Predation by Red Foxes (Vulpes vulpes) at an Outdoor Piggery
Abstract
:Simple Summary
Abstract
1. Introduction
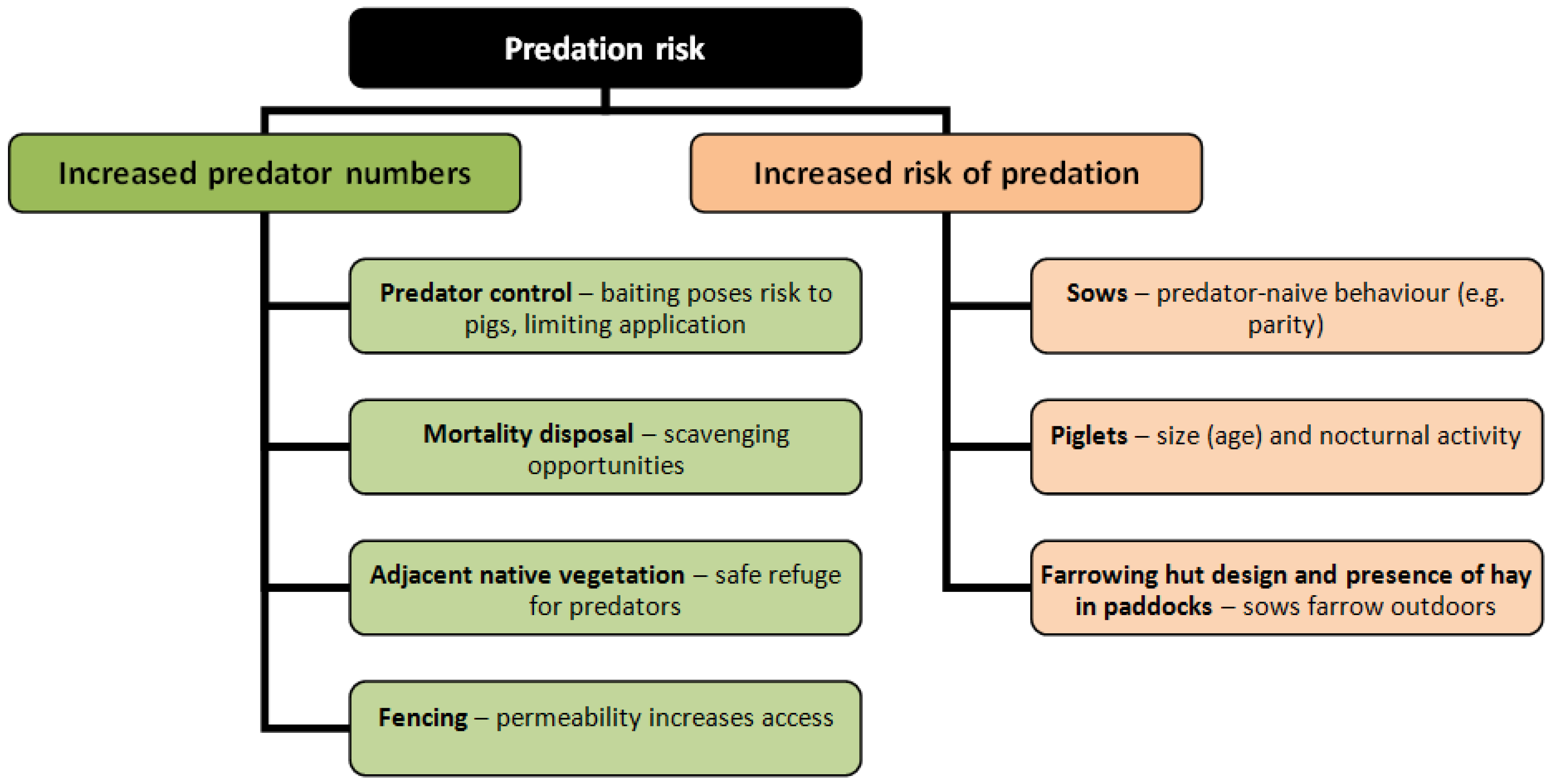
- Identify the level of threat posed by fox predation using infra-red camera trap monitoring to determine the level of fox presence in and around the outdoor piggery, and test whether there was a temporal and spatial overlap between piglets and foxes.
