Geometric Morphometric Analysis of Sexual Dimorphism in the Bill of the White Stork (Ciconia ciconia)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Sex Identification
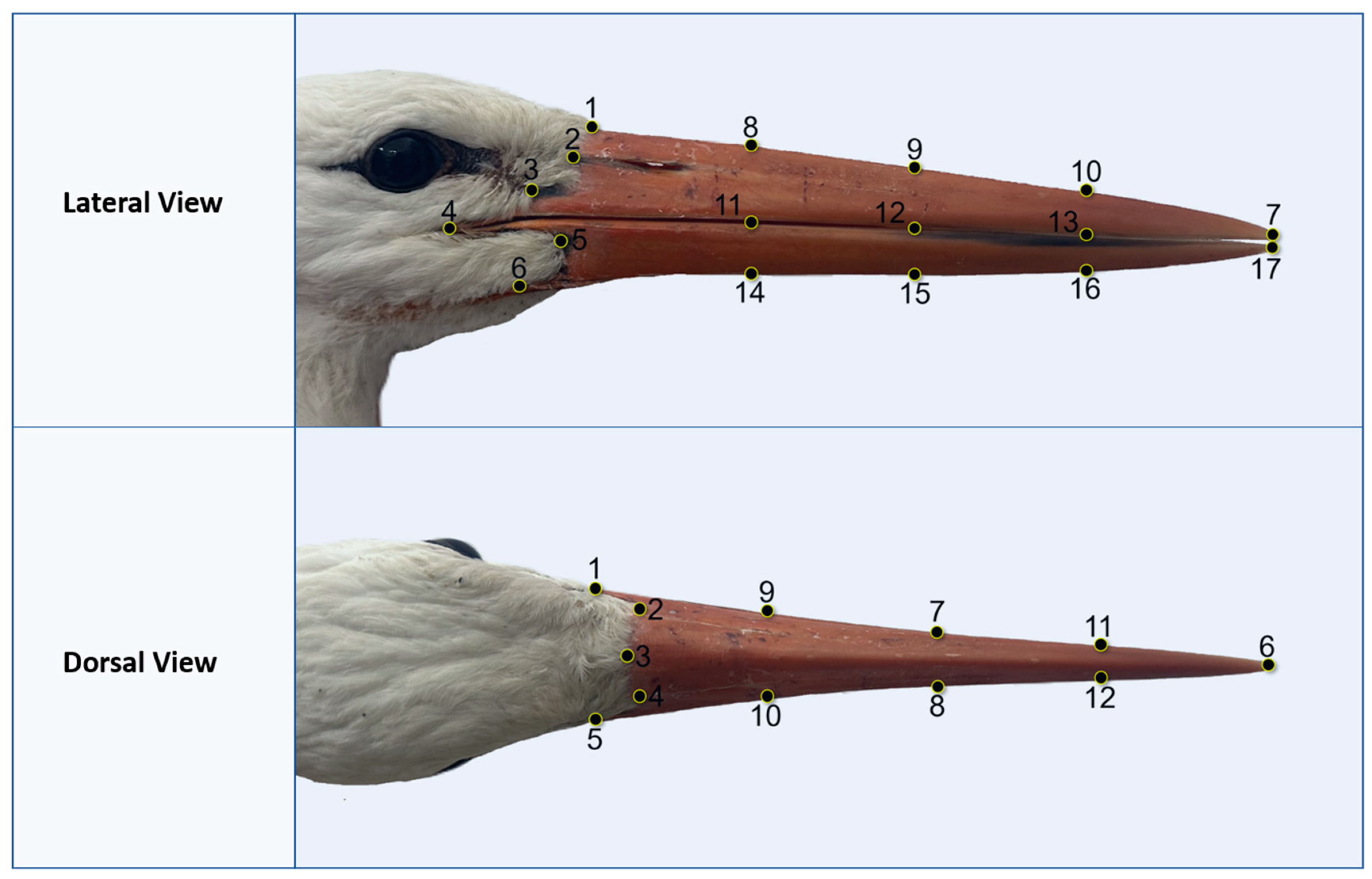
2.3. Data Collection and Analysis
3. Results
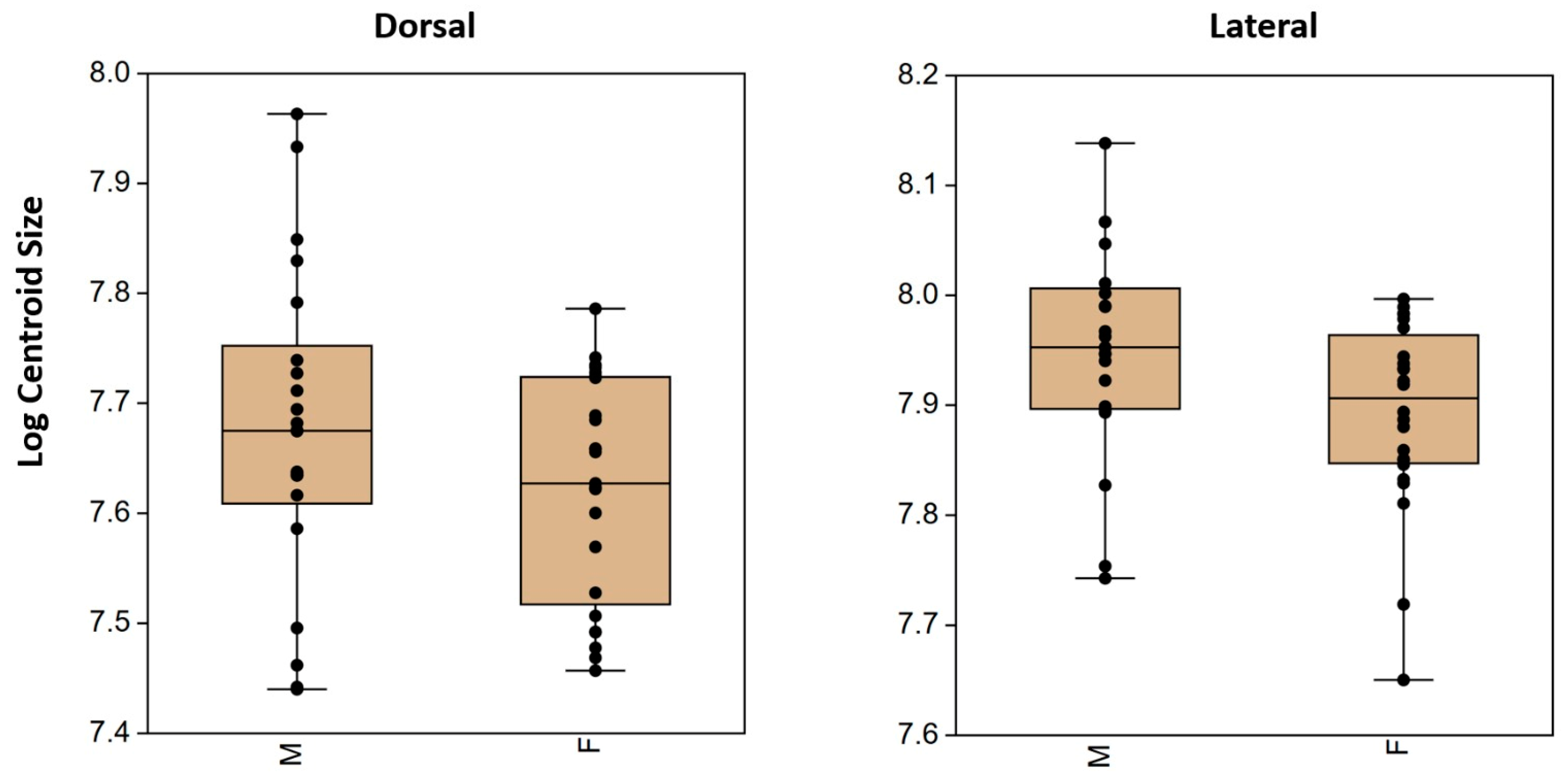
3.1. Size
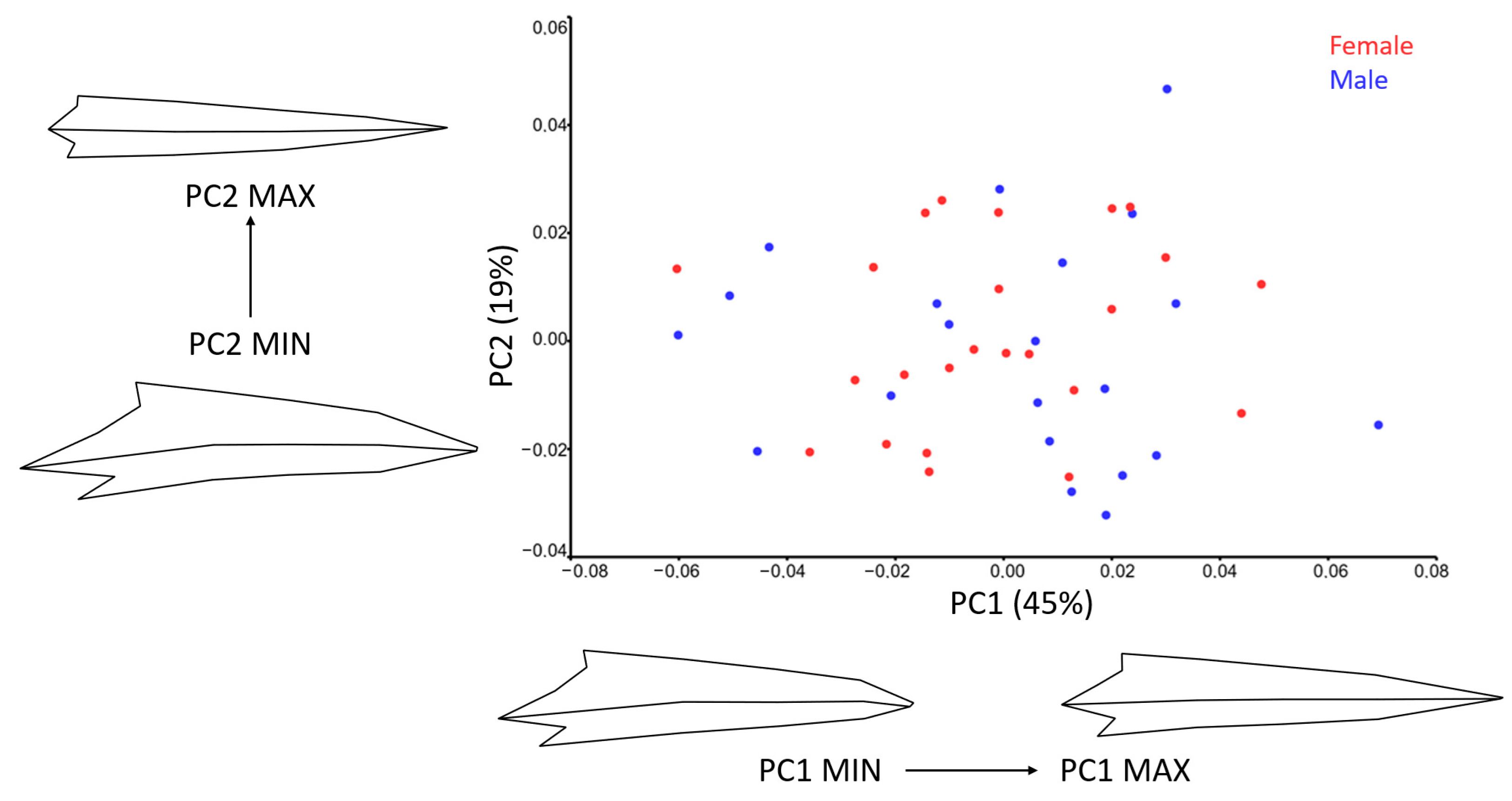
3.2. Shape
3.2.1. Dorsal View
3.2.2. Lateral View
3.3. Discriminant Function Analysis
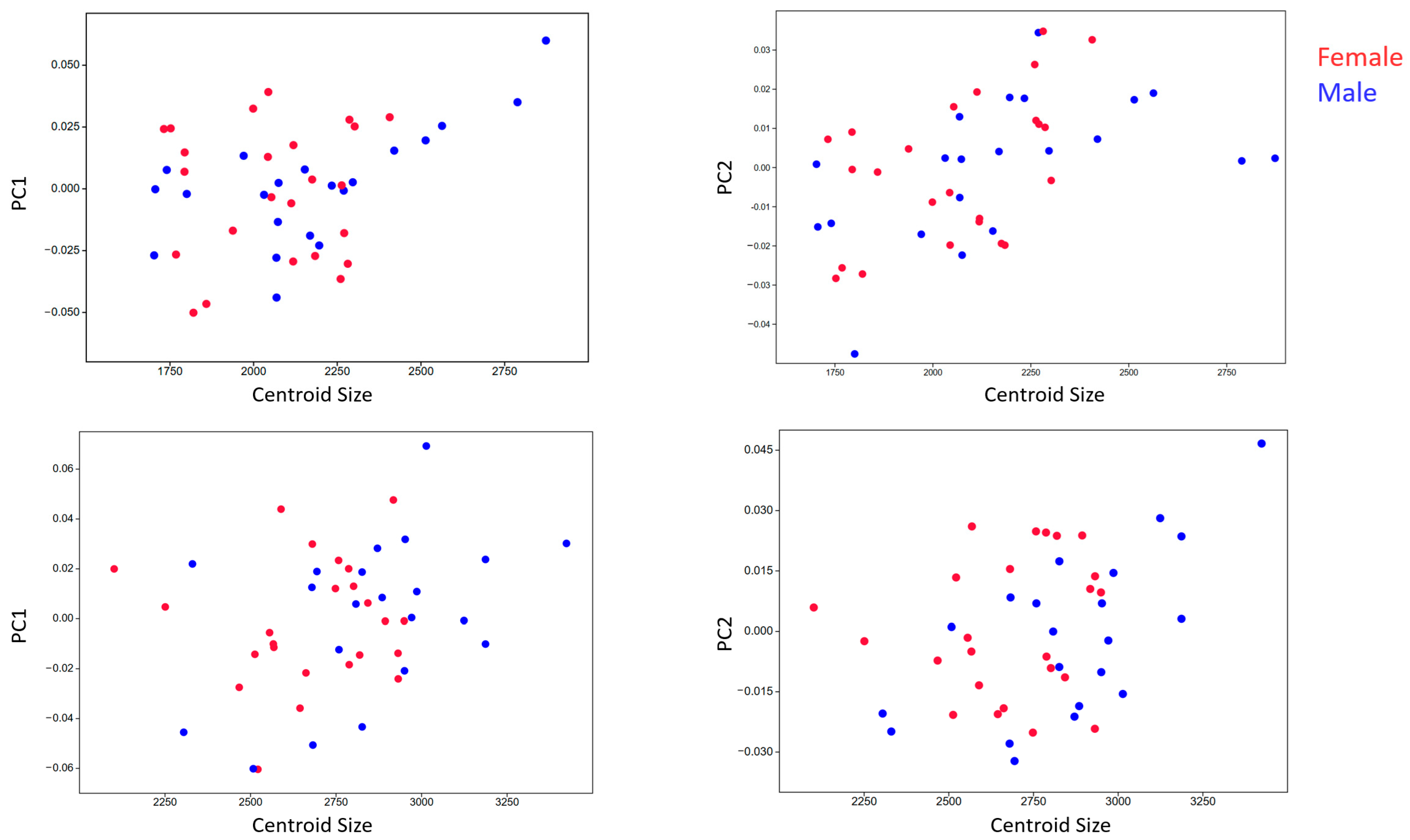
3.4. Allometry
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Süel, H. Türkiye’de leylek (Ciconia ciconia Linnaeus, 1758) dağılımının iklim değişikliğine göre kestirimi. Turk. J. 2019, 20, 243–249. [Google Scholar]
- Göcek, Ç.; Çiftçi, A.; Siki, M.; Tryjanowski, P. Breeding ecology of the white stork Ciconia ciconia in two localities of Turkey. Sandgrouse 2010, 32, 156–162. [Google Scholar]
- Hall, M.R.; Gwinner, E.; Bloesch, M. Annual cycles in moult, body mass, luteinizing hormone, prolactin, and gonadal steroids during the development of sexual maturity in the white stork (Ciconia ciconia). J. Zool. 1987, 211, 467–486. [Google Scholar] [CrossRef]
- Kahl, M.P. An overview of the storks of the world. Colon. Waterbirds 1987, 10, 131–134. [Google Scholar] [CrossRef]
- Leshem, Y.; Yom-Tov, Y. Routes of migrating soaring birds. Ibis 1998, 140, 41–52. [Google Scholar] [CrossRef]
- Darwin, C. The Descent of Man and Selection in Relation to Sex, 2nd ed.; John Murray: London, UK, 1874. [Google Scholar]
- Berns, C.M.; Adams, D.C. Becoming different but staying alike: Patterns of sexual size and shape dimorphism in bills of hummingbirds. Evol. Biol. 2012, 40, 246–260. [Google Scholar] [CrossRef]
