Evidence for a Role of Prolactin in Mediating Effects of Photoperiod during the Dry Period
Abstract
:1. Introduction
2. Materials and Methods
2.1. General
| Far off Diet d −60 to d −22 | Close up Diet d −21 to 0 | Lactation Diet d 1 to 120 | |
|---|---|---|---|
| Ingredients % of DM | |||
| Corn Silage | 21.1 | 38.2 | 24.7 |
| Alfalfa Silage | 41.7 | 6.6 | 15.6 |
| Alfalfa Hay | -- | 10.1 | 7.8 |
| Protein Blend | 11.1 | 45.2 | 7.1 |
| Wheat Straw | 26.2 | -- | -- |
| Soy Hulls | -- | -- | 4.0 |
| Wet Brewers Grains | -- | -- | 12.3 |
| Cotton Seed | -- | -- | 7.6 |
| Ground Corn | -- | -- | 20.8 |
| Chemical Composition | |||
| Dry matter | 54.6 | 56.0 | 52.2 |
| Crude Protein | 11.9 | 15.2 | 17.7 |
| Available Protein | 10.9 | 14.3 | 16.7 |
| ADICP | 1.0 | 0.8 | 1.0 |
| Adjusted CP | 11.9 | 15.2 | 17.7 |
| ADF | 38.9 | 28.3 | 26.5 |
| NDF | 57.0 | 42.9 | 39.1 |
| TDN | 63.8 | 68.3 | 68.9 |
| NEG or NEL (Mcal/kg) | 0.81 | 0.96 | 1.62 |
| Calcium | 0.8 | 0.9 | 0.8 |
| Phosphorus | 0.2 | 0.3 | 0.4 |
| Magnesium | 0.2 | 0.3 | 0.3 |
| Potassium | 1.9 | 1.5 | 1.4 |
| Sodium | 0.1 | 0.1 | 0.4 |
| Iron (PPM) | 500 | 418 | 279 |
| Zinc (PPM) | 83 | 129 | 100 |
| Copper (PPM) | 15.5 | 18.5 | 17.5 |
| Manganese (PPM) | 115.5 | 94.5 | 83.5 |
| Molybdenum (PPM) | 0.6 | 0.7 | 0.9 |
2.2. Blood Collection and Assays
2.3. Non-Esterified Fatty Acids (NEFA) and Beta-Hydroxybutyrate (BHBA) Assays
2.4. Implants
2.5. Milk Production and Sampling
2.6. Physical Exams
2.7. Mammary Biopsy
2.8. Mammary Proliferation Assay
2.9. DNA Assay
2.10. Statistical Analysis
3. Results
3.1. Prolactin
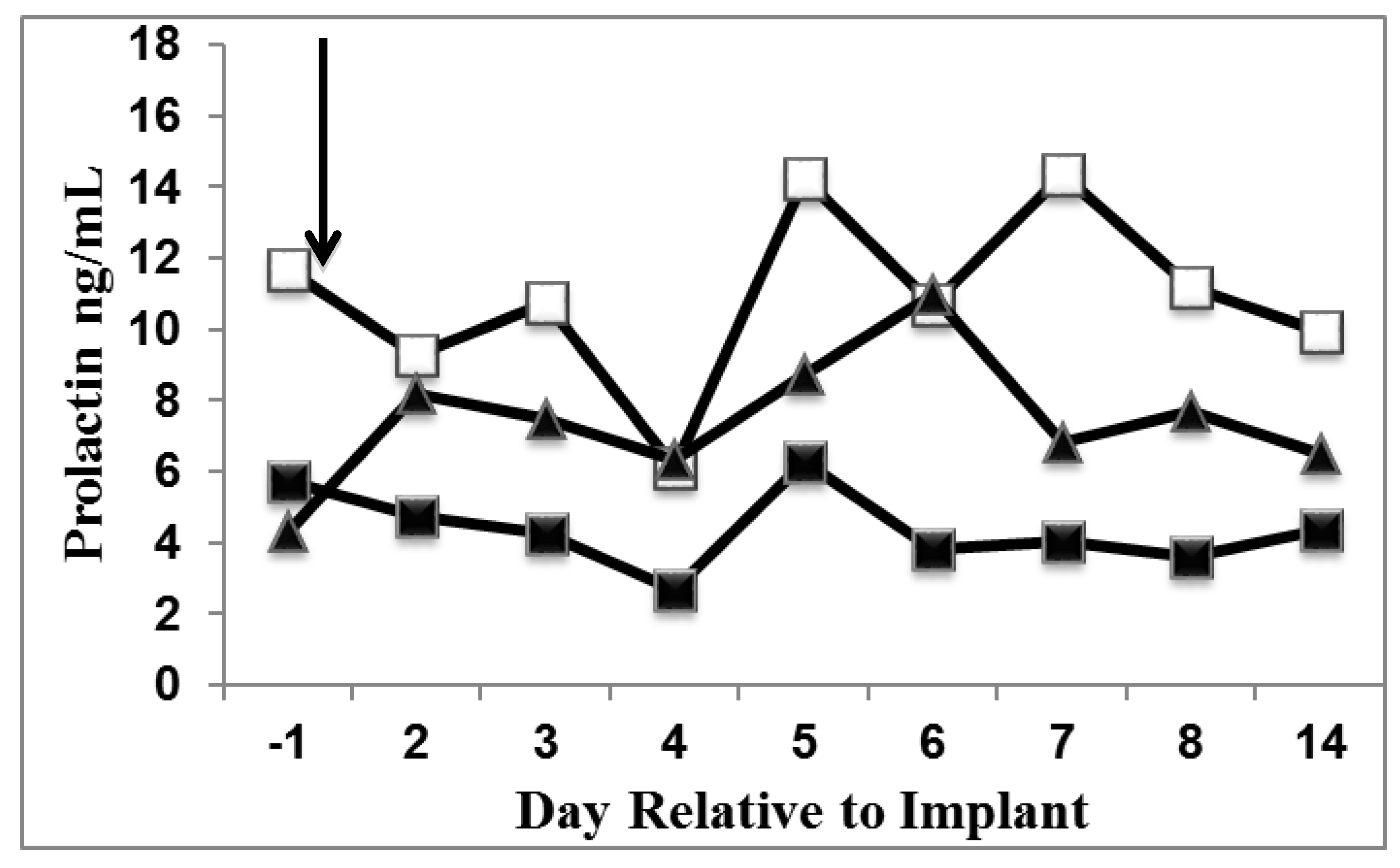
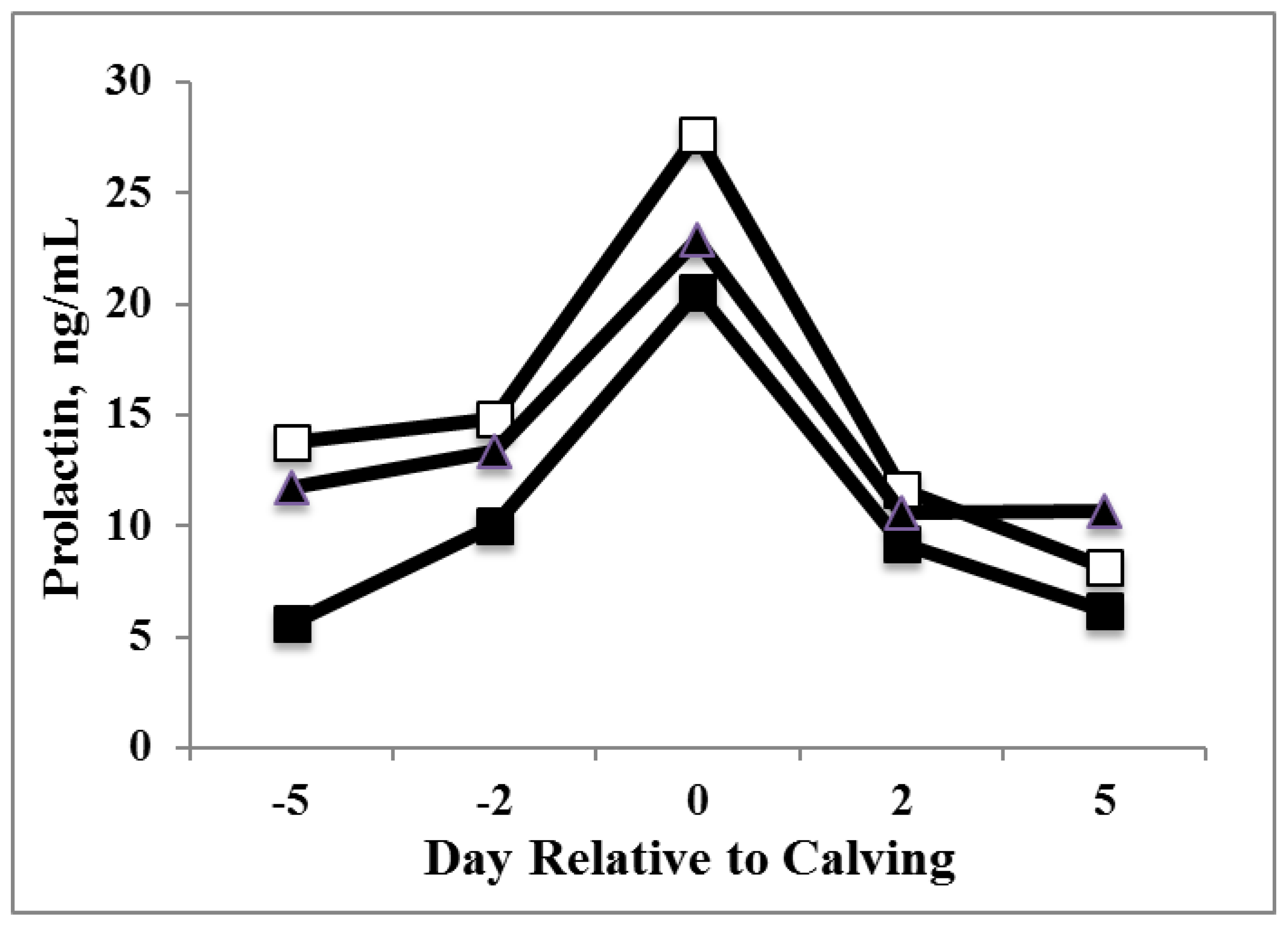
3.2. Milk Production and DMI
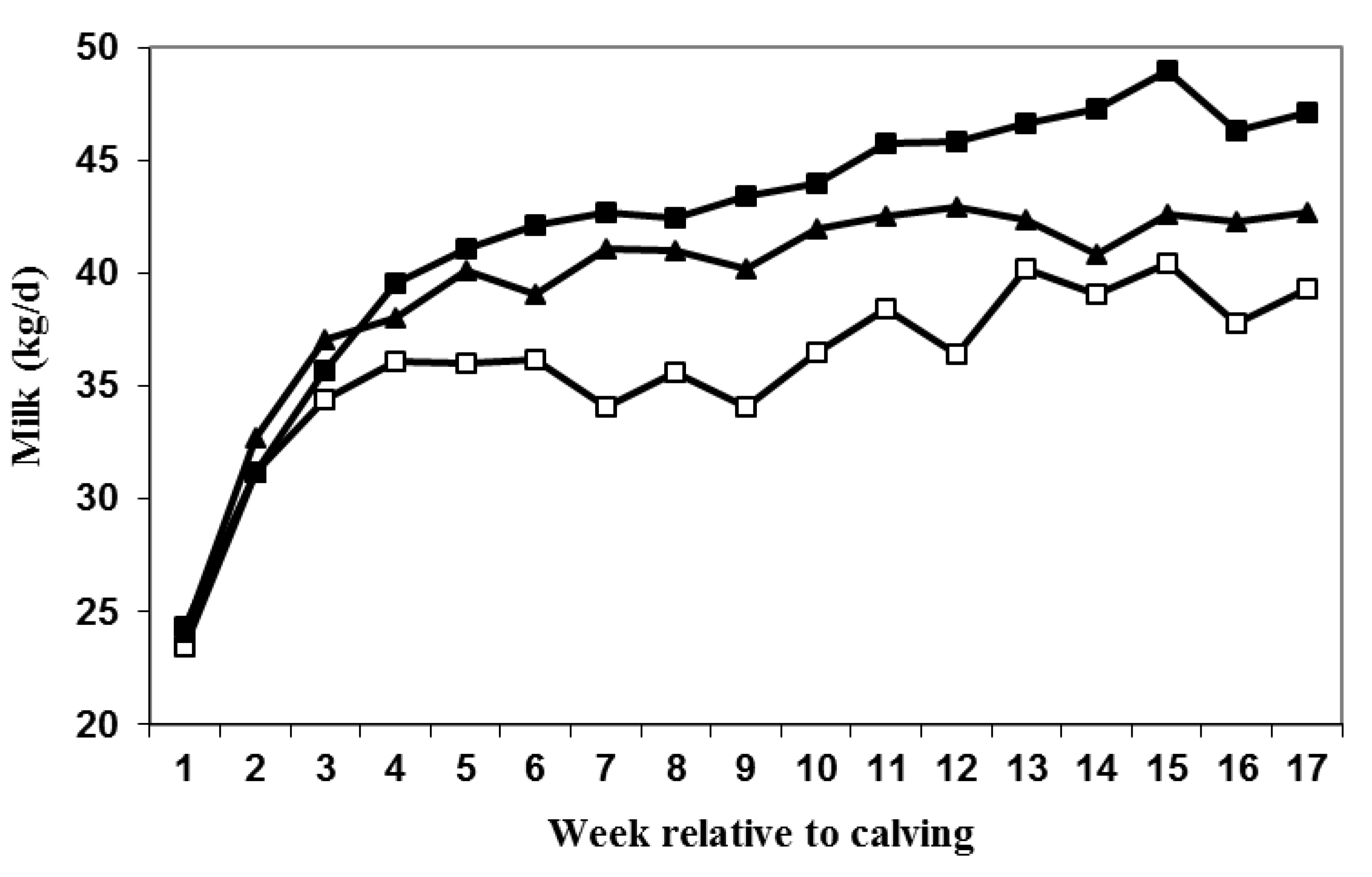