- Use piggery records of births and weaning to identify whether piglets were more vulnerable to fox predation at a particular age, whether predation risk increased with increased proximity to native vegetation, or between first time (gilts) and experienced sows.
- Carry out a preliminary study to identify factors that would influence the efficiency of control baiting.
2. Methods
2.1. Identify the Level of Threat Posed by Fox Predation
2.2. Farrowing and Weaning Records—Factors that Increase Risk of Fox Predation
2.3. Preliminary Trials to Test Bait Presentation and Placement
3. Results
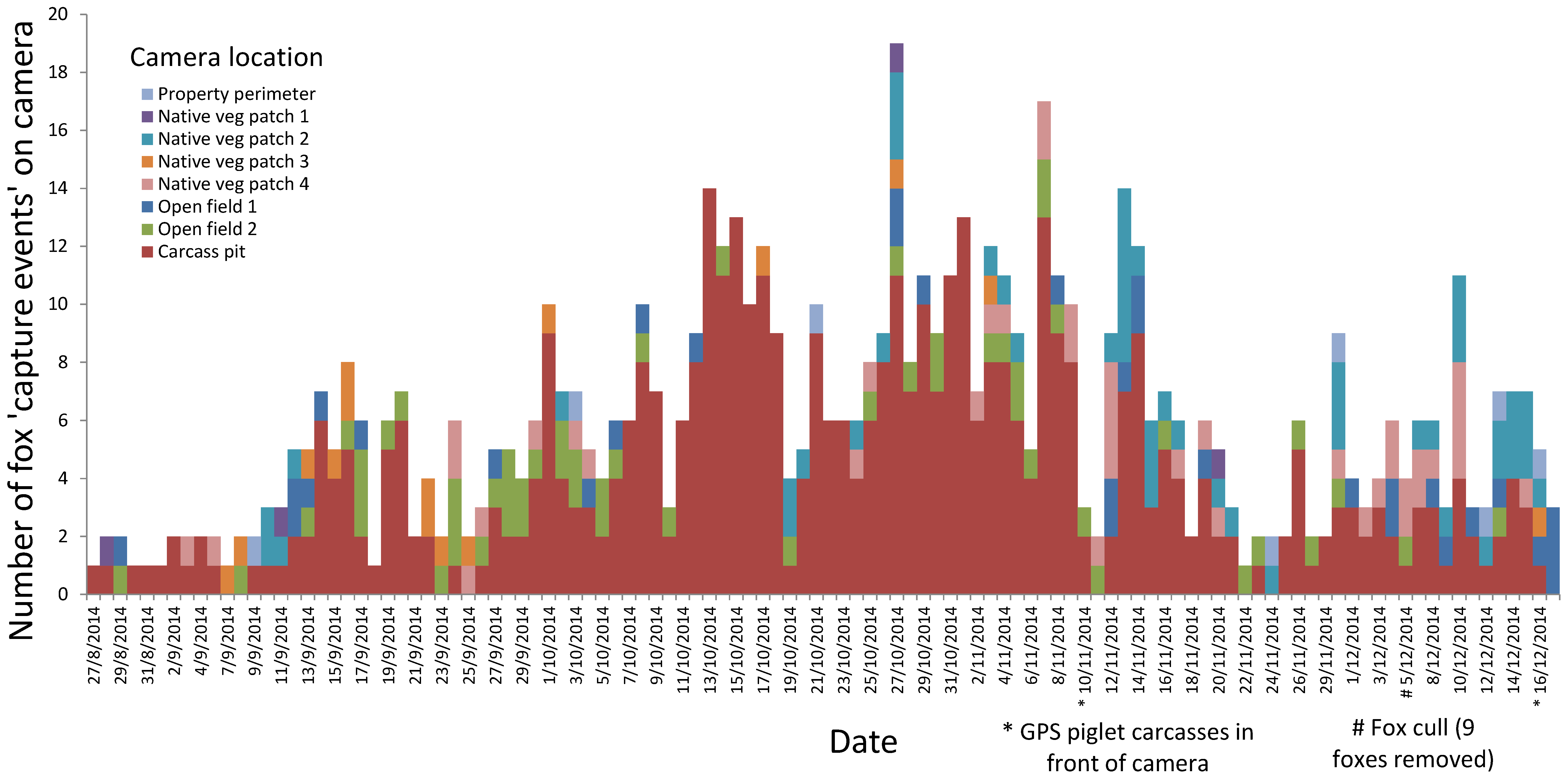
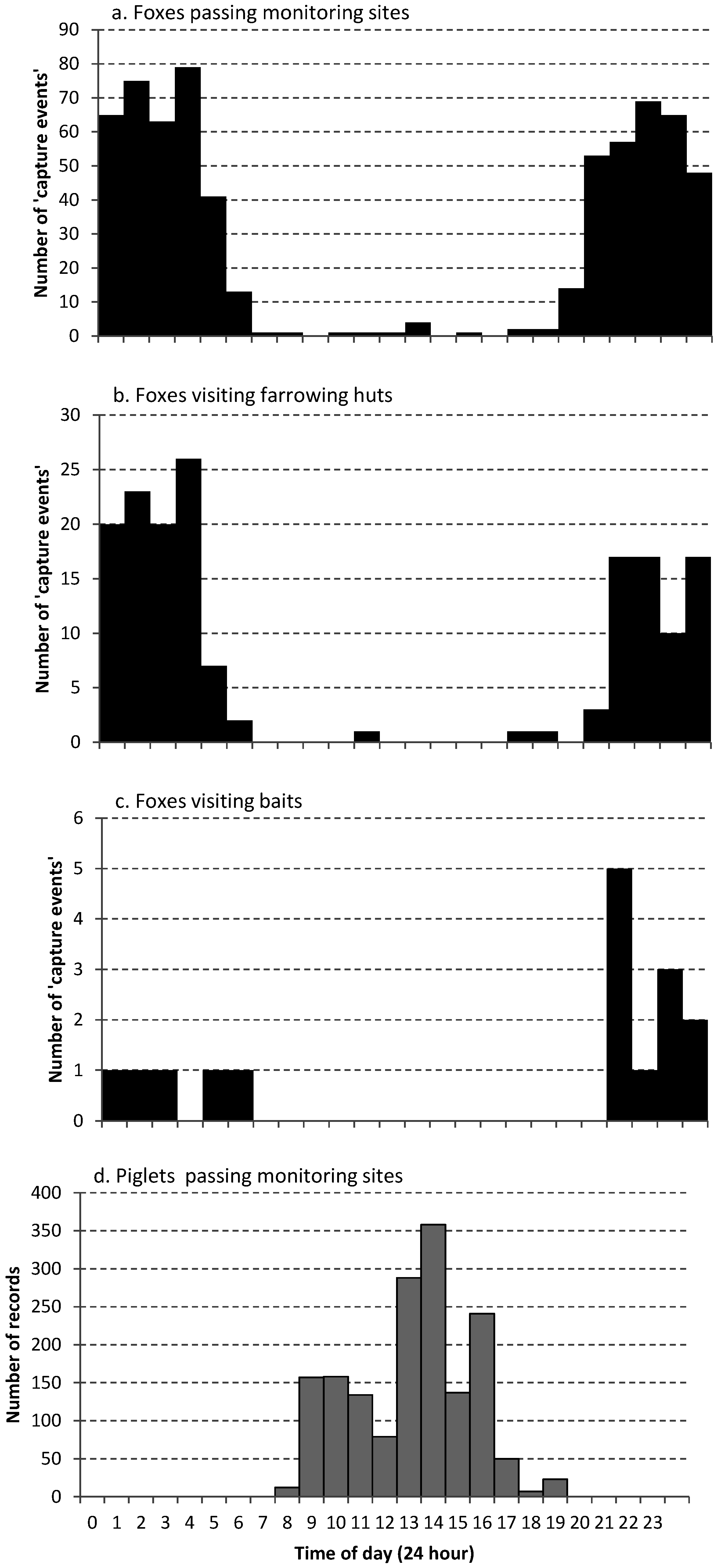
3.1. Identify the Level of Threat Posed by Fox Predation
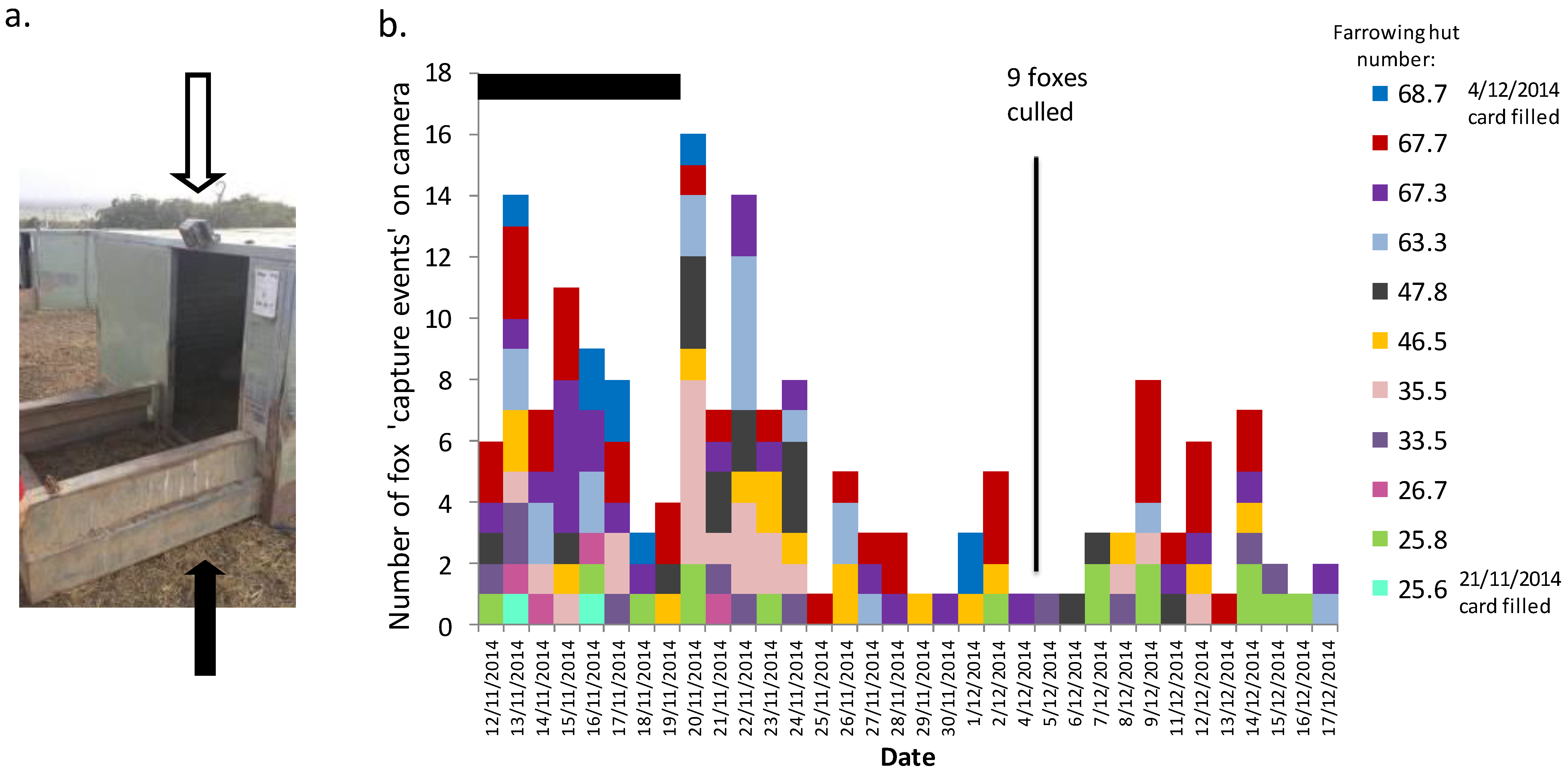
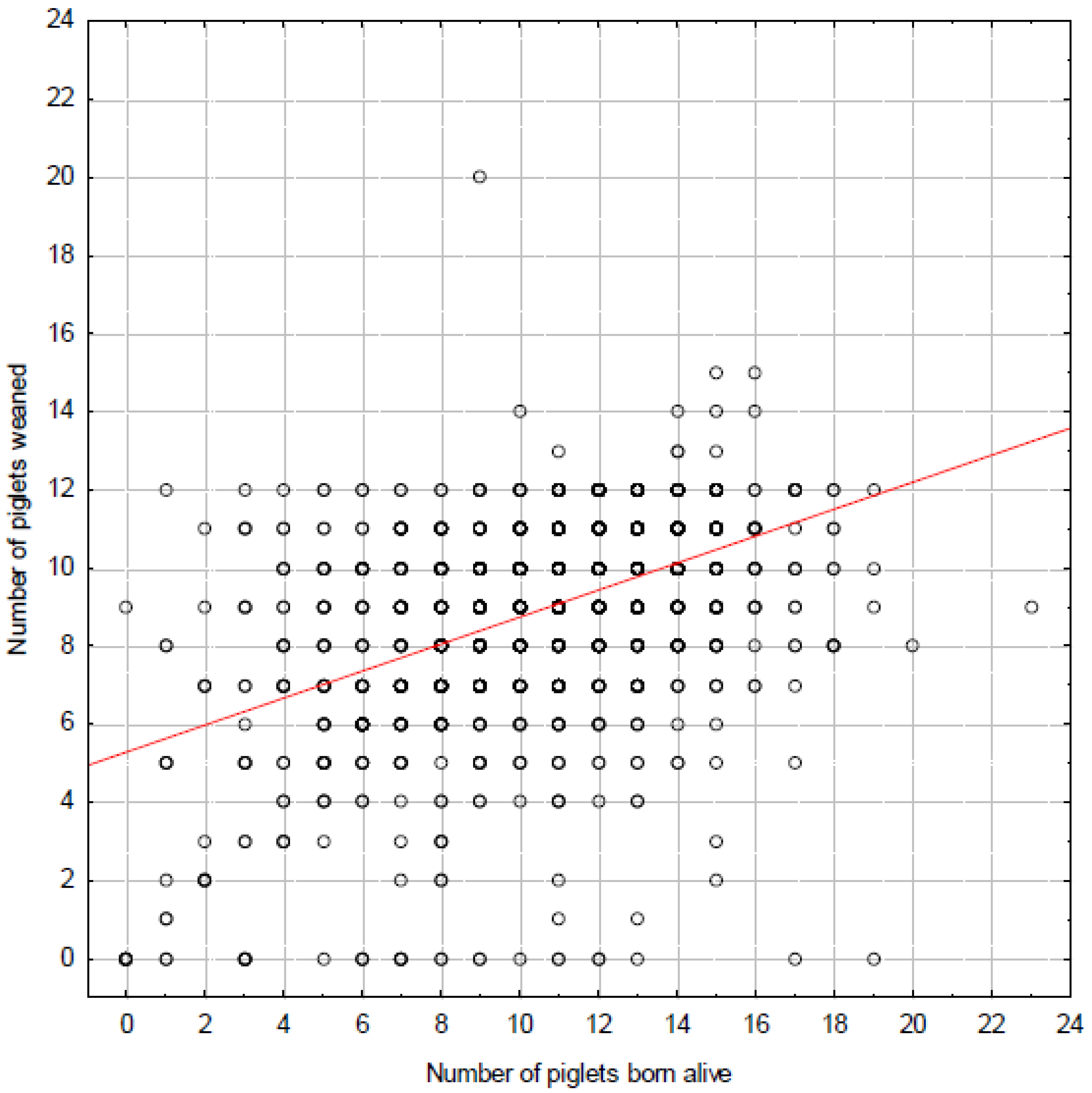
3.2. Farrowing and Weaning Records—Factors that Increase Risk of Fox Predation
3.3. Preliminary Trials to Test Bait Presentation and Placement
