An Anatomical Study on Canine Cadavers Investigating the Caudolateral Approach Involving the Elevation of the Anconeus Muscle and Splitting of the Triceps Brachii Muscle for the Potential Treatment of T-Y Humeral Fractures
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
Statistical Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Böhme, B.; d’Otreppe, V.; Ponthot, J.-P.; Balligant, M. Intraosseous stress distribution and bone interaction during load application across the canine elbow joint: A preliminary finite element analysis for determination of condylar fracture pathogenesis in immature and mature dogs. Res. Vet. Sci. 2016, 106, 143–1484. [Google Scholar]
- Jarvis, J.G.; D’Astous, J.L. The pediatric T-supracondylar fracture. J. Pediatr. Orthop. 1984, 4, 697–699. [Google Scholar] [CrossRef] [PubMed]
- Guiot, L.P.; Guillou, R.P.; Dejardin, L.M. Minimally invasive percutaneous medial plate rod osteosynthesis for treatment of bicondylar humeral fractures in dogs: Surgical technique and case report. Vet. Surg. 2019, 38, 34–40. [Google Scholar]
- McKee, W.M.; Macias, C.; Innes, J.F. Bilateral fixation of Y-T humeral condyle fractures via medial and lateral approaches in 29 dogs. J. Small Anim. Pract. 2005, 46, 217–226. [Google Scholar] [PubMed]
- Martini, F.M.; Boschi, P.; Lusetti, F.; Eid, C.; Bonardi, A. Treatment of Y-T Humeral Fractures with Polyaxial Locking Plate System (PAX) in 14 Dogs. Vet. Sci. 2022, 9, 310. [Google Scholar] [CrossRef]
- Böhmer, E.; Matis, U.; Waibl, H. Zur operativen Darstellung des Processus anconaeus ulnae beim Hund (Modifikation des Zugangs von Chalman und Slocum). Tierärztl. Prax. 1987, 15, 425–429. [Google Scholar]
