Butyrate Derivatives Exhibited Anti-Inflammatory Effects and Enhanced Intestinal Barrier Integrity in Porcine Cell Culture Models
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Butyrate Derivatives
2.2. Cell Culture Conditions
2.2.1. IPEC-J2
2.2.2. Porcine Alveolar Macrophages
2.3. Cell Viability Assays
2.4. TEER Analysis
2.5. Proinflammatory Cytokines Analysis
2.6. Statistical Analyses
3. Results
3.1. Cell Viability of IPEC-J2
3.2. TEER
3.3. Pro-Inflammatory Cytokines from Porcine Alveolar Macrophages
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| CHOs | Chinese hamster ovary cells |
| DMEM | Dulbecco modified eagle medium |
| DMSO | Dimethyl sulfoxide |
| DNA | Deoxyribonucleic acid |
| ELISA | Enzyme-linked immunosorbent assay |
| FBS | Fetal bovine serum |
| GPR | G protein-coupled receptor |
| HDAC | Histone deacetylase |
| IL-1β | Interleukin 1-beta |
| IPEC-J2 | Intestinal porcine enterocyte cell line |
| IκB | Inhibitor of Kappa B |
| LPS | Lipopolysaccharide |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NLRP3 | Nod-like receptor protein 3 |
| OD | Optical density |
| PAMs | Porcine alveolar macrophages |
| PBS | Phosphate buffered saline |
| RPMI | Roswell Park Memorial Institute |
| SOD | Superoxide dismutase |
| SP1 | Specificity protein 1 |
| TEER | Transepithelial electrical resistance |
| TLR | Toll-like receptor |
| TNF-α | Tumor necrosis factor-alpha |
References
- Partanen, K.H.; Mroz, Z. Organic acids for performance enhancement in pig diets. Nutr. Res. Rev. 1999, 12, 117–145. [Google Scholar] [CrossRef] [PubMed]
- Corrêa-Oliveira, R.; Fachi, J.L.; Vieira, A.; Sato, F.T.; Vinolo, M.A.R. Regulation of immune cell function by short-chain fatty acids. Clin. Transl. Immunol. 2016, 5, e73. [Google Scholar] [CrossRef] [PubMed]
- Donohoe, D.R.; Collins, L.B.; Wali, A.; Bigler, R.; Sun, W.; Bultman, S.J. The Warburg Effect Dictates the Mechanism of Butyrate-Mediated Histone Acetylation and Cell Proliferation. Mol. Cell 2012, 48, 612–626. [Google Scholar] [CrossRef] [PubMed]
- Steliou, K.; Boosalis, M.S.; Perrine, S.P.; Sangerman, J.; Faller, D.V. Butyrate Histone Deacetylase Inhibitors. BioResearch Open Access 2012, 1, 192–198. [Google Scholar] [CrossRef]
- Yuille, S.; Reichardt, N.; Panda, S.; Dunbar, H.; Mulder, I.E. Human gut bacteria as potent class I histone deacetylase inhibitors in vitro through production of butyric acid and valeric acid. PLoS ONE 2018, 13, e0201073. [Google Scholar] [CrossRef]
- Vinolo, M.A.R.; Rodrigues, H.G.; Hatanaka, E.; Sato, F.T.; Sampaio, S.C.; Curi, R. Suppressive effect of short-chain fatty acids on production of proinflammatory mediators by neutrophils. J. Nutr. Biochem. 2011, 22, 849–855. [Google Scholar] [CrossRef]
- Arpaia, N.; Campbell, C.; Fan, X.; Dikiy, S.; van der Veeken, J.; deRoos, P.; Liu, H.; Cross, J.R.; Pfeffer, K.; Coffer, P.J.; et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 2013, 504, 451–455. [Google Scholar] [CrossRef]
- Sunkara, L.T.; Jiang, W.; Zhang, G. Modulation of Antimicrobial Host Defense Peptide Gene Expression by Free Fatty Acids. PLoS ONE 2012, 7, e49558. [Google Scholar] [CrossRef]
