Population Dynamics and Survival Strategies of Two Endangered Ungulates in a Low Water-Availability Site of the Maya Forest of Mexico
Simple Summary
Abstract
1. Introduction
- How the population index of relative abundance and the occupancy of the two species change over the years and over the sites?
- Are fluctuations of WLP and Tapir populations in phase with variations in water availability?
- What are the pond visitation patterns of the two species over ten years and what is affecting these patterns?
- Finally, what are the differences in activity patterns of the two species?
2. Materials and Methods
2.1. Study Area
2.2. Camera Trapping
2.3. Relative Abundance
2.4. Occupancy
2.5. Activity Patterns
3. Results
3.1. Relative Abundance Index
3.2. Occupancy Models
3.3. Water and WLP and Tapir Relationships
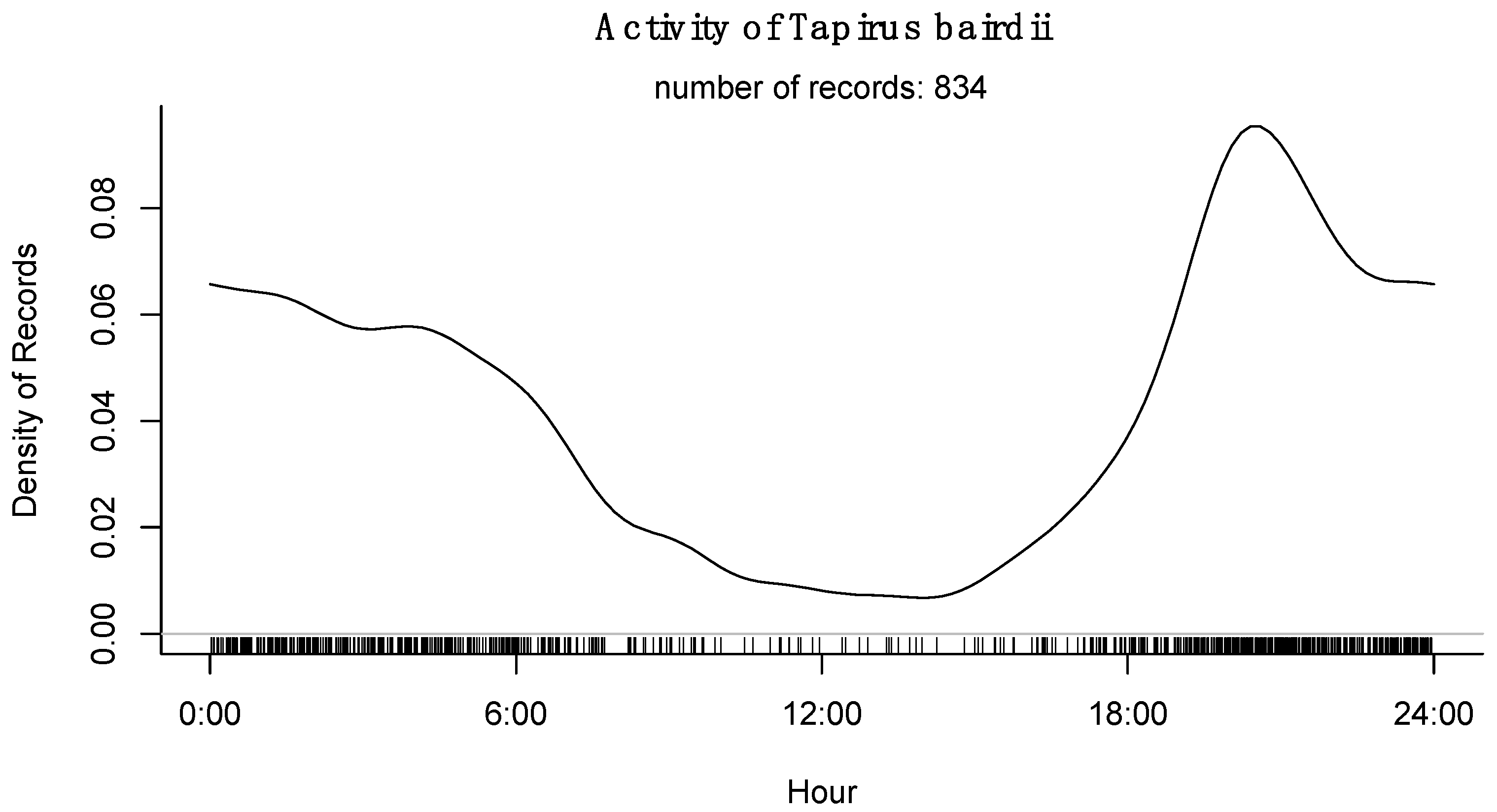
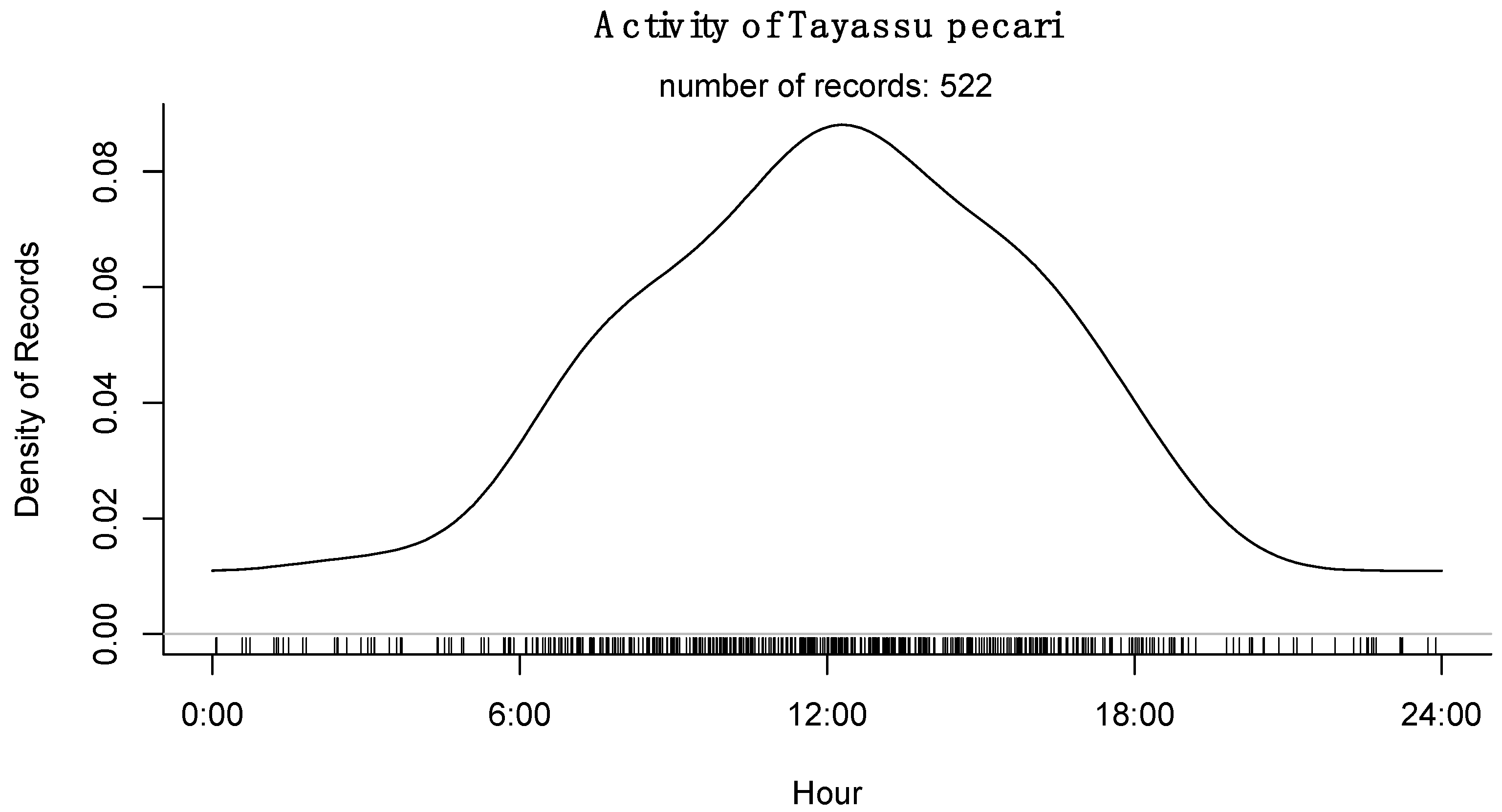
3.4. Activity Patterns
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reyna-Hurtado, R.; Chapman, C.A.; Calme, S.; Pedersen, E. Searching in heterogeneous environments: Foraging strategies in the white-lipped peccary (Tayassu pecari). J. Mammal. 2012, 93, 124–133. [Google Scholar] [CrossRef]
- McShea, W.J. Ecology and management of white-tailed deer in a changing world. Ann. N. Y. Acad. Sci. 2012, 1249, 45–56. [Google Scholar] [CrossRef]
- Fortes, V.B.; Bicca-Marques, J.C.; Urbani, B.; Fernández, V.A.; da Silva Pereira, T. Ranging behavior and spatial cognition of howler monkeys. In Howler Monkeys: BEC; Springer: Berlin/Heidelberg, Germany, 2015; pp. 219–255. [Google Scholar]
- Gillespie, T.R.; Chapman, C.A. Determinants of group size in the red colobus monkey (Procolobus badius): An evaluation of the generality of the ecological-constraints model. Behav. Ecol. Sociobiol. 2001, 50, 329–338. [Google Scholar] [CrossRef]
- Chapman, C.A.; Chapman, L.J. Constraints on group size in red colobus and red-tailed guenons: Examining the generality of the ecological constraints model. Int. J. Primatol. 2000, 21, 565–585. [Google Scholar] [CrossRef]
- Reyna-Hurtado, R.; O’Farrill, G.; Sima, D.; Andrade, M.; Padilla, A.; Sosa, L. Las aguadas de Calakmul, reservorios de fauna Silvestre y de la riqueza natural de México. Biodiversitas 2010, 93, 1–6. [Google Scholar]
- Reyna-Hurtado, R.; Rojas-Flores, E.; Tanner, G.W. Home range and habitat preferences of white-lipped peccaries (Tayassu pecari) in Calakmul Campeche, Mexico. J. Mammal. 2009, 90, 1199–1209. [Google Scholar] [CrossRef]
- Reyna-Hurtado, R.; Sanvicente-López, M.; Pérez-Flores, J.; Carrillo-Reyna, N.; Calmé, S. Insights into the multiannual home range of a Baird’s tapir (Tapirus bairdii) in the Maya Forest. Therya 2016, 7, 271–276. [Google Scholar] [CrossRef]