- Sibley, C.G. The evolutionary and taxonomic significance of sexual dimorphism and hybridization in birds. Condor 1957, 59, 166–191. [Google Scholar] [CrossRef]
- Price, T.D. The evolution of sexual size dimorphism in Darwin’s finches. Am. Nat. 1984, 123, 500–518. [Google Scholar] [CrossRef]
- Wang, C.; Fang, Z. Ontogenetic variation and sexual dimorphism of bills among four cephalopod species based on geometric morphometrics. Animals 2023, 13, 752. [Google Scholar] [CrossRef]
- Székely, T.; Lislevand, T.; Figuerola, J. Sexual size dimorphism in birds. In Sex, Size and Gender Roles: Evolutionary Studies of Sexual Size Dimorphism; Fairbairn, D.J., Blanckenhorn, W.U., Székely, T., Eds.; Oxford University Press: Oxford, UK, 2007; pp. 27–37. [Google Scholar]
- Çakar, B.; Bulut, E.Ç.; Kahvecioğlu, O.; Günay, E.; Ruzhanova-Gospodinova, I.S.; Szara, T. Bill shape variation in selected species in birds of prey. Anat. Histol. Embryol. 2024, 53, e13085. [Google Scholar] [CrossRef]
- Özkan, E.; Günay, E.; Deveci, E.İ.; Manuta, N.; Çakar, B. Geometric morphometric analysis of bill shape of Columbimorphae (Columbas, Van, Mardin and Dönek). Anat. Histol. Embryol. 2024, 53, e13094. [Google Scholar] [CrossRef] [PubMed]
- Snow, D.; Perrins, C. The Birds of the Western Palearctic; Oxford University Press: Oxford, UK, 1998. [Google Scholar]
- Griffiths, R. Sex identification in birds. Semin. Avian Exot. Pet Med. 2000, 9, 14–26. [Google Scholar] [CrossRef]
