Rabbit Feces as Feed for Ruminants and as an Energy Source
Abstract
:Simple Summary
Abstract
1. Introduction
2. Experimental Section
2.1. Data Sets and Analyses
| Reference | Raw material | Diets | Feces |
|---|---|---|---|
| [23] | False flax a | 3 | 30 |
| [24] | Chia b | 3 | 12 |
| [25] | Golden flaxseed c | 3 | 12 |
| [26] | Spirulina d | 4 | 12 |
| [27] | DHEA/Spirulina/fat level e | 8 | 24 |
| [28] | Perilla f | 3 | 12 |
| [19] | Curcuma/oils g | 4 | 16 |
| [29] | Dried tomato pomace h | 3 | 16 |
| [30] | Live yeast i | 3 | 14 |
2.2. Comparative Energetics
2.3. Comparative Partial Least Square Models of Digestibility in Forages and in Rabbit Feces
2.4. Calibration and Validation by NIRS
3. Results and Discussion
| Feces (F) | Ratio F/C | Crops (C) | |||||
|---|---|---|---|---|---|---|---|
| Mean | SD | CV | Mean | SD | CV | ||
| Composition | |||||||
| Gross Energy (MJ/kg DM) | 17.85 | 1.76 | 0.10 | 10% | 16.27 | 0.88 | 0.05 |
| Ash (% DM) | 10.37 | 2.58 | 0.25 | −26% | 13.95 | 3.61 | 0.26 |
| Crude Protein (% DM) | 15.50 | 1.91 | 0.12 | 18% | 13.15 | 3.47 | 0.26 |
| Ether Extract (% DM) | 2.04 | 0.48 | 0.24 | −15% | 2.42 | 0.55 | 0.23 |
| Crude Fiber (% DM) | 32.44 | 1.96 | 0.06 | 39% | 23.31 | 9.46 | 0.41 |
| N-free Extract (% DM) | 39.78 | 2.69 | 0.07 | −16% | 47.17 | 9.44 | 0.20 |
| Residual Fraction (N) | 0.40 | 0.05 | 0.11 | −27% | 0.55 | 0.06 | 0.11 |