- García, J.; Yeadon, R.; Solano, M.A. Bilateral locking compression plate and transcondylar screw fixation for stabilization of canine bicondylar humeral fractures. Vet. Surg. 2020, 49, 1183–1194. [Google Scholar] [CrossRef]
- Bryan, R.S.; Morray, B.F. Extensive posterior exposure of the elbow. A triceps sparing approach. Clin. Orthop. Relat. Res. 1982, 166, 188–192. [Google Scholar] [CrossRef]
- Kvale, E.; Kalmukov, I.; Grassato, L.; Kalff, S.; Solano, M. Epicondylar plate fixation of humeral condylar fractures in immature French bulldogs: 45 cases (2014–2020). J. Small. Anim. Pract. 2022, 63, 532–541. [Google Scholar] [CrossRef]
- Mostosky, U.V.; Cholvin, N.R.; Brinker, W.O. Transolecranon approach to the elbow joint. Vet. Med. 1959, 54, 560–568. [Google Scholar]
- Duelan, R. Triceps tenotomy approach for distal fractures of the canine humerus. J. Am. Vet. Med. Assoc. 1974, 165, 82–86. [Google Scholar] [CrossRef]
- Lenehan, T.M.; Nunamaker, D.M. Lateral approach to the canine elbow by proximal ulnar diaphyseal osteotomy. J. Am. Vet. Med. Assoc. 1982, 180, 523–530. [Google Scholar] [CrossRef]
- Bartonicek, J.; Nanka, O. History of surgical approaches in orthopaedics. Int. Orthop. 2025, 49, 1537–1541. [Google Scholar] [CrossRef] [PubMed]
- Amman, K.; Seiferle, E.; Pelloni, G. Atlas of Topographical Surgical Anatomy of the Dog; Paul Parey: Berlin/Hamburg, Germany, 1978. [Google Scholar]
- Piermattei, D.L. An Atlas of Surgical Approaches to the Bones and Joints of the Dog and Cat, 3rd ed.; W.B Saunders Company Philadelphia: Philadelphia, PA, USA, 1993. [Google Scholar]