- Reyna-Hurtado, R.; Sima-Pantí, D.; Andrade, M.; Padilla, A.; Retana-Guaiscon, O.; Sanchez-Pinzón, K.; Martínez, W.; Meyer, N.; Moreira-Ramírez, J.F.; Carrillo-Reyna, N.; et al. Tapir population patterns under the disappearance of freestanding water. Therya 2019, 10, 353–358. [Google Scholar] [CrossRef]
- García Vetorazzi, M.J.; Manrique, R.L. Clasificación del hábitat potencial del tapir centroamericano (Tapirus bairdii Gill, 1865) para su conservación en Guatemala. Therya 2016, 7, 107–121. [Google Scholar] [CrossRef]
- Naranjo, E.J.; Amador-Alcalá, S.A.; Falconi-Briones, F.A.; Reyna-Hurtado, R.A. Distribución, abundancia y amenazas a las poblaciones de tapir (Tapirus bairdii) y pecarí de labios blancos (Tayassu pecari) en México. Therya 2015, 6, 227–249. [Google Scholar] [CrossRef]
- Reyna-Hurtado, R.; Domínguez, N.A. Baird’s Tapir social interactions, activity patterns, and site fidelity at ponds of the Maya Forest. Therya 2024, 15, 29–37. [Google Scholar] [CrossRef]
- Meyer, N.F.; Brenes-Mora, E.; Dans, A.J.; Estrada, N.; Cabrera, V.; García, M.J.; Martínez, W.; Poot, C.; Reyna-Hurtado, R.; Rivero, M.; et al. Ecology and Conservation of the Baird’s Tapir in Mesoamerica. Imperiled Encycl. Conserv. 2022, 144–154. [Google Scholar] [CrossRef]
- Sowls, L.K. Javelinas and the Other Peccaries: Their Biology, Management and Use, 2nd ed.; Texas A&M University Press: College Station, TX, USA, 1997; p. 325. [Google Scholar]
- Thornton, D.; Reyna, R.; Perera-Romero, L.; Radachowsky, J.; Hidalgo-Mihart, M.G.; Garcia, R.; McNab, R.; Mcloughlin, L.; Foster, R.; Harmsen, B.; et al. Precipitous decline of white-lipped peccary populations in Mesoamerica. Biol. Conserv. 2020, 242, 108410. [Google Scholar] [CrossRef]
- Fragoso, J.M.V. Home range and movement patterns of white-lipped peccary (Tayassu pecari) herds in the Northern Brazilian Amazon. Biotropica. 1998, 30, 458–469. [Google Scholar] [CrossRef]
- Beck, H. A review of peccary-palm interactions and their ecological ramifications across the Neotropics. J. Mammal. 2006, 87, 519–530. [Google Scholar] [CrossRef]
- Pérez-Flores, J.; Mardero, S.; López-Cen, A.; Contreras-Moreno, F.M. Human-wildlife conflicts and drought in the greater Calakmul Region, Mexico: Implications for tapir conservation. Neotrop. Biol. Conserv. 2021, 16, 539–563. [Google Scholar] [CrossRef]
- O’Connell, A.F.; Nichols, J.D.; Karanth, K.U. Camera Traps in Animal Ecology: Methods and Analyses; Springer: New York, NY, USA, 2011. [Google Scholar]
- Nichols, J.D.; O’Connell, A.F.; Ullas Karanth, K. Camera traps in animal ecology and conservation: What’s next? In Camera Traps in Animal Ecology: Methods and Analyses; Springer: Berlin/Heidelberg, Germany, 2011; pp. 253–263. [Google Scholar]
- MacKenzie, D.I. Occupancy models. In Introduction to Ecological Sampling; CRC Press: New York, NY, USA, 2015; pp. 123–143. [Google Scholar]