| NDF (% DM) | 59.81 | 4.54 | 0.08 | 30% | 45.93 | 7.35 | 0.16 |
| Digestible NDF (% DM) | 29.99 | 2.44 | 0.08 | 2% | 29.53 | 3.99 | 0.14 |
| Indigestible NDF (% DM) | 29.82 | 5.10 | 0.17 | 82% | 16.40 | 8.00 | 0.49 |
| ADF (% DM) | 39.59 | 2.22 | 0.06 | 18% | 33.47 | 6.04 | 0.18 |
| Hemicellulose (% DM) | 20.22 | 4.23 | 0.21 | 75% | 11.56 | 3.95 | 0.34 |
| Cellulose (% DM) | 28.99 | 2.28 | 0.08 | 11% | 26.19 | 5.71 | 0.22 |
| Lignin (% DM) | 10.60 | 1.86 | 0.18 | 42% | 7.45 | 1.57 | 0.21 |
| Gross Energy (MJ/kg DM) | 17.85 | 1.76 | 0.10 | 10% | 16.27 | 0.88 | 0.05 |
| Properties | |||||||
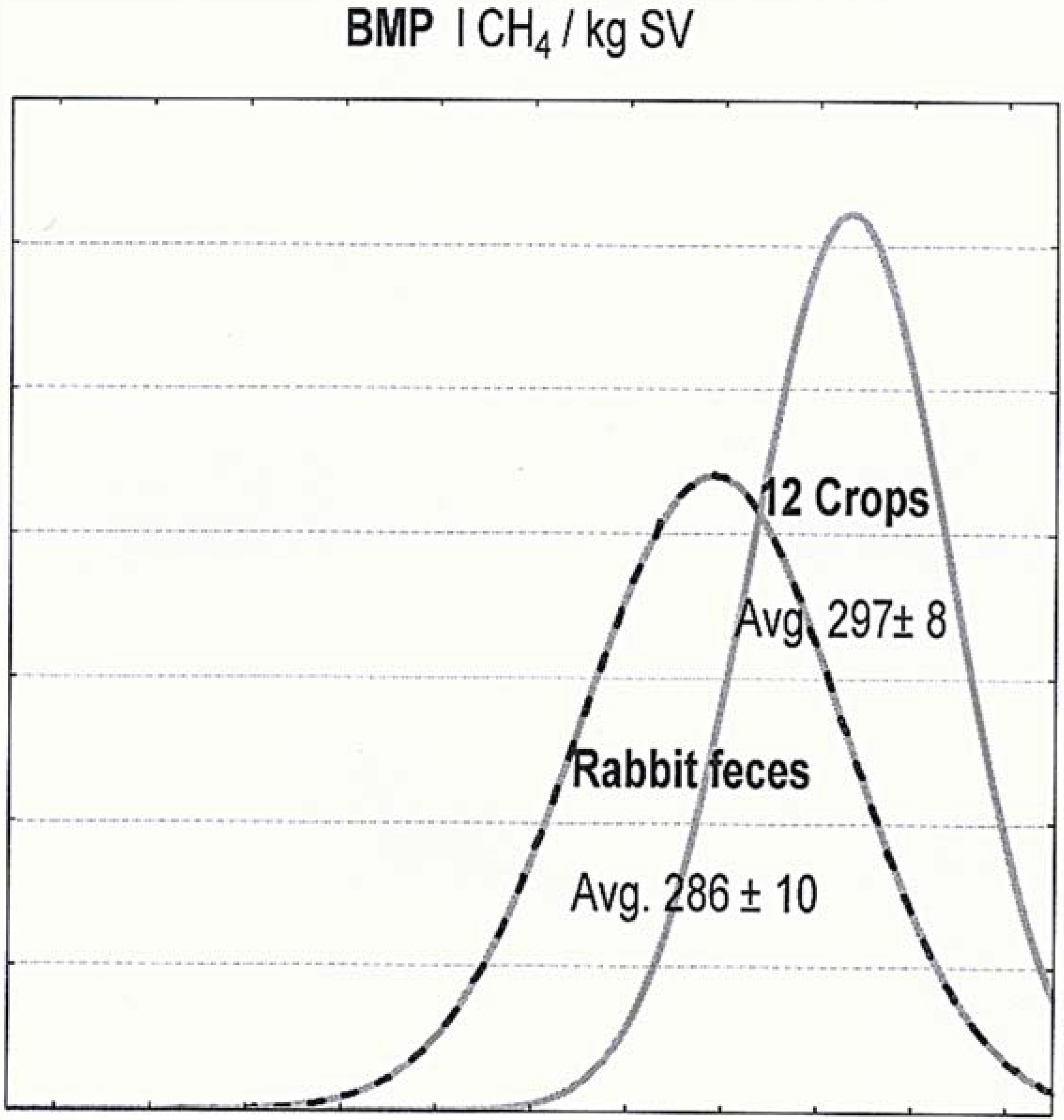
| BMP (lCH4/kg SV) | 286 | 10 | 0.04 | −4% | 297 | 8 | 0.03 |
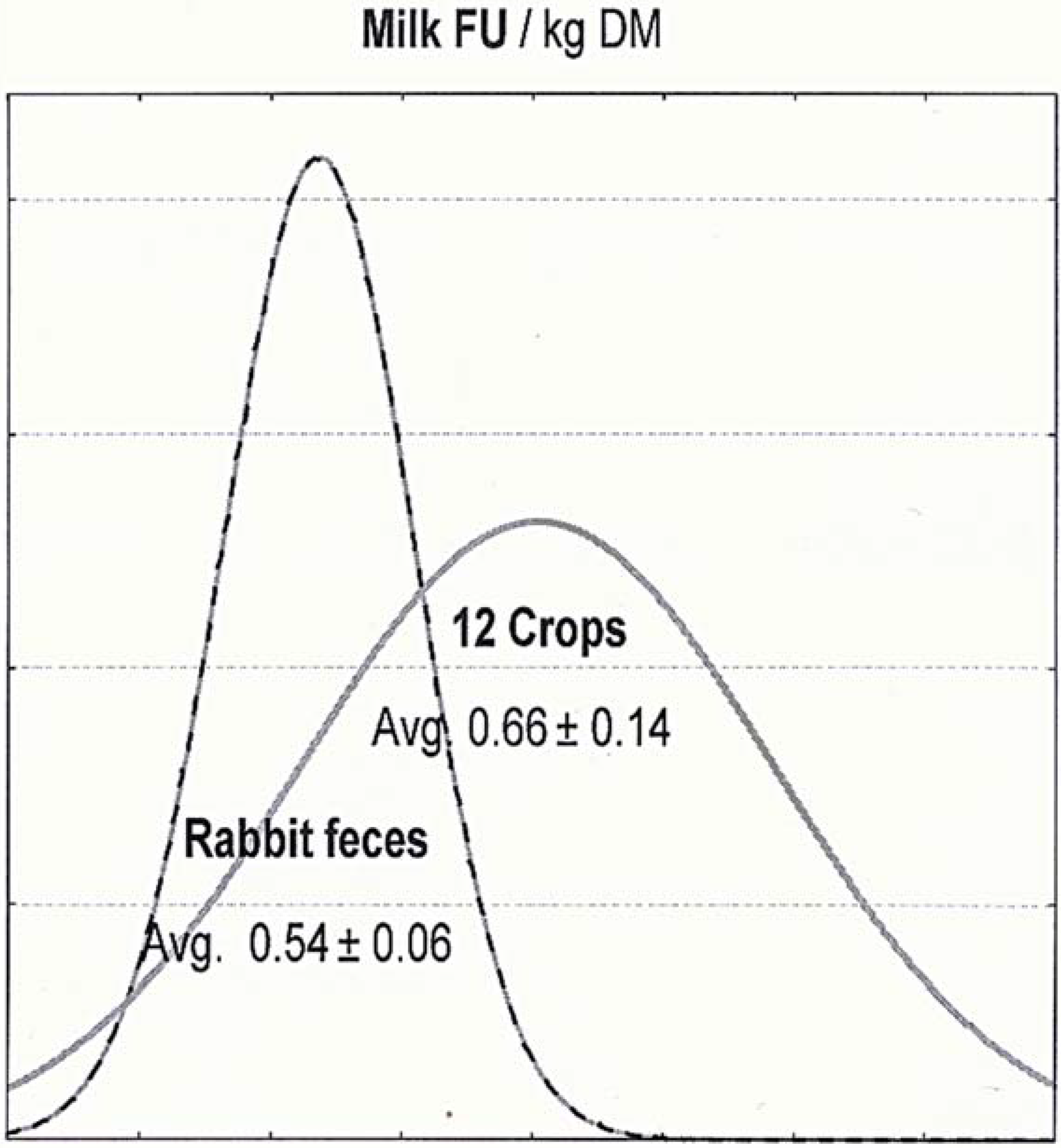
| Milk FU (/kg DM) | 0.54 | 0.06 | 0.11 | −19% | 0.66 | 0.14 | 0.21 |
| IVTD | IVTD | NDFD | NDFD | |
|---|---|---|---|---|
| Feces | Crops | Feces | Crops | |
| Ash (% DM) | −0.081 | 0.031 | −0.042 | 0.063 |
| Gross Energy (MJ/kg DM) | −0.048 | −0.030 | −0.131 | −0.053 |
| Crude protein (% DM) | 0.108 | 0.152 | 0.091 | 0.130 |
| Ether Extract (% DM) | 0.107 | 0.088 | 0.086 | 0.083 |
| Crude fiber (% DM) | −0.041 | −0.134 | −0.069 | −0.117 |
| N-free extract (% DM) | 0.027 | 0.062 | 0.025 | 0.040 |
| ADF (% OM) | −0.133 | −0.158 | −0.163 | −0.149 |
| NDF (% OM) | −0.223 | −0.214 | −0.231 | −0.180 |