- Latorre, R.; Gil, F.; Climent, S.; Lopez, O.; Henry, R.; Ayala, M.; Ramirez, G.; Martinez, F.; Vazquez, J. Color Atlas of Surgical Approaches to the Bones and Joints of the Dog and Cat. Fore Limb and Hind Limb; Inter-Medica: Buenos Aires, Argentina, 2010. [Google Scholar]
- Wilkinson, J.M.; Stanley, D. Posterior surgical approach to the elbow: A comparative anatomic study. J. Shoulder Elb. Surg. 2001, 10, 380–382. [Google Scholar] [CrossRef]
- Ganta, A.; Goldstein, A.; Lezak, B.; Campbell, H.; Egol, K.; Konda, S. Triceps-sparing versus triceps-splitting approaches for OTA 12A-C and 13A2-3 distal-third humeral shaft fractures have similar 1 year functional outcomes. Eur. J. Orthop. Surg. Traumatol. 2025, 35, 328. [Google Scholar] [CrossRef] [PubMed]
- Rankin, I.A.; Dixon, J.; Goffin, J.; Johnstone, A.J. A modified surgical approach to the distal humerus: The triceps bundle technique. Strateg. Trauma Limb Reconstr. 2024, 19, 99. [Google Scholar] [CrossRef]
- Jitpraikulsarn, S.; Hirunyhanawiwat, T.; Patamamongkonchal, C.; Thremthakanpon, W.; Gromprasit, A. Triceps reflecting anconeus pedicle approach: A versatile exposure for intercondylar fractures of the humerus. Acta Orthop. Belg 2023, 89, 217–224. [Google Scholar] [CrossRef]
- Halling, K.B.; Lewis, D.D.; Cross, A.A.R.; Kerwin, S.C.; Smith, B.A.; Kubilis, P.S. Complication rate and factors affecting outcome of olecranon osteotomies repaired with pin and tension-band fixation in dogs. Can. Vet. J. 2002, 43, 528–534. [Google Scholar] [PubMed]
- Coles, C.P.; Barei, D.P.; Nork, S.E.; Taitsman, L.A.; Hanel, D.P.; Henley, M.B. The olecranon osteotomy: A six-year experience in the treatment of intraarticular fractures of the distal humerus. J. Orthop. Trauma 2006, 20, 163–170. [Google Scholar] [CrossRef]