- Schulz, H. Ciconia ciconia white stork. In The Birds of the Western Palearctic: Update 2; Oxford University Press: Oxford, UK, 1998; pp. 69–105. [Google Scholar]
- Elliott, A. Family Ciconiidae (storks). In Handbook of the Birds of the World, Volume 1: Ostrich to Ducks; del Hoyo, J., Elliott, A., Sargatal, J., Eds.; Lynx Edicions: Barcelona, Spain, 1992. [Google Scholar]
- Kazimirski, P.P.; Kaczmarski, M.; Zagalska-Neubauer, M.M.; Żołnierowicz, K.M.; Tobółka, M. Absence of sex differences in digit ratio in nestlings of the White Stork Ciconia ciconia, a monomorphic bird species. Bird Study 2019, 66, 503–509. [Google Scholar]
- Purwaningrum, M.; Nugroho, H.A.; Asvan, M.; Karyanti, K.; Alviyanto, B.; Kusuma, R.; Haryanto, A. Molecular techniques for sex identification of captive birds. Vet. World 2019, 12, 1506–1513. [Google Scholar] [CrossRef]
- Fridolfsson, A.K.; Ellegren, H. A simple and universal method for molecular sexing of non-ratite birds. J. Avian Biol. 1999, 30, 116–121. [Google Scholar] [CrossRef]
- Urfi, A.J. Sexual size dimorphism and mating patterns. In The Painted Stork; Springer: New York, NY, USA, 2011. [Google Scholar] [CrossRef]
- Hancock, J.A.; Kushlan, J.A.; Kahl, M.P. Storks, Ibises and Spoonbills of the World; Academic Press: London, UK, 1992. [Google Scholar]
- Komiya, T.; Sugita, H.; Matsushima, K. Sex determination in the eastern white stork, Ciconia ciconia boyciana, by morphological measurement. J. Jpn. Assoc. Zool. Aquar. 1986, 28, 55–60, (In Japanese with English summary). [Google Scholar]