| Lignin (% OM) | 0.078 | 0.024 | 0.043 | −0.004 |
| Hemicellulose (% OM) | −0.169 | −0.048 | −0.163 | −0.032 |
| Cellulose (% OM) | −0.194 | −0.076 | −0.193 | −0.093 |
| XResidual (N) | 0.223 | 0.098 | 0.231 | 0.110 |
| BMP (lCH4/kg SV) | −0.129 | −0.070 | −0.095 | −0.046 |
| Milk FU (/kg DM) | 0.180 | 0.182 | 0.194 | 0.165 |
| R2 | 0.23 | 0.84 | 0.10 | 0.78 |
| Standard Error of Calibration | 7.50 | 2.98 | 8.45 | 5.97 |
| Mean of experimental | 66.9 | 83.2 | 51.1 | 65.8 |
| SD of experimental | 8.5 | 7.4 | 8.9 | 12.6 |
| Mean of potential in feces | 74.4 | 50.2 | ||
| SD of potential in feces | 3.0 | 4.5 | ||
| r | 0.85 | 0.89 | ||
| Variable | NIRS Spectra | Method | Mean | SD | RSQ | SECV | 1-VR | RPD |
|---|---|---|---|---|---|---|---|---|
| BMP | Feces | Thomsen et al. [32] | 288.6 | 6.01 | 0.53 | 5.28 | 0.23 | 1.1 |
| Feces and Crops | 292.2 | 7.16 | 0.87 | 4.63 | 0.58 | 1.5 | ||
| Milk FU | Feces | Sauvant et al. [31] | 0.5 | 0.06 | 0.98 | 0.03 | 0.72 | 1.9 |
| Feces and Crops | 0.6 | 0.10 | 0.94 | 0.04 | 0.85 | 2.6 | ||
| IVTD | Feces | Daisy | 71.6 | 6.37 | 0.86 | 4.90 | 0.41 | 1.3 |
| Feces | Potential | 74.5 | 2.88 | 0.91 | 1.65 | 0.67 | 1.7 | |
| Feces and Crops | Potential | 77.4 | 6.39 | 0.94 | 2.03 | 0.90 | 3.2 | |
| NDFD | Feces | Daisy | 50.1 | 10.36 | 0.64 | 8.32 | 0.36 | 1.2 |