4. Discussion
4.1. Identify the Level of Threat
4.2. Farrowing and Weaning Records—Factors that Increase Risk of Fox Predation
4.3. Preliminary Trials to Test Bait Presentation and Placement
4.4. Other Management Options to Reduce Predation Losses
4.4.1. Sows Farrowing in Paddocks at Night and Sows Feeding Piglets outside Farrowing Huts at Night
4.4.2. Carcass Pit
4.4.3. Property and Paddock Fencing
4.4.4. Guardian Animals
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rousing, T.; Bonde, M.; Sørensen, J.T. Aggregating welfare indicators into an operational welfare assessment system: A bottom-up approach. Acta Agric. Scand. Sec. A Anim. Sci. 2001, 51, 53–57. [Google Scholar]
- Colditz, I.G.; Ferguson, D.M.; Collins, T.; Matthews, L.; Hemsworth, P.H. A prototype tool to enable farmers to measure and improve the welfare performance of the farm animal enterprise: The unified field index. Animals 2014, 4, 446–462. [Google Scholar] [CrossRef] [PubMed]
- Feenstra, A.A. A health monitoring study in organic pig herds. In Proceedings of the NJF-Seminar No. 303; Ecological Animal Husbandry in the Nordic Countries, Horsens, Denmark, 16–17 September 1999; Danish Research Centre for Organic Farming: Foulum, Denmark, 2000. [Google Scholar]
- Prunier, A.; Lubac, S.; Mejer, H.; Roepstorff, A.; Edwards, S. Health, welfare and production problems in organic suckling piglets. Org. Agric. 2014, 4, 107–121. [Google Scholar] [CrossRef]
- Kijlstra, A.; Eissen, O.A.; Cornelissen, J.; Munniksma, K.; Eijck, I.; Kortbeek, T. Toxoplasma gondii infection in animal-friendly pig production systems. Investig. Ophthalmol. Vis. Sci. 2004, 45, 3165–3169. [Google Scholar] [CrossRef] [PubMed]
- Phillips, N.D.; La, T.; Adams, P.J.; Harland, B.L.; Fenwick, S.G.; Hampson, D.J. Detection of Brachyspira hyodysenteriae, Lawsonia intracellularis and Brachyspira pilosicoli in feral pigs. Vet. Microbiol. 