- MacKenzie, D.I.; Nichols, J.D.; Hines, J.E.; Knutson, M.G.; Franklin, A.B. Estimating site occupancy, colonization, and local extinction when a species is detected imperfectly. Ecology 2003, 84, 2200–2207. [Google Scholar] [CrossRef]
- Andrade-Ponce, R.; Mandujano, G.P.S.; Dáttilo, W.; Farías-González, V.; Jiménez, J.; Velásquez-C, K.; Zavaleta, A. A framework to interpret co-occurrence patterns from camera trap data: The case of the gray fox, the bobcat, and the eastern cottontail rabbit in a tropical dry habitat. J. Zool. 2022, 318, 91–103. [Google Scholar] [CrossRef]
- Mandujano, S. Índice de Abundancia Relativa: RAI. In Fototrampeo en R: Organización y Análisis de Datos Volúmen 1; Mandujano Rodríguez, S., Pérez Solano, L.A., Eds.; Instituto de Ecología A.C. Xalapa: Veracruz, Mexico, 2019; pp. 137–152. [Google Scholar]
- Carabias Lillo, J.; Provencio, E.; de la Maza Elvira, J.; Rodríguez de la Gala Méndez, J.B. Programa de Manejo de la Reserva de la Biosfera Calakmul; Instituto Nacional de Ecología Estado de México: Mexico City, México, 1999. [Google Scholar]
- Martínez, E.; Leal, C.G. La vegetación de Calakmul, Campeche, México: Clasificación, descripción y distribución. Bot. Sci. 2002, 71, 7–32. [Google Scholar] [CrossRef]
- Pérez-Cortez, S.; Enríquez, P.L.; Sima-Panti, D.; Reyna-Hurtado, R.; Naranjo, E.J. Influencia de la disponibilidad de agua en la presencia y abundancia de Tapirus bairdii en la selva de Calakmul Campeche, México. Rev. Mex. Biodivers. 2012, 83, 753–761. [Google Scholar] [CrossRef]
- Tobler, M.W.; Carrillo-Percastegui, S.E.; Leite Pitman, R.; Mares, R.; Powell, G. An evaluation of camera traps for inventorying large- and medium-sized terrestrial rainforest mammals. Anim. Conserv. 2008, 11, 169–178. [Google Scholar] [CrossRef]
- O’Brien, T.G. Abundance, Density and Relative Abundance: A conceptual framework. In Camera Traps in Animal Ecology: Methods and Analyses; O’Connell, A.F., Nichols, J.D., Karanth, K.U., Eds.; Springer: New York, NY, USA, 2011; pp. 71–96. [Google Scholar]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference; Springer: New York, NY, USA, 2002. [Google Scholar]
- Ridout, M.; Linkie, M. Estimating overlap of daily activity patterns from camera trap data. J. Agric. Biol. Environ. Stat. 2009, 14, 322–337. [Google Scholar] [CrossRef]
- Rowcliffe, M. Activity: Animal Activity Statistics. R Package Version 1.3.4. 2023. Available online: https://CRAN.R-project.org/package=activity (accessed on 25 October 2024).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Marschner, I.; Donoghoe, M.W.; Donoghoe, M.M.W. Package ‘glm2’. 2018. Available online: https://CRAN.R-project.org/package=glm2 (accessed on 20 February 2024).