| Feces | Potential | 50.5 | 4.44 | 0.92 | 2.43 | 0.70 | 1.8 | |
| Feces and Crops | Potential | 55.2 | 10.33 | 0.93 | 3.52 | 0.88 | 2.9 |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Thornton, P.K. Livestock production: Recent trends, future prospect. Philos. T. Roy. Soc. B 2010, 365, 2853–2867. [Google Scholar] [CrossRef]
- Bórquez, J.L.; Pinos-Rodriguez, J.M.; González, S.S.; Domínguez, I.; Bárcena, R.; Mendoza, G.; Cobos, M. Use of different kind of silage dairy cattle manure in lamb nutrition. Ital. J. Anim. Sci. 2010, 9, 129–133. [Google Scholar]
- El Jalil, M.H.; Faid, M.; Elyachioui, M. A biotechnological process for treatment and recycling poultry wastes manure as a feed ingredient. Biomass Bioener. 2001, 21, 301–309. [Google Scholar]
- Feedipedia. Manure and Poultry Litter. Available online: http://www.feedipedia.org/node/66 (accessed on 5 May 2014).
- Onimisi, P.A.; Omage, J.J. Evaluation of poultry litter as feedstuff for growing rabbits. Livest. Res. Rural Develop. 2006, 18. Available online: http://www.lrrd.org/lrrd18/11/onim18163.htm (accessed on 3 December 2014).
- Chen, S.; Liao, W.; Liu, C.; Wen, Z.; Kincaid, R.L.; Harrison, J.H. Use of animal manure as feedstock for bio-products. In Proceedings of Ninth International Animal, Agricultural and Food Processing Wastes Symposium, Research Triangle Park, NC, USA, 12–15 October 2003; pp. 50–57.