2009, 134, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Abril, C.; Thomann, A.; Grosclaude, E.; Doherr, M.G.; Boujon, P.; Ryser-Degiorgis, M.P. Risk factors for contacts between wild boar and outdoor pigs in Switzerland and investigations on potential Brucella suis spill-over. BMC Vet. Res. 2012, 8, 116. [Google Scholar] [CrossRef] [PubMed]
- Köppel, C.; Knopf, L.; Ryser, M.-P.; Miserez, R.; Thür, B.; Stärk, K.D.C. Serosurveillance for selected infectious disease agents in wild boars (Sus scrofa) and outdoor pigs in Switzerland. Eur. J. Wildl. Res. 2007, 53, 212–220. [Google Scholar] [CrossRef]
- Barnett, J.L.; Hemsworth, P.H.; Cronin, G.M.; Jongman, E.C.; Hutson, G.D. A review of the welfare issues for sows and piglets in relation to housing. Crop Pasture Sci. 2000, 52, 1–28. [Google Scholar] [CrossRef]
- Edwards, S.A.; Smith, W.J.; Fordyce, C.; MacMenemy, F. An analysis of the causes of piglet mortality in a breeding herd kept outdoors. Vet. Rec. 1994, 135, 324–327. [Google Scholar] [CrossRef] [PubMed]
- Kelly, H.R.C.; Browning, H.M.; Martins, A.P.; Pearce, G.P.; Stopes, C.; Edwards, S.A. Breeding and feeding pigs for organic production. In Proceedings of the 4th Network for Animal Health and Welfare in Organic Agriculture (NAHWOA) Workshop; Breeding and Feeding for Animal Health and Welfare in Organic Livestock Systems, Wageningen, The Netherlands, 24–27 March 2001.
- Leirs, H.; Lodal, J.; Knorr, M. Factors correlated with the presence of rodents on outdoor pig farms in Denmark and suggestions for management strategies. NJAS-Wagening. J. Life Sci. 2004, 52, 145–161. [Google Scholar] [CrossRef]
- Australian Pork Limited. Annual Report 2015–2016. Available online: http://australianpork.com.au/latest-news/annual-report-2015-2016/ (accessed on 6 October 2016).