- Louvrier, J.; Duchamp, C.; Lauret, V.; Marboutin, É.; Cubaynes, S.; Choquet, R.; Miquel, C.; Giménez, O. Mapping and explaining wolf recolonization in France using dynamic occupancy models and opportunistic data. Ecography 2017, 41, 647–660. [Google Scholar] [CrossRef]
- Jiménez-Sánchez, A.; Huerta-Rodríguez, J.O.; Poot-Sarmiento, I.; Duarte-Morales, A.; Reyna-Hurtado, R. Occupancy, relative abundance and activity patterns of three sympatric deer in ponds of Calakmul Biosphere Reserve, Campeche. Therya 2024, 15, 39–49. [Google Scholar] [CrossRef]
- Kellner, K.F.; Smith, A.D.; Royle, J.A.; Kery, M.; Belant, J.L.; Chandler, R.B. The unmarked R package: Twelve years of advances in occurrence and abundance modelling in ecology. Methods Ecol. Evol. 2023, 14, 1408–1415. [Google Scholar] [CrossRef]
- Olson, G.S.; Anthony, R.G.; Forsman, E.D.; Ackers, S.H.; Loschl, P.J.; Reid, J.A.; Dugger, K.M.; Glenn, E.M.; Ripple, W.J. Modeling of site occupancy dynamics for northern spotted owls, with emphasis on the effects of barred owls. J. Wildl. Manag. 2005, 69, 918–932. [Google Scholar] [CrossRef]
- Betts, M.G.; Rodenhouse, N.L.; Sillett, T.S.; Doran, P.J.; Holmes, R.T. Dynamic occupancy models reveal within-breeding season movement up a habitat quality gradient by a migratory songbird. Ecography 2008, 31, 592–600. [Google Scholar] [CrossRef]
- Rota, C.T.; Ferreira, M.A.R.; Kays, R.W.; Forrester, T.D.; Kalies, E.L.; McShea, W.J.; Parsons, A.W.; Millspaugh, J.J. A multispecies occupancy model for two or more interacting species. Methods Ecol Evol. 2016, 7, 1164–1173. [Google Scholar] [CrossRef]
- Fragoso, J.M.V.; Antunes, A.P.; Silvius, K.M.; Constantino, P.A.L.; Zapata-Ríos, G.; El Bizri, H.R.; Bodmer, R.E.; Camino, M.; de Thoisy, B.; Wallace, R.B.; et al. Large-scale population disappearances and cycling in the white-lipped peccary, a tropical forest mammal. PLoS ONE 2022, 17, e0276297. [Google Scholar] [CrossRef]