- Garg, A.; Yadav, U.; Rakesh, S.; Nemani, A.A.M.; Yadav, N.; Kumar, R.; Chaudhary, A.; Mishra, R.K.; Panwar, B. Distal humerus dual plating with triceps tongue technique: A case series. Int. J. Orthop. Sci. 2021, 7, 93–97. [Google Scholar] [CrossRef]
- Feinstein, S.D.; Paterno, A.V.; Allen, A.D.; Jewell, E.; Wright, S.T.; Draeger, R.W. Techniques and fixation of olecranon osteotomy: A systematic review. J. Hand Surg. Glob. Online 2023, 5, 643–649. [Google Scholar] [CrossRef]
- Ilyas, A.M.; Jupiter, J.B. Treatment of distal humerus fractures. Acta Chir. Orthop. Traumatol. Cechoslov. 2008, 75, 6–15. [Google Scholar] [CrossRef]
- Fei, T.T.; Evans, P.J.; Bafus, B.T. Triceps fascial tongue exposure for intra-articular distal humerus fracture: Revisiting the Van Gorder approach. JSES Int. 2019, 4, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Kamali, Y. Five-headed triceps brachii muscle, with an unusual communication between the musculocutaneous and median nerves in a cross-breed dog cadaver: A case report. BMC Vet. Res. 2025, 21, 130. [Google Scholar] [CrossRef]
- Rakoczy, B. Act on the protection of animals used for scientific or educational purposes-legal regulation review. Pol. Yearb. Environ. Law 2016, 5, 79–88. [Google Scholar] [CrossRef]
- Altman, D.; Machin, D.; Bryant, T.; Gardner, M. Statistics with Confidence: Confidence Intervals and Statistical Guidelines, 2nd ed.; BMJ Books: Bristol, UK, 2000; pp. 37–39, 89–92. [Google Scholar]
- Joshi, R.A.; Helambe, S.N.; Desmukh, R.R. Digital image processing based surface area calculation. Int. J. Innov. Technol. Explor. Eng. 2020, 10, 186–190. [Google Scholar] [CrossRef]
