Long-Term Immunogenicity and Protection of a rHVT-H9/Y280 Vaccine Against H9N2 Avian Influenza Virus in Commercial Layers with High Maternal Antibodies
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Chickens
2.2. Vaccines
2.3. Study I: Protective Efficacy Against Avian Influenza Virus Challenge
2.4. Study II: Duration of Immunity and Vector Kinetics
2.5. Statistical Analysis
3. Results
3.1. Humoral Immune Response to Vaccination
3.2. Efficacy Against H9N2 Challenge
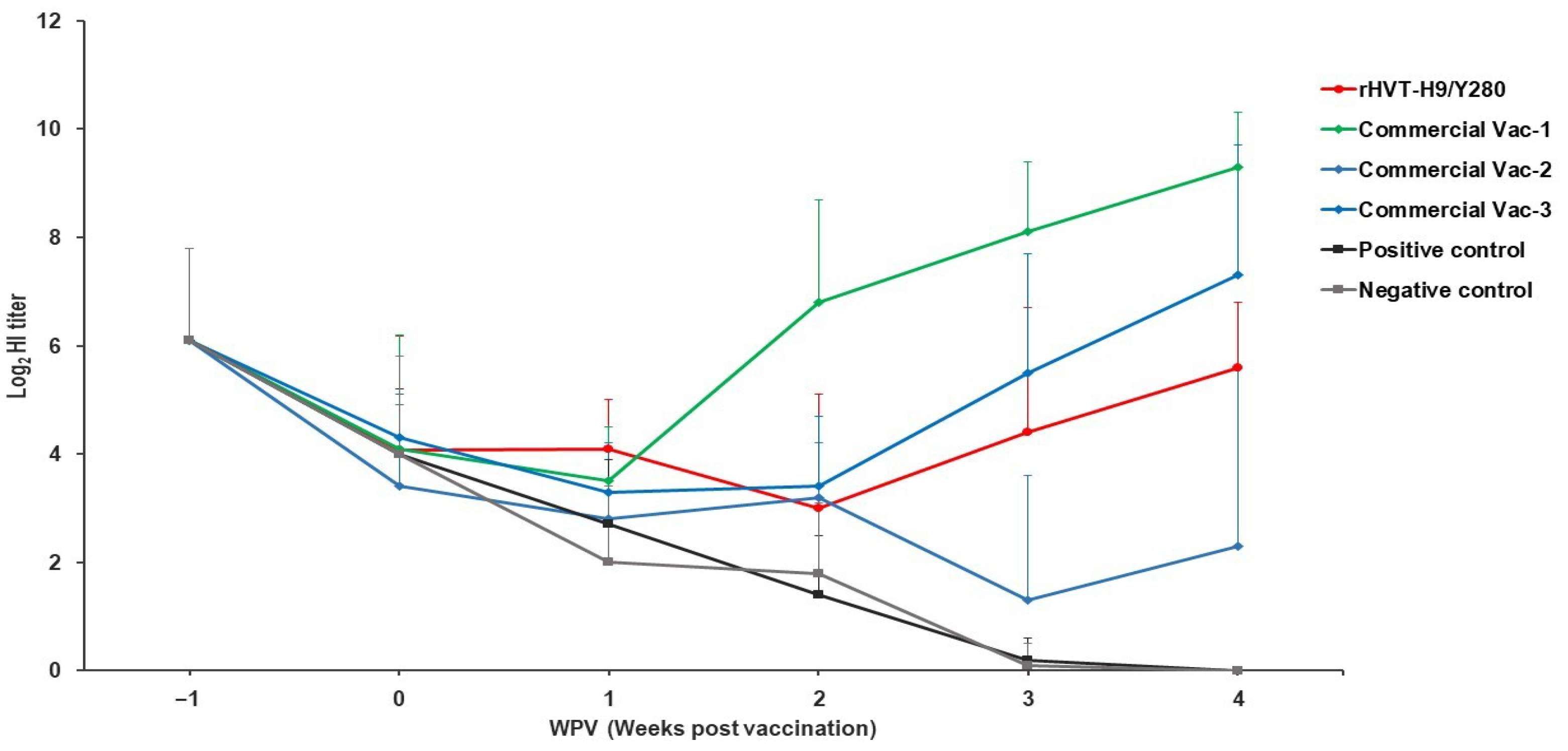
3.3. Long-Lasting Humoral Immune Response and Virus Kinetics in Layers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Homme, P.J.; Easterday, B.C. Avian influenza virus infections. I. Characteristics of influenza A-turkey-Wisconsin-1966 virus. Avian Dis. 1970, 14, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Gu, M.; Xu, L.; Wang, X.; Liu, X. Current situation of H9N2 subtype avian influenza in China. Vet. Res. 2017, 48, 49. [Google Scholar] [CrossRef] [PubMed]
- Kye, S.J.; Park, M.J.; Kim, N.Y.; Lee, Y.N.; Heo, G.B.; Baek, Y.K.; Shin, J.I.; Lee, M.H.; Lee, Y.J. Pathogenicity of H9N2 low pathogenic avian influenza viruses of different lineages isolated from live bird markets tested in three animal models: SPF chickens, Korean native chickens, and ducks. Poult. Sci. 2021, 100, 101318. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Yuan, H.; Gao, R.; Zhang, J.; Wang, D.; Xiong, Y.; Fan, G.; Yang, F.; Li, X.; Zhou, J.; et al. Clinical and epidemiological characteristics of a fatal case of avian influenza A H10N8 virus infection: A descriptive study. Lancet 2014, 383, 714–721. [Google Scholar] [CrossRef]
- Liu, D.; Shi, W.; Shi, Y.; Wang, D.; Xiao, H.; Li, W.; Bi, Y.; Wu, Y.; Li, X.; Yan, J.; et al. Origin and diversity of novel avian influenza A H7N9 viruses causing human infection: Phylogenetic, structural, and coalescent analyses. Lancet 2013, 381, 1926–1932. [Google Scholar] [CrossRef]
- Youk, S.S.; Lee, D.H.; Jeong, J.H.; Pantin-Jackwood, M.J.; Song, C.S.; Swayne, D.E. Live bird markets as evolutionary epicentres of H9N2 low pathogenicity avian influenza viruses in Korea. Emerg. Microbes Infect. 2020, 9, 616–627. [Google Scholar] [CrossRef]
- Youk, S.; Cho, A.Y.; Lee, D.H.; Jeong, S.; Kim, Y.J.; Lee, S.; Kim, T.H.; Pantin-Jackwood, M.J.; Song, C.S. Detection of newly introduced Y280-lineage H9N2 avian influenza viruses in live bird markets in Korea. Transbound. Emerg. Dis. 2022, 69, 881–885. [Google Scholar] [CrossRef]
- Dong, J.; Zhou, Y.; Pu, J.; Liu, L. Status and Challenges for Vaccination against Avian H9N2 Influenza Virus in China. Life 2022, 12, 1326. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, D.; Zhang, J.; Huang, X.; Han, K.; Liu, Q.; Yang, J.; Zhang, L.; Li, Y. Development of an Inactivated Avian Influenza Virus Vaccine against Circulating H9N2 in Chickens and Ducks. Vaccines 2023, 11, 596. [Google Scholar] [CrossRef]