- Murata, K.; Miyashita, M.; Nagase, K.; Komiya, T.; Matsushima, K. Sex determination in the eastern white stork, Ciconia c. boyciana, by bill measurements and discriminant analysis. J. Jpn. Assoc. Zool. Aquar. 1988, 30, 43–47. [Google Scholar]
- Cwiertnia, P.; Kwieciński, Z.; Kwiecińska, H.; Wysocki, A.; Tryjanowski, P.; Olsson, O. White Stork Study in Poland: Biology, Ecology and Conservation; Bogucki Wydawnictwo Naukowe: Poznań, Poland, 2006. [Google Scholar]
- Castiglioni, R.; Santoro, R. Sexual dimorphism in the acoustic signal of the European white stork (Ciconia ciconia, L. 1758): A pilot study. In Proceedings of the XI Convegno Nazionale Della Ricerca Nei Parchi, Verona, Italy, 1–3 October 2021. [Google Scholar] [CrossRef]
- Eda-Fujiwara, H.; Yamamoto, A.; Sugita, H.; Takahashi, Y.; Kojima, Y.; Sakashita, R.; Ogawa, H.; Miyamoto, T.; Kimura, T. Sexual dimorphism of acoustic signals in the oriental white stork: Non-invasive identification of sex in birds. Zool. Sci. 2004, 21, 817–821. [Google Scholar] [CrossRef]
- Kalam, A.; Urfi, A.J. Foraging behaviour and prey size of the painted stork. J. Zool. 2008, 274, 198–204. [Google Scholar] [CrossRef]
- Foster, D.J.; Podos, J.; Hendry, A.P. A geometric morphometric appraisal of bill shape in Darwin’s finches. J. Evol. Biol. 2008, 21, 263–275. [Google Scholar] [CrossRef]
- Schoener, T.W. The newest synthesis: Understanding the interplay of evolutionary and ecological dynamics. Science 2011, 331, 426–429. [Google Scholar] [CrossRef]
- Grant, P.; Grant, R. How and Why Species Multiply: The Radiation of Darwin’s Finches; Princeton University Press: Princeton, NJ, USA, 2008. [Google Scholar]