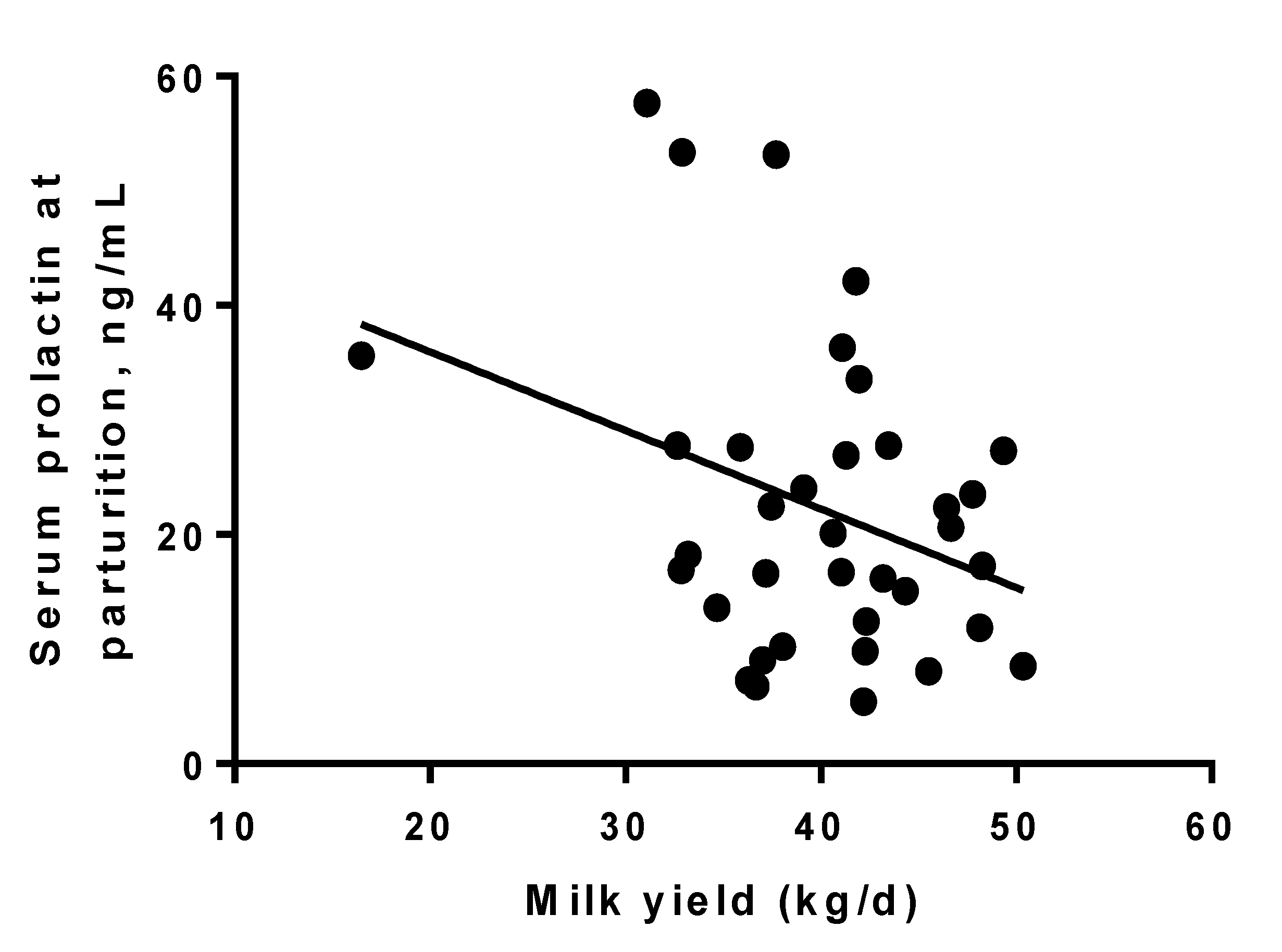
| Variable | LDPP | SDPP | SDPP+PRL | SEM | P-value |
|---|---|---|---|---|---|
| Milk yield, kg/d | 38.0 | 42.6 | 40.8 | 1.5 | 0.09 |
| Fat, % | 4.01 | 3.90 | 3.88 | 0.15 | 0.81 |
| Protein, % | 3.13 | 2.95 | 3.05 | 0.1 | 0.15 |
| Lactose, % | 4.63 | 4.81 | 4.73 | 0.08 | 0.11 |
| SCS | 4.33 | 2.80 | 3.05 | 0.5 | 0.08 |
| BW, kg | |||||
| Prepartum | 712 | 717 | 710 | 6 | 0.72 |
| Postpartum | 671 | 669 | 646 | 17 | 0.53 |
| DMI, kg/d | |||||
| Prepartum | 13.3 | 14.1 | 13.1 | 0.7 | 0.47 |
| Postpartum | 17.5 | 17.6 | 16.5 | 0.7 | 0.48 |
3.3. NEFA and BHBA
| Variable | LDPP 1 | SDPP 2 | SDPP +PRL 3 | SEM | P-value |
|---|---|---|---|---|---|
| NEFA (UeqL) | |||||
| d −7 | 138 | 112 | 105 | 40 | 0.36 |
| d 0 | 413 | 361 | 475 | 40 | 0.57 |
| d 7 | 475 | 673 | 411 | 41 | 0.06 |
| BHBA (mmol/L) | |||||
| d −7 | 0.57 | 0.66 | 0.62 | 0.15 | 0.24 |
| d 0 | 0.73 | 0.74 | 0.72 | 0.15 | 0.24 |
| d 7 | 0.59 | 0.75 | 0.88 | 0.15 | 0.36 |
3.4. Dry Period Length, Calf Weights, and Health
3.5. Mammary Tissue Proliferation
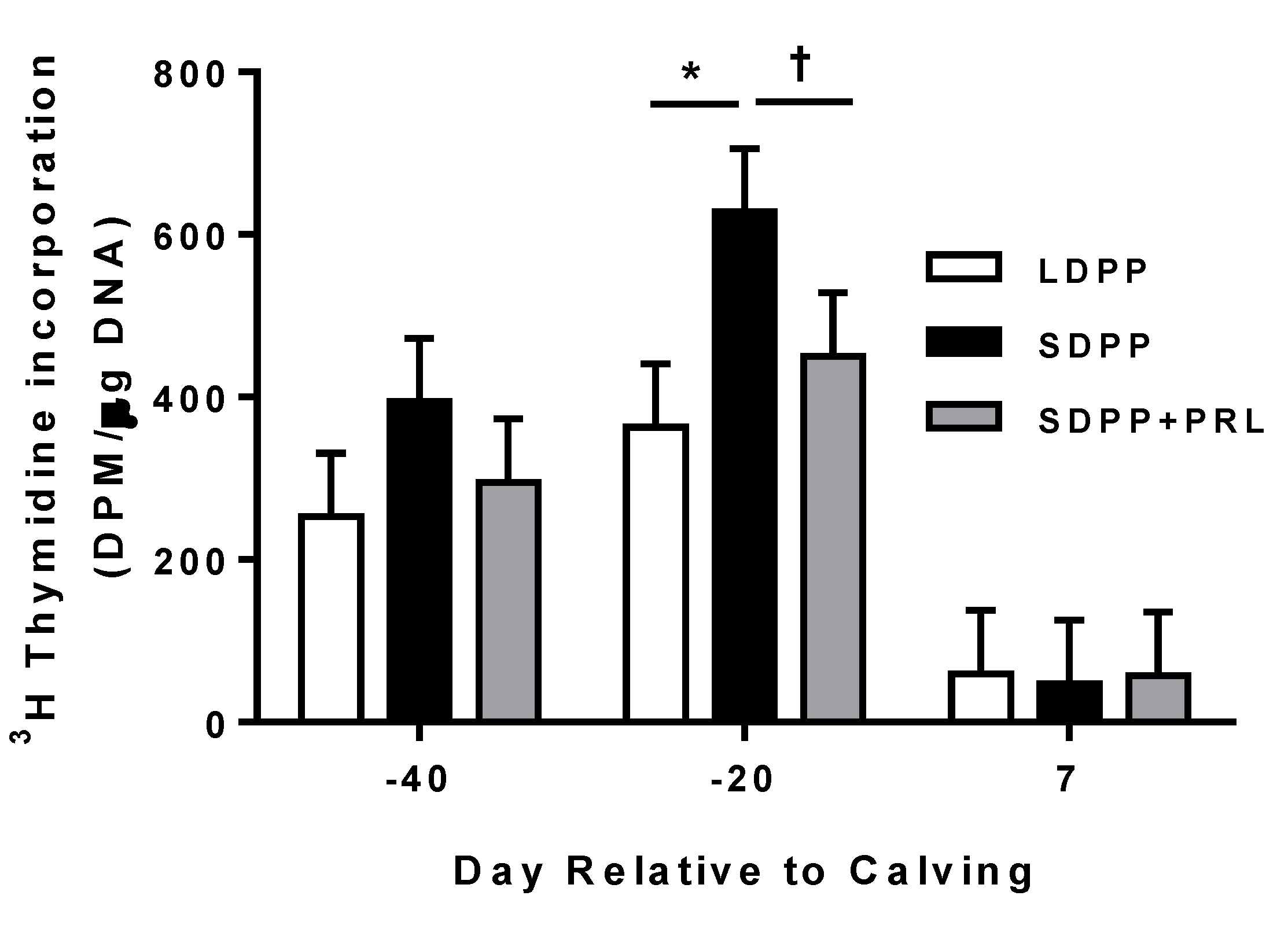
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Turner, J.D.; Huynh, H.T. Role of tissue remodeling in mammary epithelial cell proliferation and morphogenesis. J. Dairy Sci. 1991, 74, 2801–2807. [Google Scholar] [CrossRef]
- Capuco, A.V.; Akers, R.M.; Smith, J.J. Mammary growth in Holstein cows during the dry period: Quantification of nucleic acids and histology. J. Dairy Sci. 1997, 80, 477–487. [Google Scholar] [CrossRef]
- Bachman, K.C.; Schairer, M.L. Invited review: Bovine studies on optimal lengths of dry periods. J. Dairy Sci. 2003, 86, 3027–3037. [Google Scholar] [CrossRef]