- Khojastehnazhand, M.; Omid, M.; Tabatabaeefar, A. Determination of Orange Volume and Surface Area Using Image Processing Technique. Int. Agrophysics 2009, 23, 237–242. [Google Scholar]
- Bhedi, A.; Saxena, A.K.; Gadani, R.; Patel, R. Digital photography and transparency-based methods for measuring wound surface area. Indian J. Surg. 2013, 75, 111–114. [Google Scholar] [CrossRef]
- Afathi, M.; Peltier, E.; Adetchessi, T.; Graillon, T.; Dufour, H.; Fuentes, S. Minimally invasive transmuscular approach for the treatment of benign intradural extramedullary spinal cord tumours: Technical note and results. Neurochirurgie 2015, 61, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Hoh, D.J.; Wang, M.Y.; Ritland, S.L. Anatomic features of the paramedian muscle-splitting approaches to the lumbar spine. Neurosurgery 2010, 66, ons13–ons25. [Google Scholar] [CrossRef] [PubMed]
- Poetscher, A.W.; Ribas, G.C.; Yasuda, A.; Nishikuni, K. Para-muscular and trans-muscular approaches to the lumbar inter-vertebral foramen. Arq. Neuro-Psiquiatr 2005, 63, 46–49. [Google Scholar] [CrossRef]
- Tanaka, N.; Kitagawa, M.; Ito, D.; Watari, T. A modified lateral muscle-separation approach for mini-hemilaminectomy. J. Small Anim. Pract. 2013, 29, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Vezzoni, A.; Benjamino, K. Canine elbow dysplasia: Ununited anconeal process, osteochondritis dissecans, and medial coronoid process disease. Vet. Clin. Small Anim. Pract. 2021, 51, 439–474. [Google Scholar] [CrossRef]
- Chalman, J.A.; Slocum, B. The caudolateral approach to the canine elbow joint. J. Am. Anim. Hosp. Assoc. 1983, 19, 637–641. [Google Scholar]