- Rohlf, F.J.; Marcus, L.F. A revolution in morphometrics. Trends Ecol. Evol. 1993, 8, 129–132. [Google Scholar] [CrossRef] [PubMed]
- Rohlf, F.J.; Slice, D. Extensions of the Procrustes method for the optimal superimposition of landmarks. Syst. Zool. 1990, 39, 40–59. [Google Scholar] [CrossRef]
- Bookstein, F.L. Morphometric Tools for Landmark Data: Geometry and Biology; Cambridge University Press: Cambridge, UK, 1991. [Google Scholar]
- Klingenberg, C.P. Evolution and development of shape: Integrating quantitative approaches. Nat. Rev. Genet. 2010, 11, 623–635. [Google Scholar] [CrossRef] [PubMed]
- Slice, D.E. Landmark coordinates aligned by Procrustes analysis do not lie in Kendall’s shape space. Syst. Biol. 2001, 50, 141–149. [Google Scholar] [CrossRef]
- Fang, Z.; Chen, X.; Su, H.; Thompson, K.; Chen, Y. Evaluation of stock variation and sexual dimorphism of bill shape of neon flying squid, Ommastrephes bartramii, based on geometric morphometrics. Hydrobiologia 2017, 784, 367–380. [Google Scholar] [CrossRef]
- Szara, T.; Günay, E.; Boz, I.; Batmankaya, B.; Gencer, H.; Gün, G.; Vatansever Çelik, E.C.; Gündemir, O. Bill shape variation in African penguin (Spheniscus demersus) held captive in two zoos. Diversity 2023, 15, 945. [Google Scholar] [CrossRef]
- Benítez, H. Sexual dimorphism using geometric morphometric approach. In Sexual Dimorphism; InTechOpen: London, UK, 2013; pp. 35–50. [Google Scholar] [CrossRef]
- Tyler, J.; Hocking, D.P.; Younger, J.L. Intrinsic and extrinsic drivers of shape variation in the albatross compound bill. R. Soc. Open Sci. 2023, 10, 230751. [Google Scholar] [CrossRef]