- Akers, R.M. Lactation and the Mammary Gland, 1st ed.; Iowa State Press: Ames, IA, USA, 2002. [Google Scholar]
- Capuco, A.V.; Ellis, S.E.; Hale, S.A.; Long, E.; Erdman, R.A.; Zhao, X.; Paape, M.J. Lactation persistency: Insights from mammary cell proliferation studies. J. Anim. Sci. 2003, 81, 18–31. [Google Scholar] [PubMed]
- Boutinaud, M.; Guinard-Flamenta, J.; Jammes, H. The number and activity of mammary epithelial cells, determining factors for milk production. Repr. Nut. Dev. 2004, 44, 499–508. [Google Scholar] [CrossRef]
- Dahl, G.E.; Buchanan, B.A.; Tucker, H.A. Photoperiodic effects on dairy cattle: A review. J. Dairy Sci. 2000, 83, 885–893. [Google Scholar] [CrossRef]
- Peters, R.R.; Chapin, L.T.; Emery, R.S.; Tucker, H.A. Milk yield, feed intake, prolactin, growth hormone, and glucocorticoid response of cows to supplemented light. J. Dairy Sci. 1981, 64, 1671–1678. [Google Scholar] [CrossRef]
- Bilodeau, P.P.; Petitclerc, D.; St. Pierre, N.; Pelletier, G.; St. Laurent, G.J. Effects of photoperiod and pair-feeding on lactation of cows fed corn or barley grain in total mixed rations. J. Dairy Sci. 1989, 72, 2999–3005. [Google Scholar] [CrossRef]
- Evans, N.M.; Hacker, R.R. Effect of chronobiological manipulation of lactation in the dairy cow. J. Dairy Sci. 1989, 72, 2921–2927. [Google Scholar] [CrossRef]
- Miller, A.R.E.; Stanisiewski, E.P.; Erdman, R.A.; Douglass, L.W.; Dahl, G.E. Effects of long daily photoperiod and bovine somatotropin (Trobest) on milk yield in cows. J. Dairy Sci. 1999, 82, 1716–1722. [Google Scholar] [CrossRef]
- Miller, A.R.E.; Erdman, R.A.; Douglass, L.W.; Dahl, G.E. Effects of photoperiod manipulation during the dry period of dairy cows. J. Dairy Sci. 2000, 83, 962–967. [Google Scholar] [CrossRef]