- Flachowsky, G. Animal excreta as feedstuff for ruminants—A review. J. Appl. Anim. Res. 1997, 12, 1–40. [Google Scholar] [CrossRef]
- Cornejo, C.; Wilkie, A.C. Greenhouse gas emissions and biogas potential from livestock in Ecuador. Ener. Sustainable Dev. 2010, 14, 256–266. [Google Scholar] [CrossRef]
- Ward, A.J.; Hobbs, P.J.; Holliman, P.J.; Jones, D.L. Optimisation of the anaerobic digestion of agricultural resources. Bioresour. Technol. 2008, 99, 7928–7940. [Google Scholar] [CrossRef] [PubMed]
- Orskov, B.; Yongabi, K.; Subedi, M.; Smith, J. Overview of holistic application of biogas for small scale farmers in Sub-Saharan Africa. Biomass Bioener. 2014, 70, 4–16. [Google Scholar] [CrossRef]
- Castillo-Rodríguez, S.P.; Aguilar-Reyes, J.M.; Lucero-Magaña, F.A.; Martínez-Gonzáles, J.C. Substitution of commercial feed for manure in the diet of rabbits in growth. Av. Investig. Agropecu. 2007, 11, 41–48. [Google Scholar]
- Lall, D.; Kishan, J.; Negi, S.S. Feeding value of dried rabbit excreta in the ration of sheep. Indian J. Anim. Sci. 1984, 54, 1005–1007. [Google Scholar]
- Swick, R.A.; Cheeke, P.R.; Patton, N.M. Evaluation of dried rabbit manure as a feed for rabbits. Can. J. Anim. Sci. 1978, 58, 753–757. [Google Scholar] [CrossRef]
- Núñez-Sánchez, N.; Martinez Marin, A.L.; Perez Hernandez, M.; Carrion, D.; Gomez Castro, G.; Perez Alba, L.M. Faecal near infrared spectroscopy (NIRS) as a tool to asses rabbit’s feed digestibility. Livest. Sci. 2012, 150, 386–390. [Google Scholar] [CrossRef]
- Smith, L.W. Research needs on the utilization aspects of the feeding of animal wastes. J. Anim. Sci. 1981, 52, 902–905. [Google Scholar]
- Preston, T.R.; Rodriguez, L. Recent developments in the recycling of livestock excreta; an essential feature of sustainable farming systems in the tropics. Available online: http://betuco.be/dieren/Recycling%20Livestock%20Wastes%20Fao.pdf (accessed on 5 May 2014).
- Gidenne, T.; Lapanouse, A.; Segura, M.; Bannelier, C.; Tartié, V. Effect of the dietary ratio “digestible fiber/crude protein” on rabbit nitrogen balance and on health status. In Proceedings of European Meeting COST848, Joint Workshop on Reproduction and Nutrition, Varese, Italy, 24–25 October 2002; p. 28.
- Masoero, G.; Baricco, G.; Cherubini, R.; Barge, P.; Sala, G.; de Poi, E. Very low protein, aminoacid-supplied diet for heavy broiler rabbits: Effects on nitrogen metabolism, and digital evaluation of excreta and products. In Proceeding of the 9th World Rabbit Congress, Verona, Italy, 10–13 June 2008; pp. 729–734.