- Rohlf, F.J. TpsDig, version 1.4; Department of Ecology and Evolution, State University of New York: Stony Brook, NY, USA, 2004. [Google Scholar]
- Rohlf, F.J. TpsDig, Digitize Landmarks and Outlines, version 2.05; Department of Ecology and Evolution, State University of New York: Stony Brook, NY, USA, 2006. [Google Scholar]
- Klingenberg, C.P. MorphoJ: An integrated software package for geometric morphometrics. Mol. Ecol. Resour. 2011, 11, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Dryden, I.L.; Mardia, K.V. Statistical Shape Analysis; Wiley: Chichester, UK, 1998. [Google Scholar]
- Floate, K.D.; Fox, A.S. Flies under stress: A test of fluctuating asymmetry as a biomonitor of environmental quality. Ecol. Appl. 2000, 10, 1541–1550. [Google Scholar] [CrossRef]
- Klingenberg, C.P.; McIntyre, G.S. Geometric morphometrics of developmental instability: Analyzing patterns of fluctuating asymmetry with Procrustes methods. Evolution 1998, 52, 1363–1375. [Google Scholar] [CrossRef] [PubMed]
- Savaş, T.; Erdem, H. Sexual dimorphism in body size and some exterior traits of pigeon breed groups. J. Poult. Res. 2022, 19, 68–77. [Google Scholar] [CrossRef]
- Selander, R.K. Sexual dimorphism and differential niche utilization in birds. Condor 1966, 68, 113–151. [Google Scholar] [CrossRef]
- Sandercock, B.K. Assortative mating and sexual size dimorphism in Western and Semipalmated Sandpipers. Auk 1998, 115, 786–791. [Google Scholar] [CrossRef]
- Chardine, J.W.; Morris, R.D. Sexual size dimorphism and assortative mating in the Brown Noddy. Condor 1989, 91, 868–874. [Google Scholar] [CrossRef]