- Lacasse, P.; Vinet, C.M.; Petitclerc, D. Effect of prepartum photoperiod and melatonin feeding on milk production and prolactin concentration in dairy heifers and cows. J. Dairy Sci. 2014, 97, 3589–3598. [Google Scholar] [CrossRef] [PubMed]
- Wall, E.H.; Auchtung, T.L.; Dahl, G.E.; Ellis, S.E.; McFadden, T.B. Exposure to short day photoperiod during the dry period enhances mammary growth in dairy cows. J. Dairy Sci. 2005, 88, 1994–2003. [Google Scholar] [CrossRef]
- Newbold, J.A.; Chapin, L.T.; Zinn, S.A.; Tucker, H.A. Effects of photoperiod on mammary development and concentration of hormones in serum of pregnant dairy heifers. J. Dairy Sci. 1991, 74, 100–108. [Google Scholar] [CrossRef]
- Auchtung, T.L.; Kendall, P.E.; Salak-Johnson, J.L.; McFadden, T.B.; Dahl, G.E. Photoperiod and bromocriptine treatment effects on expression of prolactin receptor mRNA in bovine liver, mammary gland and peripheral blood lymphocytes. J. Endocrinol. 2003, 179, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Auchtung, T.L.; Rius, A.G.; Kendall, P.E.; McFadden, T.B.; Dahl, G.E. Effects of photoperiod during the dry period on prolactin, prolactin receptor and milk production of dairy cows. J. Dairy Sci. 2005, 88, 121–127. [Google Scholar] [CrossRef]
- Barash, I.; Madar, Z.; Gertler, A. Down-regulation of prolactin receptors in the liver, mammary gland and kidney of female virgin rat, infused with ovine prolactin or human growth hormone. Biochem. Biophys. Res. Commun. 1983, 116, 644–650. [Google Scholar] [CrossRef]