- Zunino, V.; Meineri, G.; Peiretti, P.G. Curcuma longa and dietary plant oils for growing rabbits: Effect on apparent digestibility. J. Food Agric. Environ. 2010, 8, 435–438. [Google Scholar]
- Perez, J.M.; Lebas, F.; Gidenne, T.; Maertens, L.; Xiccato, G.; Parigi-Bini, R.; Dalle Zotte, A.; Cossu, M.E.; Carazzolo, A.; Villamide, M.J.; et al. European reference method for in vivo determination of diet digestibility in rabbits. World Rabbit Sci. 1995, 3, 41–43. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3591. [Google Scholar] [CrossRef] [PubMed]
- Robinson, P.H.; Campbell, M.; Fadel, J.G. Influence of storage time and temperature on in vitro digestion of neutral detergent fiber at 48 h, and comparison to 48 h in sacco neutral detergent fiber digestion. Anim. Feed Sci. Technol. 1999, 80, 257–266. [Google Scholar] [CrossRef]
- Peiretti, P.G.; Mussa, P.P.; Meineri, G.; Perona, G. Apparent digestibility of mixed feed with increasing levels of false flax (Camelina sativa L.) seeds in rabbit diets. J. Food Agr. Environ. 2007, 5, 85–88. [Google Scholar]
- Meineri, G.; Peiretti, P.G. Apparent digestibility of mixed feed with increasing levels of chia (Salvia hispanica L.) seeds in rabbit diets. Ital. J. Anim. Sci. 2007, 6, 778–780. [Google Scholar]
- Peiretti, P.G.; Meineri, G. Effects of golden flaxseed supplementation on the performance and feed digestibility of rabbits. J. Anim. Vet. Adv. 2008, 7, 56–60. [Google Scholar]
- Peiretti, P.G.; Meineri, G. Effects of diets with increasing levels of Spirulina platensis on the performance and apparent digestibility in growing rabbits. Livest. Sci. 2008, 118, 173–177. [Google Scholar] [CrossRef]
- Meineri, G.; Ingravalle, F.; Radice, E.; Aragno, M.; Peiretti, P.G. Effects of high fat diets and Spirulina platensis supplementation in New Zealand White Rabbits. J. Anim. Vet. Adv. 2009, 8, 2735–2744. [Google Scholar]
- Peiretti, P.G.; Gai, F.; Meineri, G.; Zoccarato, I.; Gasco, L. Apparent digestibility of compound diets with increasing levels of perilla (Perilla frutescens L.) seeds in rabbits. Ital. J. Anim. Sci. 2010, 9, 425–428. [Google Scholar]
- Peiretti, P.G.; Gai, F.; Rotolo, L.; Gasco, L. Effects of diets with increasing levels of dried tomato pomace on the performances and apparent digestibility of growing rabbits. Asian J. Anim. Vet. Adv. 2012, 7, 521–527. [Google Scholar] [CrossRef]
- Rotolo, L.; Gai, F.; Peiretti, P.G.; Ortoffi, M.; Zoccarato, I.; Gasco, L. Live yeast (Saccharomyces cerevisiae var. boulardii) supplementation in fattening rabbit diet: Effect on productive performance and meat quality. Livest. Sci. 2014, 162, 178–184. [Google Scholar] [CrossRef]
- Sauvant, D.; Perez, J.M.; Tran, G. Tables de Composition et de Valeur Nutritive des Matières Premières Destinées Aux Animaux D’élevage: Porcs, Volailles, Bovins, Ovins, Caprins, Lapins, Chevaux, Poisons, INRA ed.; Inra-Quae: Versailles, France, 2002. [Google Scholar]
- Thomsen, S.T.; Spliid, H.; Østergård, H. Statistical prediction of biomethane potentials based on the composition of lignocellulosic biomass. Bioresour. Technol. 2014, 154, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Tassone, S.; Masoero, G.; Peiretti, P.G. Vibrational spectroscopy to predict in vitro digestibility and crop maturity index of different forages during the growing cycle and after freeze- or oven-drying. Anim. Feed Sci. Technol. 2014, 194, 12–25. [Google Scholar] [CrossRef]
- Raju, C.S.; Ward, A.J.; Nielsen, L.; Bjarne Møller, H. Comparison of near infra-red spectroscopy, neutral detergent fiber assay and in vitro organic matter digestibility assay for rapid determination of the biochemical methane potential of meadow grasses. Bioresour. Technol. 2011, 102, 7835–7839. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peiretti, P.G.; Tassone, S.; Gai, F.; Gasco, L.; Masoero, G. Rabbit Feces as Feed for Ruminants and as an Energy Source. Animals 2014, 4, 755-766. https://doi.org/10.3390/ani4040755
Peiretti PG, Tassone S, Gai F, Gasco L, Masoero G. Rabbit Feces as Feed for Ruminants and as an Energy Source. Animals. 2014; 4(4):755-766. https://doi.org/10.3390/ani4040755
Chicago/Turabian StylePeiretti, Pier Giorgio, Sonia Tassone, Francesco Gai, Laura Gasco, and Giorgio Masoero. 2014. "Rabbit Feces as Feed for Ruminants and as an Energy Source" Animals 4, no. 4: 755-766. https://doi.org/10.3390/ani4040755