- Bildstein, K.L. Energetic consequences of sexual size dimorphism in white ibises (Eudocimus albus). Auk 1987, 104, 771–775. [Google Scholar] [CrossRef]
- Vergara, P.; Gordo, O.; Aguirre, J.I. Nest size, nest building behaviour and breeding success in a species with nest reuse: The white stork Ciconia ciconia. Ann. Zool. Fenn. 2010, 47, 184–194. [Google Scholar] [CrossRef]
- Wagner, R.H. Sexual size dimorphism and assortative mating in razorbills (Alca torda). Auk 1999, 116, 542–544. [Google Scholar] [CrossRef]
- Helfenstein, F.; Danchin, E.; Wagner, R.H. Assortative mating and sexual size dimorphism in black-legged kittiwakes. Waterbirds 2004, 27, 350–354. [Google Scholar] [CrossRef]
- Indykiewicz, P.; Manuta, N.; Bonecka, J.; Gündemir, O.; Szara, T. Is the skull of the black-headed gull dimorphic? Analysis of shape variation. Eur. Zool. J. 2025, 92, 174–181. [Google Scholar] [CrossRef]
- Bright, J.A.; Marugán-Lobón, J.; Cobb, S.N.; Rayfield, E.J. The shapes of bird bills are highly controlled by nondietary factors. Proc. Natl. Acad. Sci. USA 2016, 113, 5352–5357. [Google Scholar] [CrossRef] [PubMed]
- Cheong, S.; Sung, H.-C.; Park, S.-R. A new method for sexing oriental white storks. J. Field Ornithol. 2007, 78, 329–333. [Google Scholar] [CrossRef]




| Sum of Squares | Mean Square | F Statistic | p-Value | |
|---|---|---|---|---|
| Dorsal | 159,819.346 | 159,819.346 | 2.2284 | 0.1428 |
| Lateral | 306,785.1804 | 306,785.1804 | 5.0804 | 0.02935 |
| Dorsal | Lateral | |||||
|---|---|---|---|---|---|---|
| PC | Eigenvalue % | Variance % | Cumulative % | Eigenvalues % | Variance % | Cumulative % |