- Schaaf, O.R.; Roennfeldt-Philip, T.; Webster, N.S. Anconeus muscle injury in a juvenile greyhound. Vet. Surg. 2020, 49, 1648–1657. [Google Scholar] [CrossRef]
- Van der Werf, B.A.; Hidaka, T.; Creed, K.E. Continence and some properties of the urethral striated muscle of male greyhounds. BJU Int. 2000, 85, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Osiak-Wicha, C.; Kras, K.; Arciszewski, M.B. Comparative analysis of muscle fibers in selected muscles of working and companion dog breeds. Animals 2024, 14, 3576. [Google Scholar] [CrossRef] [PubMed]
- Illical, E.M.; Farrel, D.J.; Siska, P.A.; Evans, A.R.; Gruen, G.S.; Tarkin, I.S. Comparison of outcomes after triceps split versus sparing surgery for extra-articular distal humerus fractures. Injury 2014, 45, 1545–1548. [Google Scholar] [CrossRef]
- Yeh, P.C.; El Attrache, N.S.; Mazzocca, A.; Katie, V.; Sethi, P.M. Distal Triceps Tendon Repair Using an Anatomic Footprint Repair. Tech. Shoulder Elb. Surg. 2011, 12, 62–66. [Google Scholar] [CrossRef]
- Earley, N.F.; Ellse, G.; Wallace, A.M.; Parsons, K.J.; Voss, K.; Pugliese, L.C.; Moores, A.P.; Whitelock, R.; Stork, C.; Langley-Hobbs, S.J.; et al. Complications and outcomes associated with 13 cases of triceps tendon disruption in dogs and cats (2003–2014). Vet. Rec. 2018, 182, 108. [Google Scholar] [CrossRef]
- Perez-Merino, E.M.; Uson-Gargallo, J.; Sanchez-Margallo, F.M.; Uson-Casans, J.M. Comparison of the use of fresh-frozen canine cadavers and a realistic composite ex vivo simulator for training in small animal flexible gastrointestinal endoscopy. J. Am. Vet. Med. Assoc. 