- Hu, Z.; Ai, H.; Wang, Z.; Huang, S.; Sun, H.; Xuan, X.; Chen, M.; Wang, J.; Yan, W.; Sun, J.; et al. Impact of inactivated vaccine on transmission and evolution of H9N2 avian influenza virus in chickens. Npj Vaccines 2025, 10, 67. [Google Scholar] [CrossRef]
- Alqazlan, N.; Astill, J.; Raj, S.; Sharif, S. Strategies for enhancing immunity against avian influenza virus in chickens: A review. Avian Pathol. 2022, 51, 211–235. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, J.; Zhang, J.; Ly, H. Advances in Development and Application of Influenza Vaccines. Front. Immunol. 2021, 12, 711997. [Google Scholar] [CrossRef] [PubMed]
- Kamel, M.; El-Sayed, A. Utilization of herpesviridae as recombinant viral vectors in vaccine development against animal pathogens. Virus Res. 2019, 270, 197648. [Google Scholar] [CrossRef] [PubMed]
- Reemers, S.; Verstegen, I.; Basten, S.; Hubers, W.; van de Zande, S. A broad spectrum HVT-H5 avian influenza vector vaccine which induces a rapid onset of immunity. Vaccine 2021, 39, 1072–1079. [Google Scholar] [CrossRef]
- Roh, J.H.; Kang, M.; Wei, B.; Yoon, R.H.; Seo, H.S.; Bahng, J.Y.; Kwon, J.T.; Cha, S.Y.; Jang, H.K. Efficacy of HVT-IBD vector vaccine compared to attenuated live vaccine using in-ovo vaccination against a Korean very virulent IBDV in commercial broiler chickens. Poultry Sci. 2016, 95, 1020–1024. [Google Scholar] [CrossRef]
- Kapczynski, D.R.; Esaki, M.; Dorsey, K.M.; Jiang, H.; Jackwood, M.; Moraes, M.; Gardin, Y. Vaccine protection of chickens against antigenically diverse H5 highly pathogenic avian influenza isolates with a live HVT vector vaccine expressing the influenza hemagglutinin gene derived from a clade 2.2 avian influenza virus. Vaccine 2015, 33, 1197–1205. [Google Scholar] [CrossRef]
- Lee, J.; Lee, C.W.; Suarez, D.L.; Lee, S.A.; Kim, T.; Spackman, E. Efficacy of commercial recombinant HVT vaccines against a North American clade 2.3.4.4b H5N1 highly pathogenic avian influenza virus in chickens. PLoS ONE 2024, 19, e0307100. [Google Scholar] [CrossRef]
- Kilany, W.H.; Hassan, M.K.; Safwat, M.; Mohammed, S.; Selim, A.; VonDobschuetz, S.; Dauphin, G.; Lubroth, J.; Jobre, Y. Comparison of the effectiveness of rHVT-H5, inactivated H5 and rHVT-H5 with inactivated H5 prime/boost vaccination regimes in commercial broiler chickens carrying MDAs against HPAI H5N1 clade 2.2.1 virus. Avian Pathol. 2015, 44, 333–341. [Google Scholar] [CrossRef]
- Pan, X.; Liu, Q.; Niu, S.; Huang, D.; Yan, D.; Teng, Q.; Li, X.; Beerens, N.; Forlenza, M.; de Jong, M.C.M.; et al. Efficacy of a recombinant turkey herpesvirus (H9) vaccine against H9N2 avian influenza virus in chickens with maternal-derived antibodies. Front. Microbiol. 2022, 13, 1107975. [Google Scholar] [CrossRef]