- Akers, R.M.; Bauman, D.E.; Capuco, A.V.; Goodman, G.T.; Tucker, H.A. Prolactin regulation of milk secretion and biochemical differentiation of mammary epithelial cells in periparturient cows. Endocrinology 1981, 109, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Akers, R.M.; Bauman, D.E.; Goodman, G.T.; Capuco, A.V.; Tucker, H.A. Prolactin regulation of cytological differentiation of mammary epithelial cells in periparturient cows. Endocrinology 1981, 109, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Ormandy, C.J.; Naylor, M.; Harris, J.; Robertson, F.; Horseman, N.D.; Lindeman, G.J.; Visvader, J.; Kelly, P.A. Investigation of the transcriptional changes underlying functional defects in the mammary glands of prolactin receptor knockout mice. Recent Prog. Horm. Res. 2003, 58, 297–323. [Google Scholar] [CrossRef] [PubMed]
- Dahl, G.E. Effects of short day photoperiod on prolactin signaling in dry cows: A common mechanism among tissues and environments? J. Anim. Sci. 2008, 86, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.M.; Peters, J.P. Technical note: An improved method to quantify nonesterified fatty acids in bovine plasma. J. Anim Sci. 1993, 71, 753–756. [Google Scholar] [PubMed]
- Litherland, N.B.; Thire, S.; Beaulieu, A.D.; Reynolds, C.K.; Benson, J.A.; Drackley, J.K. Dry matter intake is decreased more by abomasal infusion of unsaturated free fatty acids than by unsaturated triglycerides. J. Dairy Sci. 2005, 88, 632–643. [Google Scholar] [CrossRef]
- Farr, V.C.; Stelwagen, K.; Cate, L.R.; Molenaar, A.J.; McFadden, T.B.; Davis, S.R. An improved method for the routine biopsy of bovine mammary tissue. J. Dairy Sci. 1996, 79, 543–549. [Google Scholar] [CrossRef]
- Labarca, C.; Paigen, K. A simple, rapid, and sensitive DNA assay procedure. Anal. Biochem. 1980, 102, 344–352. [Google Scholar] [CrossRef]
- Velasco, J.M.; Reid, E.D.; Fried, K.K.; Gressley, T.F.; Wallace, R.L.; Dahl, G.E. Short day photoperiod increases milk yield in cows with a reduced dry period length. J. Dairy Sci. 2008, 91, 3467–3473. [Google Scholar] [CrossRef] [PubMed]