2018, 252, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Eisma, R.; Mahendran, S.; Majumdar, S.; Smith, D.; Soames, R.W. A comparison of Thiel and formalin embalmed cadavers for thyroid surgery training. Surgeon 2011, 9, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Lewis, C.E.; Peacock, W.J.; Tillou, A.; Hines, O.J.; Hiatt, J.R. A novel cadaver-based educational program in general surgery training. J. Surg. Educ. 2012, 69, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Denny, H.R. Condylar fractures of the humerus in the dog. A review of 133 cases. J. Small. Anim. Pract. 1983, 24, 185–197. [Google Scholar] [CrossRef]
- Chevalier, L.; Bouvy, B.; Hamon, M.; Picavet, P.P. Buried transcondylar locking screws ensure stable fixation and favorable owner-reported outcomes in canine humeral condylar fractures. Am. J. Vet. Res. 2025. [Google Scholar] [CrossRef]
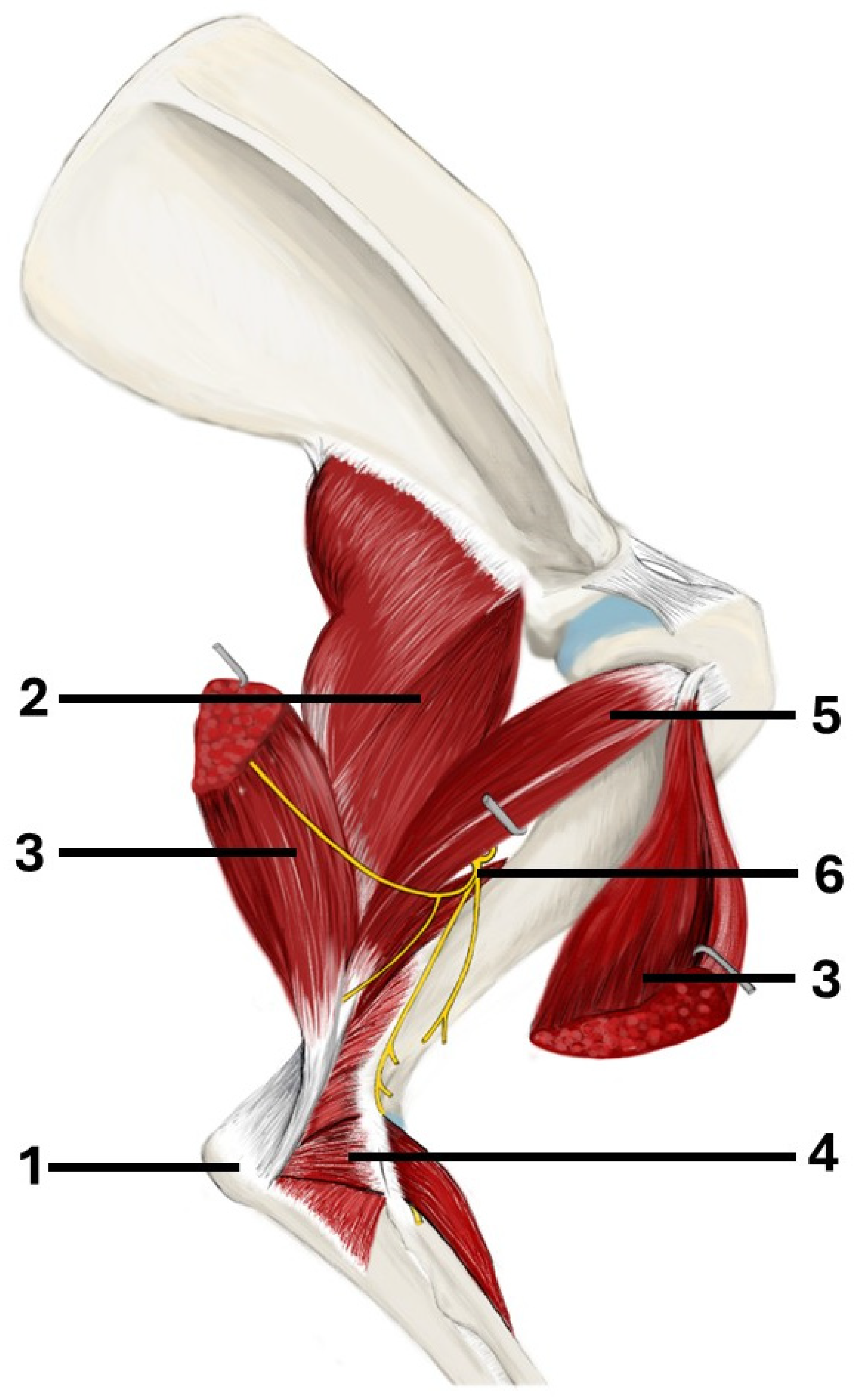
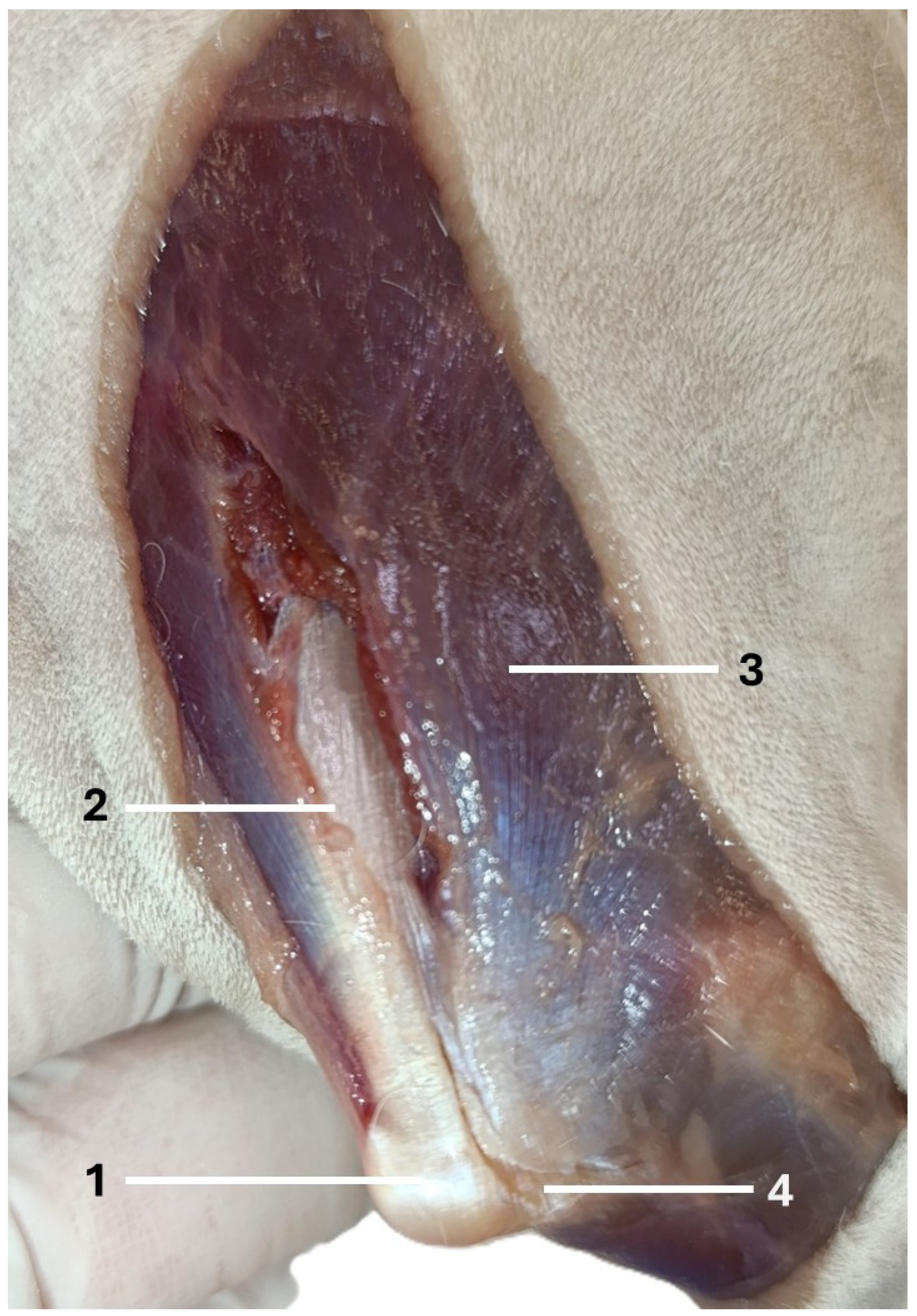
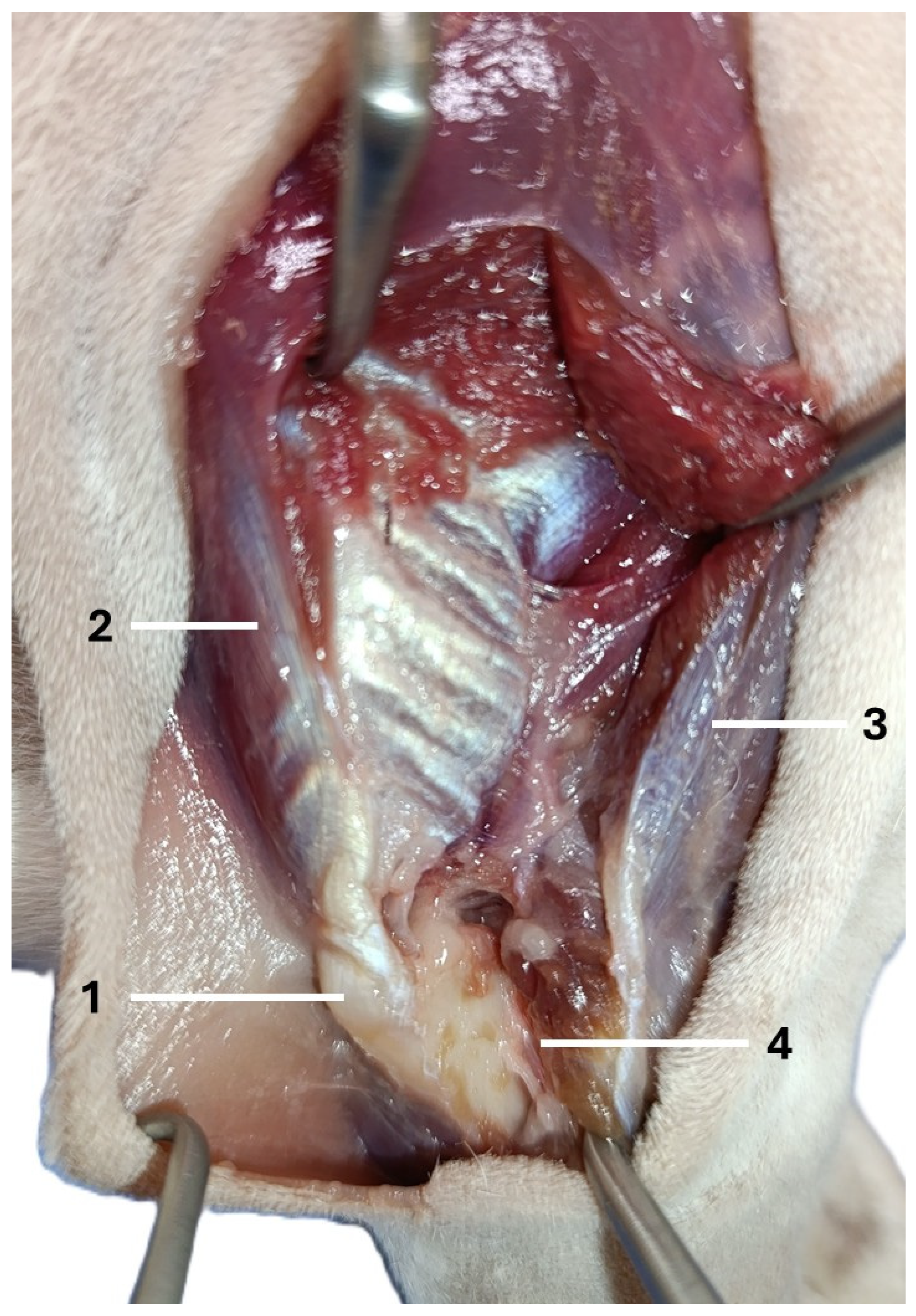
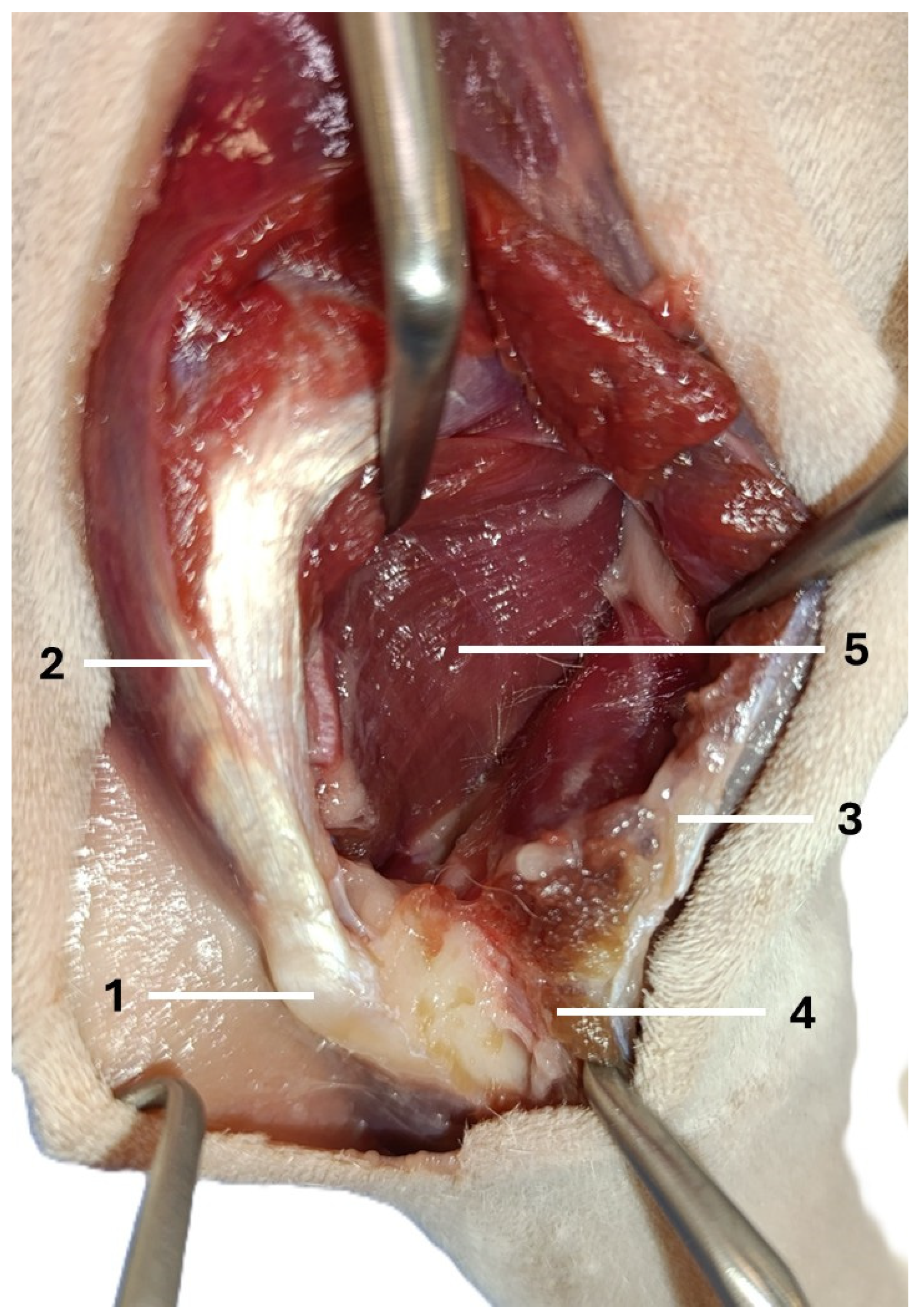
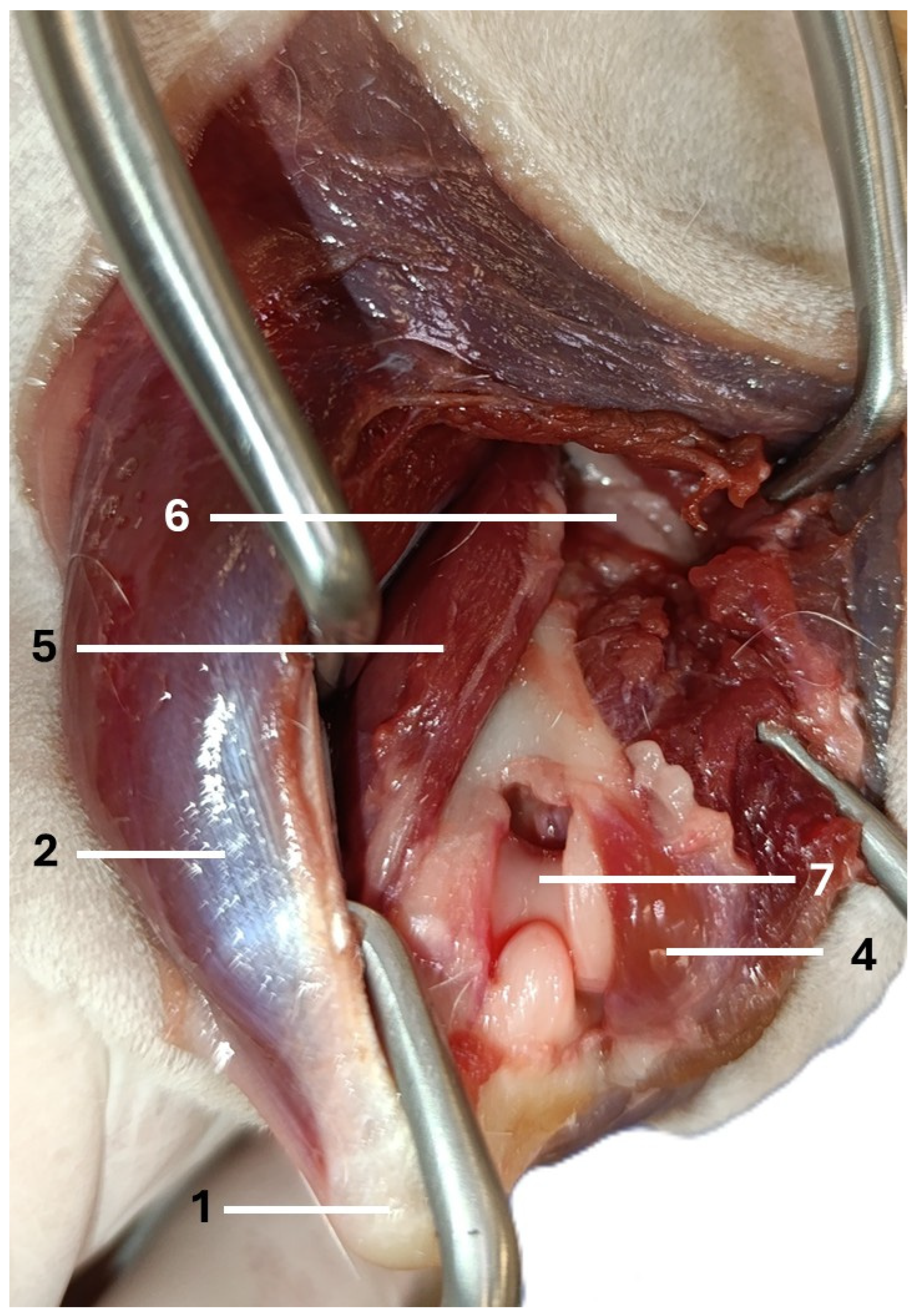
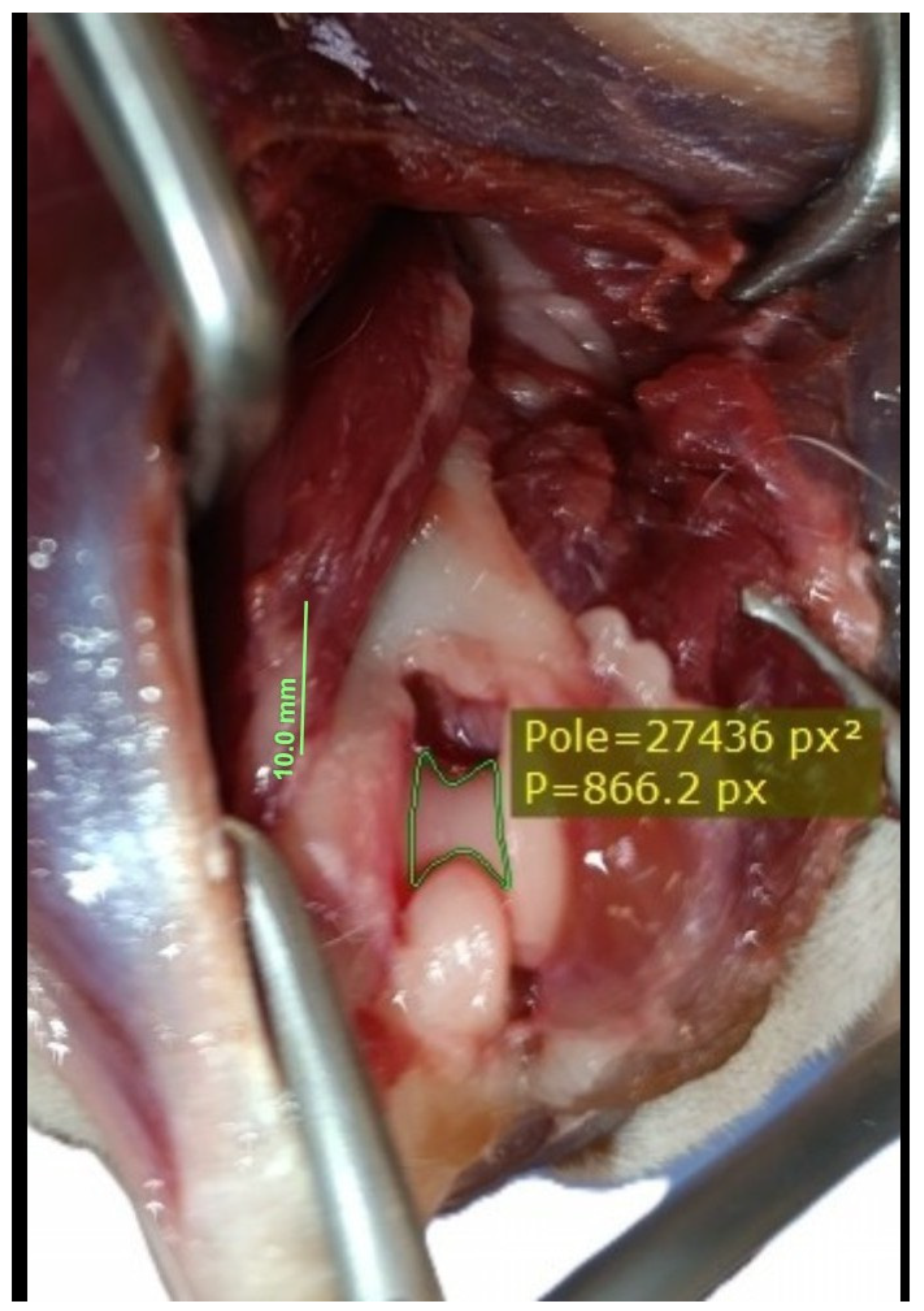
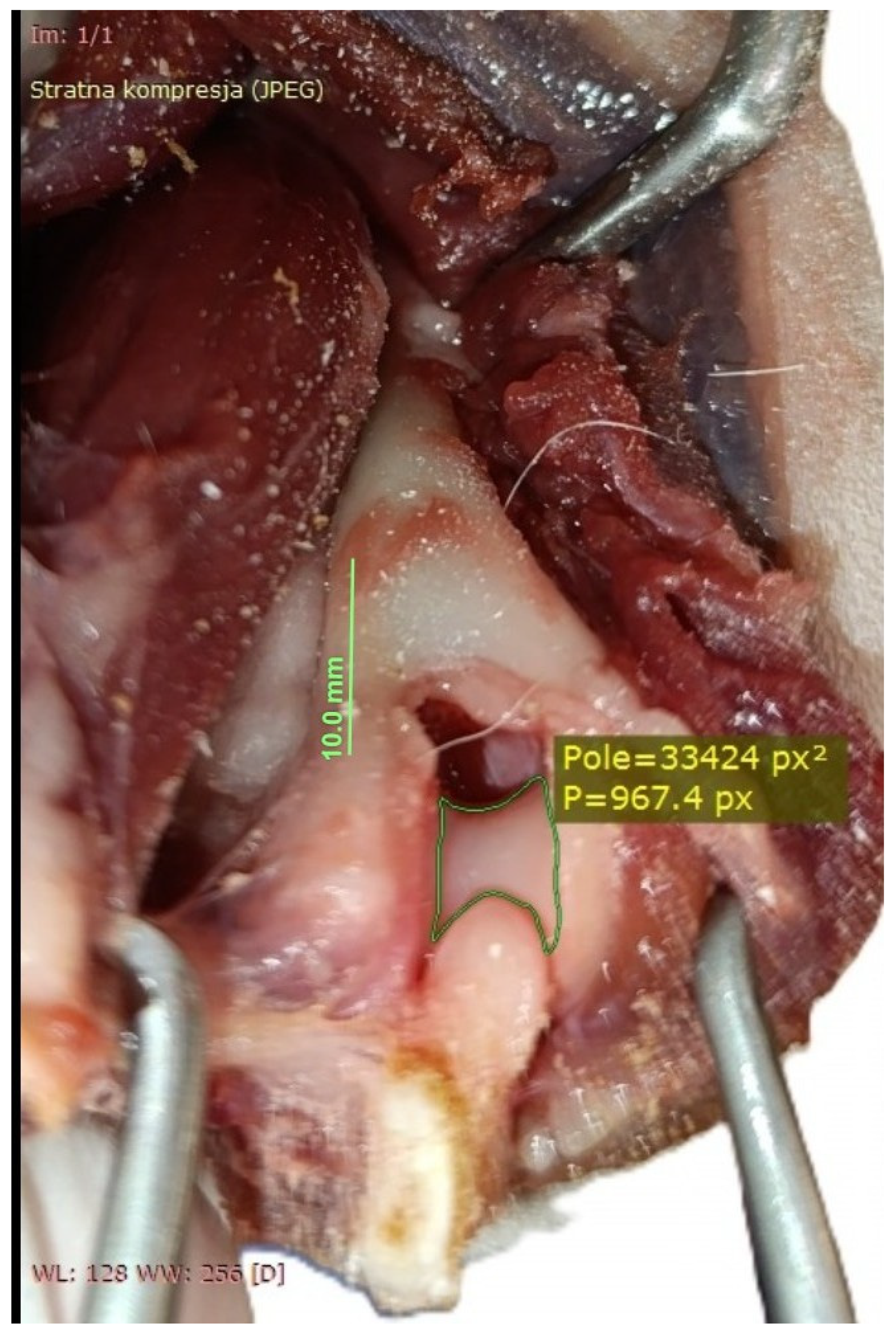
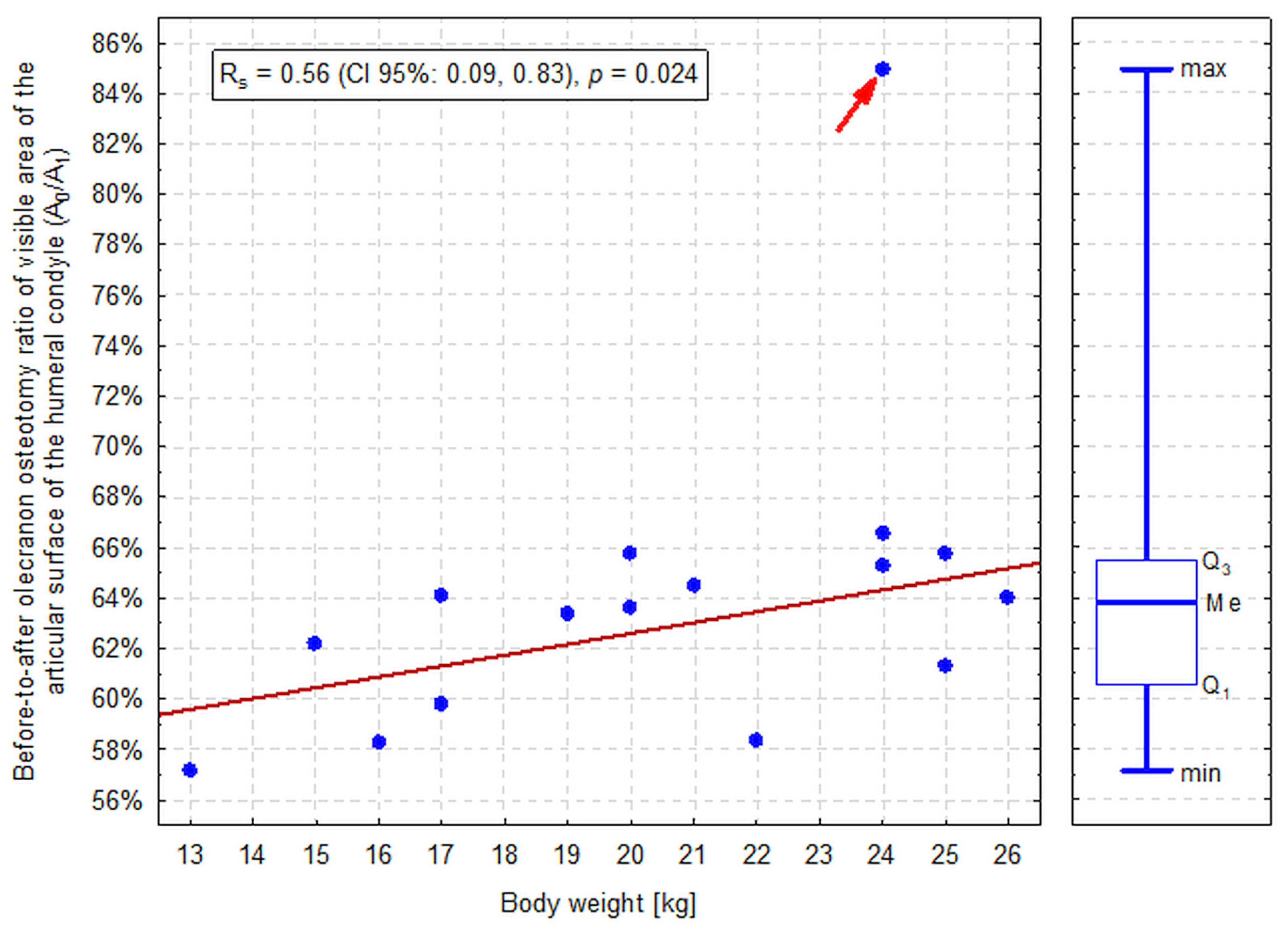
| Visible Area | Left Elbow (n = 16) | Right Elbow (n = 16) | Median Difference (CI 95%) | p-Value a |
|---|---|---|---|---|
| Before olecranon osteotomy (A0) [×103 px2] | 38.7, 25.0–40.3 (16.5–41.4) | 38.0, 27.1–39.6 (17.0–41.0) | 0.36 (−0.65, 0.76) | 0.698 |
| After olecranon osteotomy (A1) [×103 px2] | 59.2, 41.9–61.5 (29.6–66.2) | 59.1, 41.4–61.1 (29.0–66.0) | 0.14 (−0.50, 0.67) | 0.605 |