- Aussie Pig Farming (APL), 2016. Available online: http://aussiepigfarmers.com.au/ (accessed on 3 October 2016).
- Welbourne, D.J.; MacGregor, C.; Paull, D.; Lindenmayer, D.B. The effectiveness and cost of camera traps for surveying small reptiles and critical weight range mammals: A comparison with labour-intensive complementary methods. Wildl. Res. 2015, 42, 414–425. [Google Scholar] [CrossRef]
- Bateman, P.W.; Fleming, P.A. Big city life: Carnivores in urban environments. J. Zool. Lond. 2012, 287, 1–23. [Google Scholar] [CrossRef]
- Andersson, A.; Valros, A.; Rombin, J.; Jensen, P. Extensive infanticide in enclosed European wild boars (Sus scrofa). Appl. Anim. Behav. Sci. 2011, 134, 184–192. [Google Scholar] [CrossRef]
- Rowley, I. An evaluation of predation by ‘crows’ on young lambs. Wildl. Res. 1969, 14, 153–179. [Google Scholar] [CrossRef]
- Martin, J.M.; French, K.; Major, R.E. The pest status of Australian white ibis (Threskiornis molucca) in urban situations and the effectiveness of egg-oil in reproductive control. Wildl. Res. 2007, 34, 319–324. [Google Scholar] [CrossRef]
- Carrick, R. The food and feeding habits of the Straw-necked Ibis, Threskiornis spinicollis (Jameson), and the White Ibis, T. molucca (Cuvier) in Australia. Wildl. Res. 1959, 4, 69–92. [Google Scholar] [CrossRef]
- Williams, A.J.; Ward, V.L. Sacred Ibis and Gray Heron predation of Cape Cormorant eggs and chicks; and a review of Ciconiiform birds as seabird predators. Waterbirds 2006, 29, 321–327. [Google Scholar] [CrossRef]
- Pearson, H. Understanding and mitigating the risk of pathogen transmission from wild animals to domestic pigs in Australia. In Faculty of Veterinary Science; University of Sydney: Sydney, Australia, 2012. [Google Scholar]
- Brooker, M.G.; Ridpath, M.G. The diet of the Wedge-Tailed Eagle, Aquila audax, in Western Australia. Wildl. Res. 1980, 7, 433–452. [Google Scholar] [CrossRef]
- Leopole, A.S.; Wolfe, T.O. Food habits of nesting Wedge-Tailed Eagles, Aquila audax, in south-eastern Australia. Wildl. Res. 1970, 15, 1–17. [Google Scholar] [CrossRef]
- Olsen, J.; Fuentes, E.; Rose, A.B. Trophic relationships between neighbouring White-bellied Sea-Eagles (Haliaeetus leucogaster) and Wedge-tailed Eagles (Aquila audax) breeding on rivers and dams near Canberra. Emu 2006, 106, 193–201. [Google Scholar] [CrossRef]
- Fleming, P.J.S. Uptake of baits by red foxes (Vulpes vulpes): Implications for rabies contingency planning in Australia. Wildl. Res. 1997, 24, 335–346. [Google Scholar] [CrossRef]
- Towerton, A.L.; Dickman, C.R.; Kavanagh, R.P.; Penman, T.D. Control of the red fox in remnant forest habitats. Wildl. Res. 2016, 43, 169–177. [Google Scholar] [CrossRef]
- Gentle, M.N.; Saunders, G.R.; Dickman, C.R. Poisoning for production: How effective is fox baiting in south-eastern Australia? Mamm. Rev. 2007, 37, 177–190. [Google Scholar] [CrossRef]
- Coman, B.J. The age structure of a sample of red foxes (Vulpes vulpes L.) taken by hunters in Victoria. Wildl. Res. 1988, 15, 223–229. [Google Scholar] [CrossRef]
- Saunders, G.; Coman, B.; Kinnear, J.; Braysher, M. Managing Vertebrate Pests: Foxes; Australian Government Publishing Service: Canberra, Australia, 1995.