- Medici, E.P.; Mezzini, S.; Fleming, C.H.; Calabrese, J.M.; Noonan, M.J. Movement ecology of vulnerable lowland tapirs between areas of varying human disturbance. Mov. Ecol. 2022, 10, 14. [Google Scholar] [CrossRef]
- Keuroghlian, A.; Desbiez, A.; Reyna-Hurtado, R.; Altrichter, M.; Beck, H.; Taber, A.; Fragoso, J.M.V. Tayassu pecari. IUCN: E. T41778A44051115; IUCN Red List of Threatened Species: Cambridge, UK, 2013. [Google Scholar]
- Calme, S.; O’Farrill, G.; Perez-Flores, J.; Reyna-Hurtado, R.; Sanvicente-López, M.; Ceballos, G. Ecología y Conservación del Tapir en la Región de Calakmul. In El Tapir en México: Distribución, Ecología y Conservación; Ceballos, G., Mendoza, L., O’Farrill, G., Eds.; Universidad Nacional Autónoma de México: Ciudad de México, México, 2021; p. 201. ISBN 978-607-30-3805-8. [Google Scholar]
- Giljov, A.; Karenina, K. Social arenas in the open habitat: The social role of waterholes for saiga antelope. Therya 2024, 15, 182. [Google Scholar] [CrossRef]
- O’farrill, G.; Schampaert, K.G.; Rayfield, B.; Bodin, Ö.; Calmé, S.; Sengupta, R.; Gonzalez, A. The potential connectivity of waterhole networks and the effectiveness of a protected area under various drought scenarios. PLoS ONE 2014, 9, e95049. [Google Scholar] [CrossRef] [PubMed]
- Reyna-Hurtado, R.; Huerta-Rodríguez, J.O.; Rojas-Flores, E. Extreme fighting and vocalizations in Tapirus bairdii: Observations from aguadas of Calakmul, social arenas for the species. Neotrop. Biol. Conserv. 2025, 20, 67–78. [Google Scholar] [CrossRef]
- Bernstein, L.; Bosch, P.; Canziani, O.; Chen, Z.; Christ, R.; Riahi, K. Climate Change 2007: Synthesis Report; WMO: Geneva, Switzerland, 2008. [Google Scholar]