- Zhang, J.F.; Kim, S.W.; Shang, K.; Park, J.Y.; Choi, Y.R.; Jang, H.K.; Wei, B.; Kang, M.; Cha, S.Y. Protection of Chickens against H9N2 Avian Influenza Isolates with a Live Vector Vaccine Expressing Influenza Hemagglutinin Gene Derived from Y280 Avian Influenza Virus. Animals 2024, 14, 872. [Google Scholar] [CrossRef]
- Pedersen, J.C. Hemagglutination-inhibition test for avian influenza virus subtype identification and the detection and quantitation of serum antibodies to the avian influenza virus. Methods Mol. Biol. 2008, 436, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Islam, A.; Harrison, B.; Cheetham, B.F.; Mahony, T.J.; Young, P.L.; Walkden-Brown, S.W. Differential amplification and quantitation of Marek’s disease viruses using real-time polymerase chain reaction. J. Virol. Methods 2004, 119, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Abdullatif, T.M.; Hassanin, O.; Mohamed, W.; AbdelMageed, M.; Al-Baqir, A. Investigation into the efficacy of a commercially available inactivated avian influenza virus (AIV) vaccine (H9N2) through experimental and field assessments, with an emphasis on its compatibility with recent AIV (H9N2) G1-sublineage isolates. Vet. Res. Commun. 2025, 50, 14. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, O.B.; Estevez, C.; Yu, Q.; Suarez, D.L. Passive antibody transfer in chickens to model maternal antibody after avian influenza vaccination. Vet. Immunol. Immunopathol. 2013, 152, 341–347. [Google Scholar] [CrossRef]
- Abdelwhab, E.M.; Grund, C.; Aly, M.M.; Beer, M.; Harder, T.C.; Hafez, H.M. Influence of maternal immunity on vaccine efficacy and susceptibility of one day old chicks against Egyptian highly pathogenic avian influenza H5N1. Vet. Microbiol. 2012, 155, 13–20. [Google Scholar] [CrossRef]
- Kilany, W.H.; Bazid, A.H.; Ali, A.; El-Deeb, A.H.; El-Abideen, M.A.; Sayed, M.E.; El-Kady, M.F. Comparative Effectiveness of Two Oil Adjuvant-Inactivated Avian Influenza H9N2 Vaccines. Avian Dis. 2016, 60, 226–231. [Google Scholar] [CrossRef]
- Kilany, W.H.; Ali, A.; Bazid, A.H.; El-Deeb, A.H.; El-Abideen, M.A.; Sayed, M.E.; El-Kady, M.F. A Dose-Response Study of Inactivated Low Pathogenic Avian Influenza H9N2 Virus in Specific-Pathogen-Free and Commercial Broiler Chickens. Avian Dis. 2016, 60, 256–261. [Google Scholar] [CrossRef]
- Ingrao, F.; Ngabirano, E.; Rauw, F.; Dauphin, G.; Lambrecht, B. Immunogenicity and protective efficacy of a multivalent herpesvirus vectored vaccine against H9N2 low pathogenic avian influenza in chicken. Vaccine 2024, 42, 3410–3419. [Google Scholar] [CrossRef]
- Palya, V.; Tatar-Kis, T.; Walkone Kovacs, E.; Kiss, I.; Homonnay, Z.; Gardin, Y.; Kertesz, K.; Dan, A. Efficacy of a Recombinant Turkey Herpesvirus AI (H5) Vaccine in Preventing Transmission of Heterologous Highly Pathogenic H5N8 Clade 2.3.4.4b Challenge Virus in Commercial Broilers and Layer Pullets. J. Immunol. Res. 2018, 2018, 3143189. [Google Scholar] [CrossRef]