| A0 to A1 ratio (A0/A1) | 63.3%, 58.7–64.9% (55.6–85.8%) | 63.6%, 60.1–65.4% (58.1–84.1%) | 0.3% (−3.0%, 1.4%) | 0.877 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Trębacz, P.; Frymus, J.; Czopowicz, M.; Barteczko, A.; Pawlik, M.; Kurkowska, A. An Anatomical Study on Canine Cadavers Investigating the Caudolateral Approach Involving the Elevation of the Anconeus Muscle and Splitting of the Triceps Brachii Muscle for the Potential Treatment of T-Y Humeral Fractures. Animals 2026, 16, 110. https://doi.org/10.3390/ani16010110
Trębacz P, Frymus J, Czopowicz M, Barteczko A, Pawlik M, Kurkowska A. An Anatomical Study on Canine Cadavers Investigating the Caudolateral Approach Involving the Elevation of the Anconeus Muscle and Splitting of the Triceps Brachii Muscle for the Potential Treatment of T-Y Humeral Fractures. Animals. 2026; 16(1):110. https://doi.org/10.3390/ani16010110
Chicago/Turabian StyleTrębacz, Piotr, Jan Frymus, Michał Czopowicz, Anna Barteczko, Mateusz Pawlik, and Aleksandra Kurkowska. 2026. "An Anatomical Study on Canine Cadavers Investigating the Caudolateral Approach Involving the Elevation of the Anconeus Muscle and Splitting of the Triceps Brachii Muscle for the Potential Treatment of T-Y Humeral Fractures" Animals 16, no. 1: 110. https://doi.org/10.3390/ani16010110
APA StyleTrębacz, P., Frymus, J., Czopowicz, M., Barteczko, A., Pawlik, M., & Kurkowska, A. (2026). An Anatomical Study on Canine Cadavers Investigating the Caudolateral Approach Involving the Elevation of the Anconeus Muscle and Splitting of the Triceps Brachii Muscle for the Potential Treatment of T-Y Humeral Fractures. Animals, 16(1), 110. https://doi.org/10.3390/ani16010110