- Saunders, G.R.; Gentle, M.N.; Dickman, C.R. The impacts and management of foxes Vulpes vulpes in Australia. Mamm. Rev. 2010, 40, 181–211. [Google Scholar] [CrossRef]
- Allen, L.R.; Fleming, P.J.S.; Thompson, J.A.; Strong, A. Effect of presentation on the attractiveness and palatability to wild dogs and other wildlife of two unpoisoned wild-dog bait types. Aust. Wildl. Res. 1989, 16, 593–598. [Google Scholar] [CrossRef]
- Thomson, P.C.; Kok, N.E. The fate of dried meat baits laid for fox control: The effects of bait presentation on take by foxes and non-target species, and on caching by foxes. Wildl. Res. 2002, 29, 371–377. [Google Scholar] [CrossRef]
- Moseby, K.E.; Read, J.L.; Galbraith, B.; Hill, B.M. The use of poison baits to control feral cats and red foxes in arid South Australia II. Bait type, placement, lures and non-target uptake. Wildl. Res. 2011, 38, 350–358. [Google Scholar] [CrossRef]
- Saunders, G.; McLeod, S.; Kay, B. Degradation of sodium monofluoroacetate (1080) in buried fox baits. Wildl. Res. 2000, 27, 129–135. [Google Scholar] [CrossRef]
- Tucker, R.W.; O’Keefe, M.F. National Environmental Guidelines for Rotational Outdoor Piggeries; FSA Consulting Report 7634/2; 10 August 2012; FSA Consulting: Horsham, PA, USA, 2013.
- Somers, M.J.; Hayward, M. Fencing for Conservation; Springer: New York, NY, USA, 2012. [Google Scholar]
- Mahoney, S.; Charry, A.A. The use of alpacas as new-born lamb protectors to minimise fox predation. Ext. Farming Syst. J. 2005, 1, 65–70. [Google Scholar]
- Van Bommel, L. Guardian Dogs: Best Practice Manual for the Use of Livestock Guardian Dogs; Invasive Animals Cooperative Research Centre: Canberra, Australia, 2010. [Google Scholar]
- Edwards, S.A. Perinatal mortality in the pig: Environmental or physiological solutions? Livest. Prod. Sci. 2002, 78, 3–12. [Google Scholar] [CrossRef]


| df | df | ||||
|---|---|---|---|---|---|
| Effect | Effect type | Effect | Error | F | P |
| Sow ID | Random | 455 | 936 | 1.28 | 0.001 |
| Parity | Fixed (covariate) | 1 | 467 | 0.37 | 0.544 |
| Paddock ID | Random | 68 | 869 | 1.00 | 0.475 |
| Distance to native vegetation | Fixed (covariate) | 1 | 30 | 0.06 | 0.816 |
| Position on farm | Fixed | 2 | 30 | 2.38 | 0.110 |
| Open Sites | Forest Sites | ||||
|---|---|---|---|---|---|
| Species | Action | Buried | Surface-Laid | Buried | Surface-Laid |
| Fox | Visited | 6 | 2 | 1 | 1 |
| Took bait | - | - | - | 1 | |
| Raven | Took bait | 3 | 2 | 3 | |
| Total baits deployed | 9 | 9 | 9 | 9 | |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fleming, P.A.; Dundas, S.J.; Lau, Y.Y.W.; Pluske, J.R. Predation by Red Foxes (Vulpes vulpes) at an Outdoor Piggery. Animals 2016, 6, 60. https://doi.org/10.3390/ani6100060
Fleming PA, Dundas SJ, Lau YYW, Pluske JR. Predation by Red Foxes (Vulpes vulpes) at an Outdoor Piggery. Animals. 2016; 6(10):60. https://doi.org/10.3390/ani6100060
Chicago/Turabian StyleFleming, Patricia A., Shannon J. Dundas, Yvonne Y. W. Lau, and John R. Pluske. 2016. "Predation by Red Foxes (Vulpes vulpes) at an Outdoor Piggery" Animals 6, no. 10: 60. https://doi.org/10.3390/ani6100060
APA StyleFleming, P. A., Dundas, S. J., Lau, Y. Y. W., & Pluske, J. R. (2016). Predation by Red Foxes (Vulpes vulpes) at an Outdoor Piggery. Animals, 6(10), 60. https://doi.org/10.3390/ani6100060