- Wang, H.; Tian, J.; Zhao, J.; Zhao, Y.; Yang, H.; Zhang, G. Current Status of Poultry Recombinant Virus Vector Vaccine Development. Vaccines 2024, 12, 630. [Google Scholar] [CrossRef]
- Romanutti, C.; Keller, L.; Zanetti, F.A. Current status of virus-vectored vaccines against pathogens that affect poultry. Vaccine 2020, 38, 6990–7001. [Google Scholar] [CrossRef]
- Hassanin, O.; Abdallah, F.; Mohamed, M.H.A.; Abdel Fattah, D.M. Influence of Marek’s disease virus vaccines on chicken melanoma differentiation-associated gene 5-dependent-type I interferon signal transduction pathway with a highlight on their secondary impact on the immune responses post Newcastle disease virus vaccination. Vet. Microbiol. 2019, 235, 248–256. [Google Scholar] [CrossRef]

| Group | Virus Isolation a (%) | Virus Load (log10 EID50/mL) | Protection Index (%) |
|---|---|---|---|
| G1 (rHVT-H9/Y280) | 0% (0/12) | Not Detected | 100 |
| G2 (Commercial Vac 1) | 16.7% (2/12) | 2.2 ± 0.5 | 72.2 |
| G3 (Commercial Vac 2) | 16.7% (2/12) | 2.0 ± 0.6 | 72.2 |
| G4 (Commercial Vac 3) | 25% (3/12) | 2.2 ± 0.8 | 58.3 |
| G5 (Pos Ctrl) | 60% (6/10) | 2.0 ± 0.5 | - |
| G6 (Neg Ctrl) | 0% (0/8) | Not Detected | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Kim, S.-W.; Park, J.-Y.; Son, J.-E.; Zheng, K.-Q.; Yu, C.-D.; Kim, K.-W.; Jeon, W.-B.; Choi, Y.-R.; Jang, H.-K.; Wei, B.; et al. Long-Term Immunogenicity and Protection of a rHVT-H9/Y280 Vaccine Against H9N2 Avian Influenza Virus in Commercial Layers with High Maternal Antibodies. Animals 2026, 16, 242. https://doi.org/10.3390/ani16020242
Kim S-W, Park J-Y, Son J-E, Zheng K-Q, Yu C-D, Kim K-W, Jeon W-B, Choi Y-R, Jang H-K, Wei B, et al. Long-Term Immunogenicity and Protection of a rHVT-H9/Y280 Vaccine Against H9N2 Avian Influenza Virus in Commercial Layers with High Maternal Antibodies. Animals. 2026; 16(2):242. https://doi.org/10.3390/ani16020242
Chicago/Turabian StyleKim, Sang-Won, Jong-Yeol Park, Ji-Eun Son, Kai-Qiong Zheng, Cheng-Dong Yu, Ki-Woong Kim, Won-Bin Jeon, Yu-Ri Choi, Hyung-Kwan Jang, Bai Wei, and et al. 2026. "Long-Term Immunogenicity and Protection of a rHVT-H9/Y280 Vaccine Against H9N2 Avian Influenza Virus in Commercial Layers with High Maternal Antibodies" Animals 16, no. 2: 242. https://doi.org/10.3390/ani16020242
APA StyleKim, S.-W., Park, J.-Y., Son, J.-E., Zheng, K.-Q., Yu, C.-D., Kim, K.-W., Jeon, W.-B., Choi, Y.-R., Jang, H.-K., Wei, B., & Kang, M. (2026). Long-Term Immunogenicity and Protection of a rHVT-H9/Y280 Vaccine Against H9N2 Avian Influenza Virus in Commercial Layers with High Maternal Antibodies. Animals, 16(2), 242. https://doi.org/10.3390/ani16020242