| PC1 | 0.000627 | 46.788 | 46.788 | 0.00080073 | 44.64 | 44.64 |
| PC2 | 0.00033007 | 24.63 | 71.418 | 0.0003435 | 19.15 | 63.80 |
| PC3 | 0.00015145 | 11.301 | 82.719 | 0.00015325 | 8.54 | 72.34 |
| Female-Male | Dorsal | Lateral |
|---|---|---|
| Procrustes Distance | 0.00765637 | 0.01131073 |
| p-Value | 0.5843 | 0.1590 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Günay, E.; Szara, T.; Çakar, B.; Deveci, E.İ.; Coşkun, A.S.; Gün, G.; Yiğit, F.; Gündemir, O.; Duro, S.; Spataru, M.C. Geometric Morphometric Analysis of Sexual Dimorphism in the Bill of the White Stork (Ciconia ciconia). Animals 2025, 15, 1312. https://doi.org/10.3390/ani15091312
Günay E, Szara T, Çakar B, Deveci Eİ, Coşkun AS, Gün G, Yiğit F, Gündemir O, Duro S, Spataru MC. Geometric Morphometric Analysis of Sexual Dimorphism in the Bill of the White Stork (Ciconia ciconia). Animals. 2025; 15(9):1312. https://doi.org/10.3390/ani15091312
Chicago/Turabian StyleGünay, Ebuderda, Tomasz Szara, Buket Çakar, Emine İrem Deveci, Ali Serhan Coşkun, Gökhan Gün, Funda Yiğit, Ozan Gündemir, Sokol Duro, and Mihaela Claudia Spataru. 2025. "Geometric Morphometric Analysis of Sexual Dimorphism in the Bill of the White Stork (Ciconia ciconia)" Animals 15, no. 9: 1312. https://doi.org/10.3390/ani15091312
APA StyleGünay, E., Szara, T., Çakar, B., Deveci, E. İ., Coşkun, A. S., Gün, G., Yiğit, F., Gündemir, O., Duro, S., & Spataru, M. C. (2025). Geometric Morphometric Analysis of Sexual Dimorphism in the Bill of the White Stork (Ciconia ciconia). Animals, 15(9), 1312. https://doi.org/10.3390/ani15091312