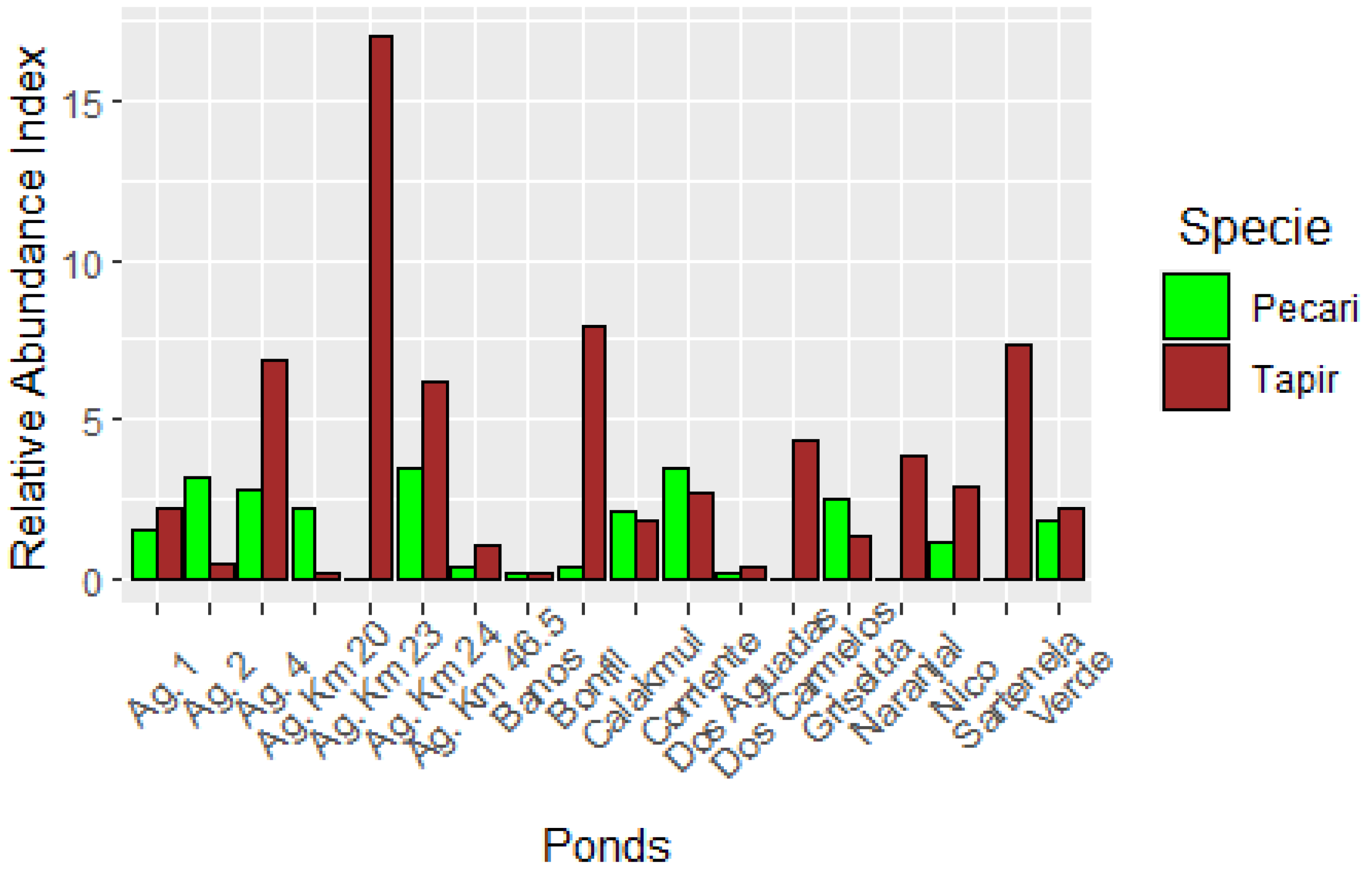
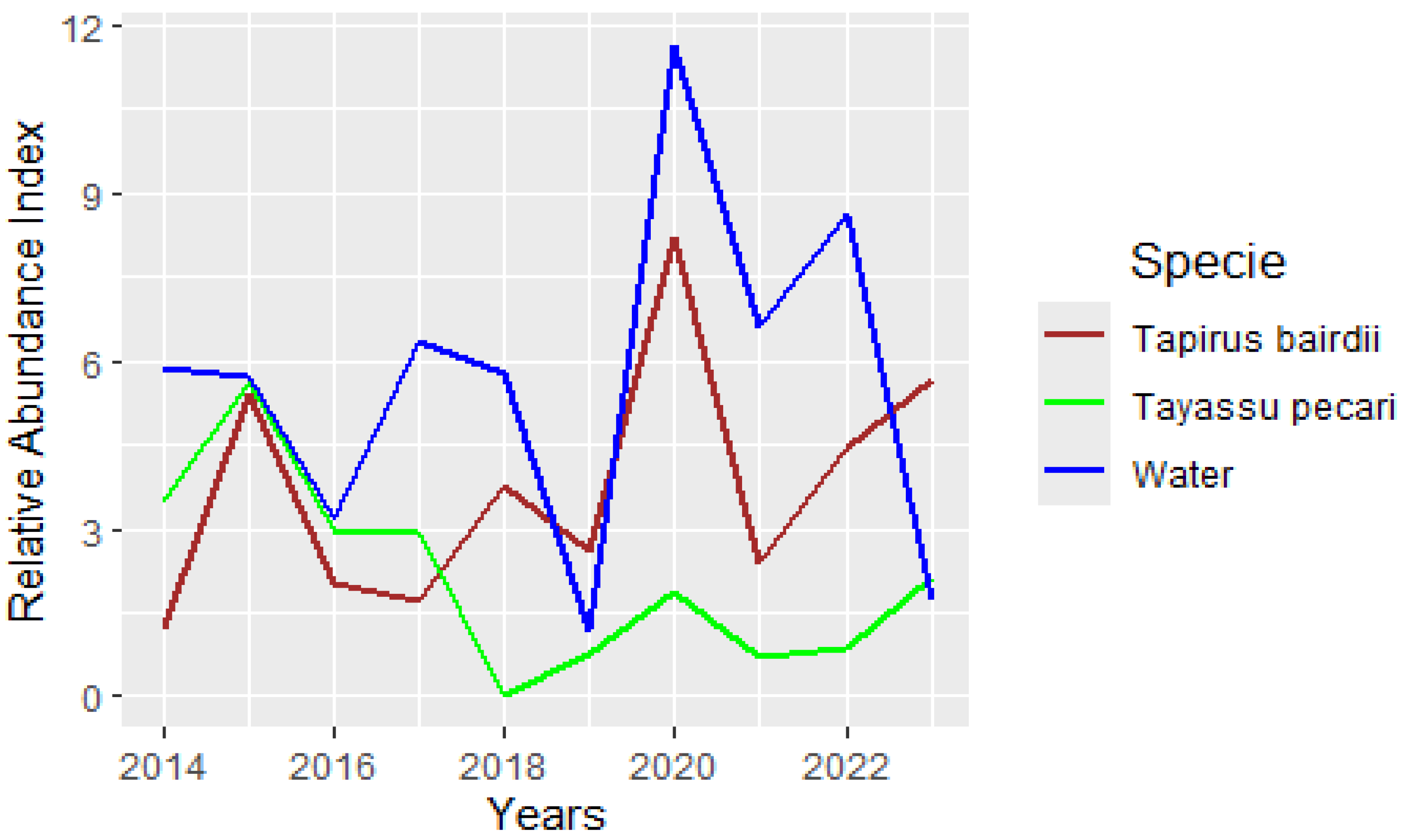
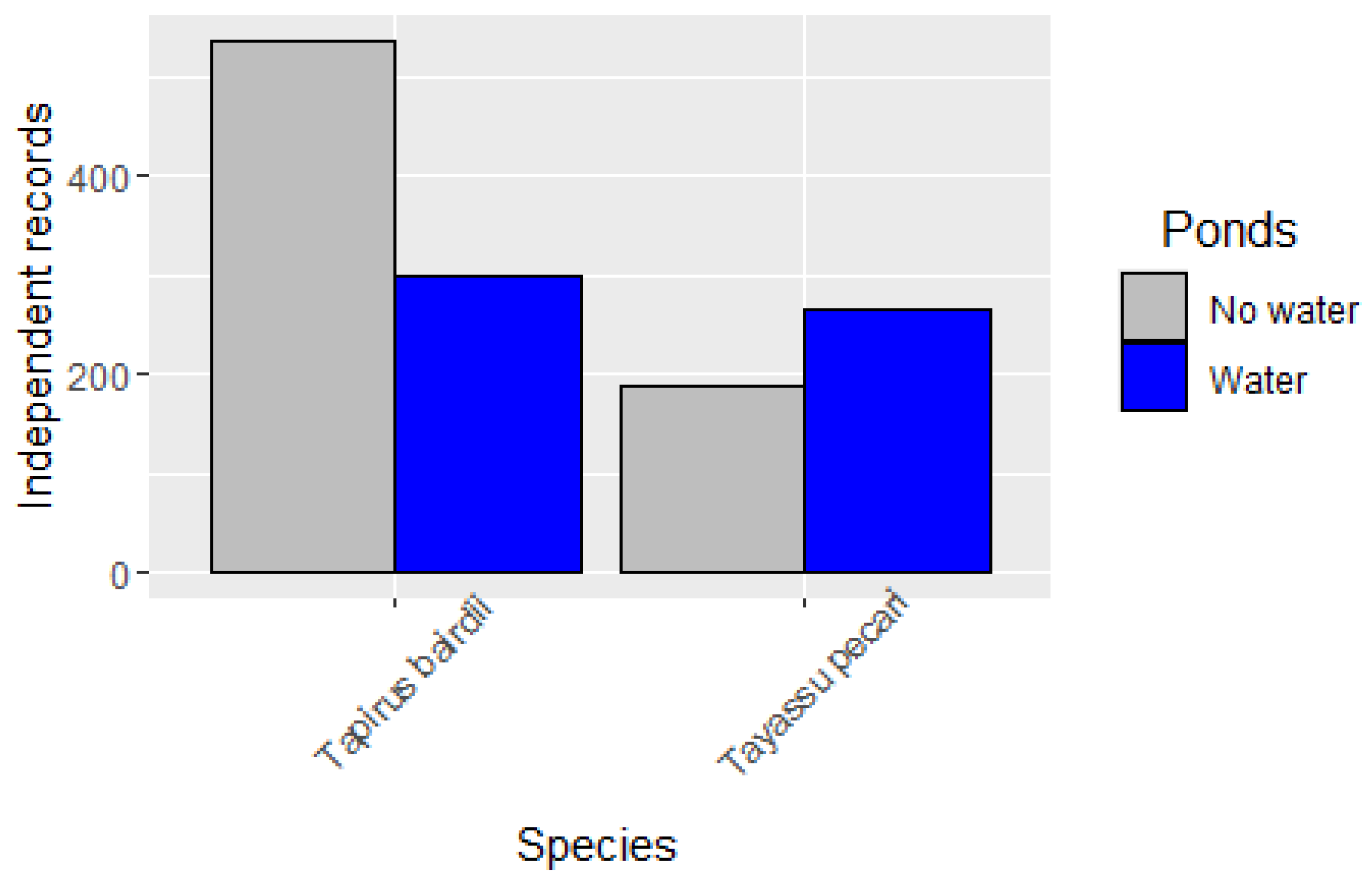
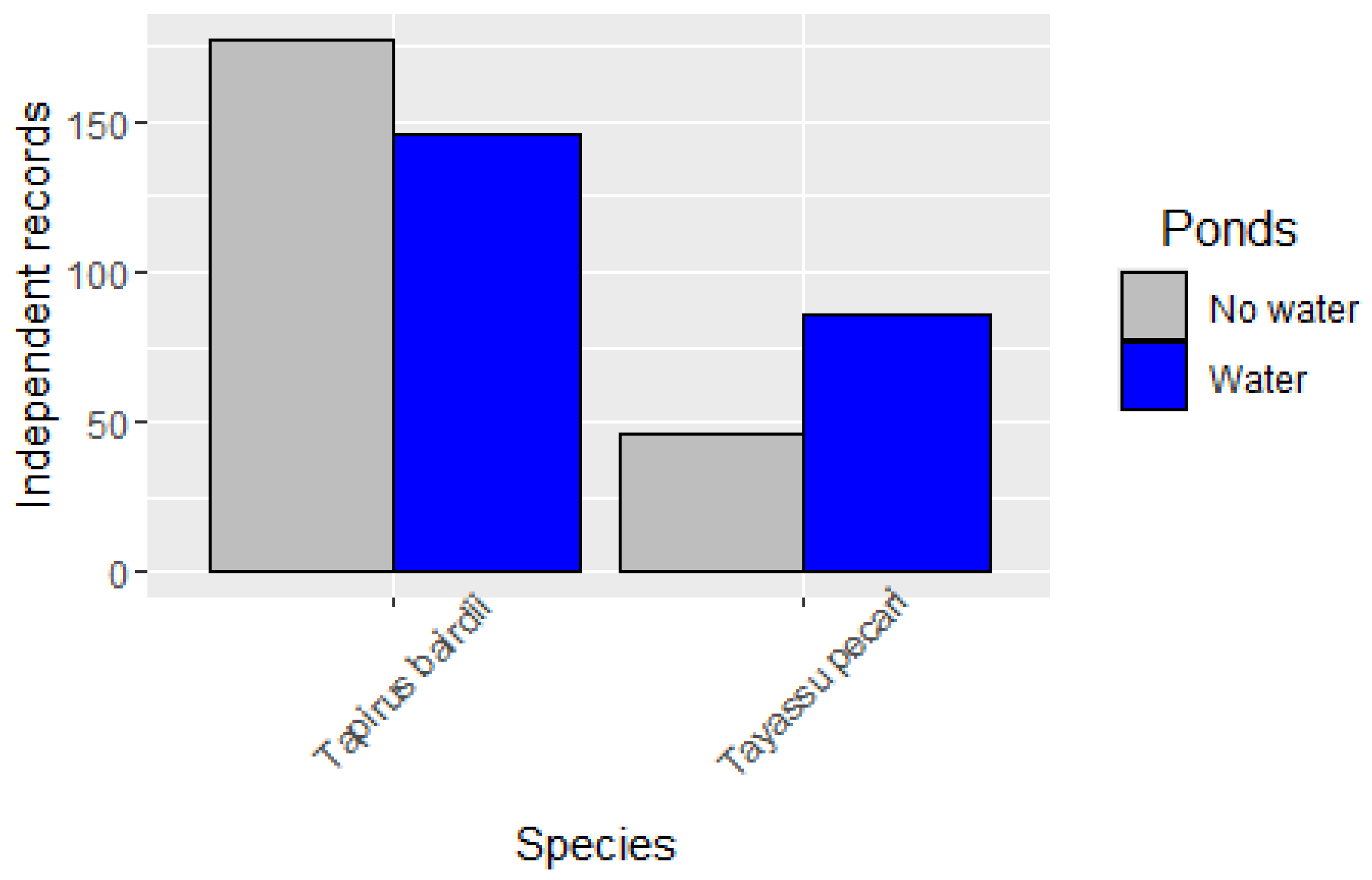
| Specie | RAI of Total Sampling Time and Sites |
|---|---|
| Tapirus bairdii | 2.49 (0.77) |
| Tayassu pecari | 1.56 (1.62) |
| Species | Models Affecting RAI | AIC | Adjusted R2 | Model p Value |
|---|---|---|---|---|
| Tapirus bairdi | RAI~Distance to Roads + Vegetation Type + Perturbation Degree | 107.5 | 0.13 | 0.34 |
| RAI~Distance to Roads + Vegetation Type | 110.2 | 0.03 | 0.51 | |
| RAI~Distance to Roads | 106.3 | 0.00 | 0.32 | |
| RAI~Vegetation Type | 109.4 | 0.01 | 0.48 | |
| RAI~Perturbation Degree | 109.2 | 0.11 | 0.87 | |
| RAI~Distance to Roads + Perturbation Degree | 109.8 | 0.10 | 0.71 | |
| RAI~Vegetation Type + Perturbation Degree | 107.3 | 0.13 | 0.31 | |
| Tayassu pecari | Models affecting RAI | AIC | Adjusted R2 | p value |
| RAI~Distance to Roads + Vegetation Type + Perturbation Degree | 69.31 | −0.07 | 0.57 | |
| RAI~Distance to Roads + Vegetation Type | 69.00 | −0.07 | 0.59 | |
| RAI~Distance to Roads | 65.43 | −0.05 | 0.77 | |
| RAI~Vegetation Type | 67.06 | 0.01 | 0.43 | |
| RAI~Perturbation Degree | 64.46 | 0.04 | 0.27 | |
| RAI~Distance to Roads + Perturbation Degree | 65.23 | 0.04 | 0.32 | |
| RAI~Vegetation Type + Perturbation Degree | 67.35 | 0.03 | 0.43 |
| Species | Models | AIC | Estimate and Prob. of Occu. Psi (Ψ) | Estimate and Prob. of Det (p) | Estimate and Prob. of Col (γ) | Estimate and Prob. of Ext (ϵ) | Model p Value |
|---|---|---|---|---|---|---|---|
| Tapirus bairdi | Null Model | 814.27 | 1.97 (0.878) | −0.51 (0.377) | 0.54 (0.632) | −1.58 (0.170) | 0.00 |
| Det (p)~Vegetation Type | 795.67 | 1.96 (0.877) | 0.87 (0.705) | 0.66 (0.66) | −1.73 (0.151) | 0.14 | |