- Auchtung, T.L.; Dahl, G.E. Prolactin mediates photoperiodic immune enhancement: Effects of administration of exogenous prolactin on circulating concentrations, receptor expression, and immune function in steers. Biol. Reprod. 2004, 71, 1913–1918. [Google Scholar] [CrossRef] [PubMed]
- Aharoni, Y.; Brosh, A.; Ezra, E. Short communication: Prepartum photoperiod effect on milk yield and composition in dairy cows. J. Dairy Sci. 2000, 83, 2779–2781. [Google Scholar] [CrossRef]
- Akers, R.M. Lactogenic hormones: Binding sites, mammary growth, secretory cell differentiation, and milk biosynthesis in ruminants. J. Dairy Sci. 1985, 68, 501–519. [Google Scholar] [CrossRef]
- Koprowski, J.A.; Tucker, H.A. Serum prolactin during various physiological states and its relationship to milk production in the bovine. Endocrinology 1973, 92, 1480–1487. [Google Scholar] [CrossRef] [PubMed]
- Tucker, H.A. Factors affecting mammary gland cell numbers. J. Dairy Sci. 1969, 52, 720–729. [Google Scholar] [CrossRef]
- Flint, D.J.; Gardner, M. Evidence that growth hormone stimulates milk synthesis by direct action on the mammary gland and that prolactin exerts effects on milk secretion by maintenance of mammary deoxyribonucleic acid content and tight junction status. Endocrinology 1994, 135, 1119–1124. [Google Scholar] [PubMed]
- Cowie, A.T. Variations in the yield and composition of the milk during lactation in the rabbit and the galactopoietic effect of prolactin. J. Endocrinol. 1969, 44, 437–450. [Google Scholar] [CrossRef] [PubMed]
- Plaut, K.; Bauman, D.E.; Agergaard, N.; Akers, R.M. Effect of exogenous prolactin administration on lactational performance of dairy cows. Domest. Anim. Endocrinol. 1987, 4, 279–290. [Google Scholar] [CrossRef]