- Zeng, X.; Sunkara, L.T.; Jiang, W.; Bible, M.; Carter, S.; Ma, X.; Qiao, S.; Zhang, G. Induction of Porcine Host Defense Peptide Gene Expression by Short-Chain Fatty Acids and Their Analogs. PLoS ONE 2013, 8, e72922. [Google Scholar] [CrossRef]
- Dou, X.; Han, J.; Song, W.; Dong, N.; Xu, X.; Zhang, W.; Shan, A. Sodium butyrate improves porcine host defense peptide expression and relieves the inflammatory response upon toll-like receptor 2 activation and histone deacetylase inhibition in porcine kidney cells. Oncotarget 2017, 8, 26532–26551. [Google Scholar] [CrossRef]
- Yan, H.; Ajuwon, K.M. Butyrate modifies intestinal barrier function in IPEC-J2 cells through a selective upregulation of tight junction proteins and activation of the Akt signaling pathway. PLoS ONE 2017, 12, e0179586. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, H.; Lin, Y.; Wang, G.; Luo, Y.; Li, X.; Wang, M.; Huai, M.; Li, L.; Barri, A. Butyrate Glycerides Protect against Intestinal Inflammation and Barrier Dysfunction in Mice. Nutrients 2022, 14, 3991. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Shen, Z.; Cao, S.; Zhang, Q.; Peng, Y.; Hong, Q.; Feng, J.; Hu, C. Effects of tributyrin on growth performance, intestinal microflora and barrier function of weaned pigs. Anim. Feed. Sci. Technol. 2019, 258, 114311. [Google Scholar] [CrossRef]
- Sotira, S.; Dell’Anno, M.; Caprarulo, V.; Hejna, M.; Pirrone, F.; Callegari, M.L.; Tucci, T.V.; Rossi, L. Effects of Tributyrin Supplementation on Growth Performance, Insulin, Blood Metabolites and Gut Microbiota in Weaned Piglets. Animals 2020, 10, 726. [Google Scholar] [CrossRef]
- Tugnoli, B.; Piva, A.; Sarli, G.; Grilli, E. Tributyrin differentially regulates inflammatory markers and modulates goblet cells number along the intestinal tract segments of weaning pigs. Livest. Sci. 2020, 234, 103996. [Google Scholar] [CrossRef]
- Piva, A.; Morlacchini, M.; Casadei, G.; Gatta, P.P.; Biagi, G.; Prandini, A. Sodium butyrate improves growth performance of weaned piglets during the first period after weaning. Ital. J. Anim. Sci. 2002, 1, 35–41. [Google Scholar] [CrossRef]
- Lu, J.J.; Zou, X.T.; Wang, Y.M. Effects of sodium butyrate on the growth performance, intestinal microflora and morphology of weanling pigs. J. Anim. Feed. Sci. 2008, 17, 568–578. [Google Scholar] [CrossRef]
- Weber, T.E.; Kerr, B.J. Effect of sodium butyrate on growth performance and response to lipopolysaccharide in weanling pigs. J. Anim. Sci. 2008, 86, 442–450. [Google Scholar] [CrossRef]
- Fang, C.L.; Sun, H.; Wu, J.; Niu, H.H.; Feng, J. Effects of sodium butyrate on growth performance, haematological and immunological characteristics of weanling piglets. J. Anim. Physiol. Anim. Nutr. 2014, 98, 680–685. [Google Scholar] [CrossRef]
- Chen, J.; Xu, Q.; Li, Y.; Tang, Z.; Sun, W.; Zhang, X.; Sun, J.; Sun, Z. Comparative effects of dietary supplementations with sodium butyrate, medium-chain fatty acids, and n-3 polyunsaturated fatty acids in late pregnancy and lactation on the reproductive performance of sows and growth performance of suckling piglets. J. Anim. Sci. 2019, 97, 4256–4267. [Google Scholar] [CrossRef]
