 0.8 | 5.6 ± 0.5 | 6.5 ± 0.5 | 0.050 | 0.016 | ns |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sales, F.; Peralta, O.A.; Narbona, E.; McCoard, S.; De los Reyes, M.; González-Bulnes, A.; Parraguez, V.H. Hypoxia and Oxidative Stress Are Associated with Reduced Fetal Growth in Twin and Undernourished Sheep Pregnancies. Animals 2018, 8, 217. https://doi.org/10.3390/ani8110217
Sales F, Peralta OA, Narbona E, McCoard S, De los Reyes M, González-Bulnes A, Parraguez VH. Hypoxia and Oxidative Stress Are Associated with Reduced Fetal Growth in Twin and Undernourished Sheep Pregnancies. Animals. 2018; 8(11):217. https://doi.org/10.3390/ani8110217
Chicago/Turabian StyleSales, Francisco, Oscar A. Peralta, Eileen Narbona, Sue McCoard, Mónica De los Reyes, Antonio González-Bulnes, and Víctor H. Parraguez. 2018. "Hypoxia and Oxidative Stress Are Associated with Reduced Fetal Growth in Twin and Undernourished Sheep Pregnancies" Animals 8, no. 11: 217. https://doi.org/10.3390/ani8110217
APA StyleSales, F., Peralta, O. A., Narbona, E., McCoard, S., De los Reyes, M., González-Bulnes, A., & Parraguez, V. H. (2018). Hypoxia and Oxidative Stress Are Associated with Reduced Fetal Growth in Twin and Undernourished Sheep Pregnancies. Animals, 8(11), 217. https://doi.org/10.3390/ani8110217