- Wall, E.H.; Crawford, H.M.; Ellis, S.E.; Dahl, G.E.; McFadden, T.B. Mammary response to exogenous prolactin or frequent milking during early lactation in dairy cows. J. Dairy Sci. 2006, 89, 4640–4648. [Google Scholar] [CrossRef]
- Lacasse, P.; Lollivier, V.; Bruckmaier, R.M.; Boisclair, Y.R.; Wagner, G.F.; Boutinaud, M. Effect of the prolactin-release inhibitor quinagolide on lactating dairy cows. J. Dairy Sci. 2008, 94, 1302–1309. [Google Scholar] [CrossRef] [PubMed]
- Kahn, C.R.; Smith, R.J.; Chin, W.W. Mechanism of Action of Hormones That Act at the Cell Surface. In Williams Textbook of Endocrinology; Wilson, J.D., Foster, D.W., Kronenberg, H.M., Larsen, P.R.W.B., Eds.; Saunders Co.: New York, NY, USA, 1998. [Google Scholar]
- Reid, E.D.; Auchtung, T.L.; Morin, D.E.; McFadden, T.B.; Dahl, G.E. Effects of 21-day short day photoperiod (SDPP) during the dry period on dry matter intake and subsequent milk production in cows. J. Dairy Sci. 2004, 87 (Suppl. 1), 424. [Google Scholar]
- Accorsi, P.A.; Pacioni, B.; Pezzi, C.; Forni, M.; Flint, D.J.; Seren, E. Role of prolactin, growth hormone and insulin-like growth factor 1 in mammary gland involution in the dairy cow. J. Dairy Sci. 2002, 85, 507–513. [Google Scholar] [CrossRef]
- Wall, E.H.; Auchtung-Montgomery, T.L.; Dahl, G.E.; McFadden, T.B. Short Communication: Short day photoperiod during the dry period decreases expression of suppressors of cytokine signaling in the mammary gland of dairy cows. J. Dairy Sci. 2005, 88, 3145–3148. [Google Scholar] [CrossRef]