| Det (p)~Perturbation Degree | 799.33 | 2.0 (0.880) | -0.39 (0.402) | 0.781 (0.686) | −1.72 (0.152) | 0.06 | |
| Psi (Ψ)~Null Col (γ)~Distance to Roads Ext (ϵ)~Distance to Roads Det (p)~Vegetation Type | 795.05 | 1.87 (0.867) | 0.87 (0.705) | 1.11 (0.752) | −1.63 (0.162) | 0.14 | |
| Psi (Ψ)~Null Col (γ)~Distance to Roads Ext (ϵ)~Distance to Roads Det (p)~Perturbation | 799.53 | 1.93 (0.874) | −0.43 (0.392) | -1.43 (0.744) | 0.52 (0.164) | 0.07 | |
| Tayassu pecari | Null model | 576.59 | 6.43 (0.998) | −1.36 (0.205) | 0.53 (0.631) | −1.03 (0.236) | 0.00 |
| Det (p)~Vegetation Type | 557.3 | 8.28 (1.00) | −0.07 (0.482) | 1.29 (0.784) | −1.13 (0.244) | 0.01 | |
| Det (p)~Perturbation Degree | 559.92 | 7.52 (0.99) | −2.65 (0.06) | 5.69 (0.997) | −1.25 (0.222) | 0.01 | |
| Psi (Ψ)~Null Col (γ)~Perturbation Degree Ext (ϵ)~Perturbation Degree Det (p)~Vegetation Type | 562.68 | 8.47 (1.00) | −0.07 (0.481) | −0.04 (0.489) | −0.19 (0.451) | 0.00 |
| Species and Water Co-Occurring | AIC | AIC Weights | Occupancy Estimates (in Logit Scale) and p Values (in Brackets) |
|---|---|---|---|
| Tapirus bairdi and water (only dry seasons) | |||
| Co-occurrence | 297.19 | 0.83 | 0.89 (0.02) |
| No Co-occurrence | 300.38 | 0.17 | |
| Tapirus bairdi and water (all year) | |||
| Co-occurrence | 3027.47 | 0.80 | 2.17 (0.00) |
| No Co-occurrence | 3030.42 | 0.19 | |
| Tapirus bairdi and Panthera onca | |||
| Co-occurrence | 2939.54 | 0.80 | 2.14 (0.00) |
| No Co-occurrence | 2942.32 | 0.20 | |
| Tapirus bairdi and Puma concolor | |||
| Co-occurrence | 3301.05 | 0.83 | 2.21 (0.00) |
| No Co-occurrence | 3304.21 | 0.17 | |
| Tayassu pecari and water (only dry seasons) | |||
| Co-occurrence | 234.74 | 0.57 | 1.46 (0.00) |
| No Co-occurrence | 235.33 | 0.43 | |