- Do Amaral, B.C.; Connor, E.E.; Tao, S.; Hayen, J.; Bubolz, J.; Dahl, G.E. Heat stress abatement during the dry period influences prolactin signaling in lymphocytes. Domest. Anim. Endo. 2010, 38, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; Bubolz, J.W.; do Amaral, B.C.; Thompson, I.M.; Hayen, M.J.; Johnson, S.E.; Dahl, G.E. Effect of heat stress during the dry period on mammary gland development. J. Dairy Sci. 2011, 94, 5976–5986. [Google Scholar]
- Tao, S.; Dahl, G.E. Invited review: Heat stress impacts during late gestation on dry cows and their calves. J. Dairy Sci. 2013, 96, 4079–4093. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; Thompson, I.M.; Monteiro, A.P.; Hayen, M.J.; Dahl, G.E. Effect of heat stress during the dry period on insulin responses of multiparous dairy cows. J. Dairy Sci. 2012, 95, 5035–5046. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crawford, H.M.; Morin, D.E.; Wall, E.H.; McFadden, T.B.; Dahl, G.E. Evidence for a Role of Prolactin in Mediating Effects of Photoperiod during the Dry Period. Animals 2015, 5, 803-820. https://doi.org/10.3390/ani5030385
Crawford HM, Morin DE, Wall EH, McFadden TB, Dahl GE. Evidence for a Role of Prolactin in Mediating Effects of Photoperiod during the Dry Period. Animals. 2015; 5(3):803-820. https://doi.org/10.3390/ani5030385
Chicago/Turabian StyleCrawford, Heather M., Dawn E. Morin, Emma H. Wall, Thomas B. McFadden, and Geoffrey E. Dahl. 2015. "Evidence for a Role of Prolactin in Mediating Effects of Photoperiod during the Dry Period" Animals 5, no. 3: 803-820. https://doi.org/10.3390/ani5030385
APA StyleCrawford, H. M., Morin, D. E., Wall, E. H., McFadden, T. B., & Dahl, G. E. (2015). Evidence for a Role of Prolactin in Mediating Effects of Photoperiod during the Dry Period. Animals, 5(3), 803-820. https://doi.org/10.3390/ani5030385