| Tayassu pecari and water (all year) | |||
| Co-occurrence | 2464.00 | 0.78 | 2.09 (0.00) |
| No Co-occurrence | 2466.53 | 0.22 | |
| Tayassu pecari and Panthera onca | |||
| Co-occurrence | 2376.02 | 0.77 | 2.07 (0.00) |
| No Co-occurrence | 2378.43 | 0.23 | |
| Tayassu pecari and Puma concolor | |||
| Co-occurrence | 2737.65 | 0.79 | 2.13 (0.00) |
| No Co-occurrence | 2740.32 | 0.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reyna-Hurtado, R.; Huerta-Rodríguez, J.O.; Duarte-Morales, A.; Poot-Sarmiento, I.; Martínez-Martínez, L.V.; Jiménez-Sánchez, M.A. Population Dynamics and Survival Strategies of Two Endangered Ungulates in a Low Water-Availability Site of the Maya Forest of Mexico. Animals 2025, 15, 1307. https://doi.org/10.3390/ani15091307
Reyna-Hurtado R, Huerta-Rodríguez JO, Duarte-Morales A, Poot-Sarmiento I, Martínez-Martínez LV, Jiménez-Sánchez MA. Population Dynamics and Survival Strategies of Two Endangered Ungulates in a Low Water-Availability Site of the Maya Forest of Mexico. Animals. 2025; 15(9):1307. https://doi.org/10.3390/ani15091307
Chicago/Turabian StyleReyna-Hurtado, Rafael, Jonathan O. Huerta-Rodríguez, Alan Duarte-Morales, Itzel Poot-Sarmiento, Lizzi Valeria Martínez-Martínez, and Manuel Alejandro Jiménez-Sánchez. 2025. "Population Dynamics and Survival Strategies of Two Endangered Ungulates in a Low Water-Availability Site of the Maya Forest of Mexico" Animals 15, no. 9: 1307. https://doi.org/10.3390/ani15091307
APA StyleReyna-Hurtado, R., Huerta-Rodríguez, J. O., Duarte-Morales, A., Poot-Sarmiento, I., Martínez-Martínez, L. V., & Jiménez-Sánchez, M. A. (2025). Population Dynamics and Survival Strategies of Two Endangered Ungulates in a Low Water-Availability Site of the Maya Forest of Mexico. Animals, 15(9), 1307. https://doi.org/10.3390/ani15091307