- Liu, Y.; Song, M.; Che, T.M.; Bravo, D.; Pettigrew, J.E. Anti-inflammatory effects of several plant extracts on porcine alveolar macrophages in vitro. J. Anim. Sci. 2012, 90, 2774–2783. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hu, S.; Liu, Q.; Li, Y.; Xu, L.; Zhang, Z.; Cai, X.; He, X. Porcine alveolar macrophage polarization is involved in inhibition of porcine reproductive and respiratory syndrome virus (PRRSV) replication. J. Vet. Med. Sci. 2017, 79, 1906–1915. [Google Scholar] [CrossRef] [PubMed]
- Hejna, M.; Kovanda, L.; Rossi, L.; Liu, Y. Mint Oils: In Vitro Ability to Perform Anti-Inflammatory, Antioxidant, and Antimicrobial Activities and to Enhance Intestinal Barrier Integrity. Antioxidants 2021, 10, 1004. [Google Scholar] [CrossRef] [PubMed]
- Vergauwen, H. The IPEC-J2 Cell Line. In The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models; Verhoeckx, K., Cotter, P., López-Expósito, I., Kleiveland, C., Lea, T., Mackie, A., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 125–134. [Google Scholar] [CrossRef]
- Schierack, P.; Nordhoff, M.; Pollmann, M.; Weyrauch, K.D.; Amasheh, S.; Lodemann, U.; Jores, J.; Tachu, B.; Kleta, S.; Blikslager, A.; et al. Characterization of a porcine intestinal epithelial cell line for in vitro studies of microbial pathogenesis in swine. Histochem. Cell Biol. 2006, 125, 293–305. [Google Scholar] [CrossRef]
- Zakrzewski, S.S.; Richter, J.F.; Krug, S.M.; Jebautzke, B.; Lee, I.F.M.; Rieger, J.; Sachtleben, M.; Bondzio, A.; Schulzke, J.D.; Fromm, M.; et al. Improved Cell Line IPEC-J2, Characterized as a Model for Porcine Jejunal Epithelium. PLoS ONE 2013, 8, e79643. [Google Scholar] [CrossRef]
- Srinivasan, B.; Kolli, A.R.; Esch, M.B.; Abaci, H.E.; Shuler, M.L.; Hickman, J.J. TEER measurement techniques for in vitro barrier model systems. J. Lab. Autom. 2015, 20, 107–126. [Google Scholar] [CrossRef]
- Kovanda, L.; Park, J.; Park, S.; Kim, K.; Li, X.; Liu, Y. Dietary butyrate and valerate glycerides impact diarrhea severity and immune response of weaned piglets under ETEC F4-ETEC F18 coinfection conditions. J. Anim. Sci. 2023, 101, skad401. [Google Scholar] [CrossRef]
- Smith, P.D.; Smythies, L.E.; Shen, R.; Greenwell-Wild, T.; Gliozzi, M.; Wahl, S.M. Intestinal macrophages and response to microbial encroachment. Mucosal Immunol. 2011, 4, 31–42. [Google Scholar] [CrossRef]
- Pelaseyed, T.; Bergström, J.H.; Gustafsson, J.K.; Ermund, A.; Birchenough, G.M.H.; Schütte, A.; van der Post, S.; Svensson, F.; Rodríguez-Piñeiro, A.M.; Nyström, E.E.L.; et al. The mucus and mucins of the goblet cells and enterocytes provide the first defense line of the gastrointestinal tract and interact with the immune system. Immunol. Rev. 2014, 260, 8–20. [Google Scholar] [CrossRef]
- Willing, B.P.; Van Kessel, A.G. Enterocyte proliferation and apoptosis in the caudal small intestine is influenced by the composition of colonizing commensal bacteria in the neonatal gnotobiotic pig. J. Anim. Sci. 2007, 85, 3256–3266. [Google Scholar] [CrossRef]
- Wang, J.; Ford, H.R.; Grishin, A.V. NF-κB-mediated expression of MAPK phosphatase-1 is an early step in desensitization to TLR ligands in enterocytes. Mucosal Immunol. 2010, 3, 523–534. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Hu, N.; Jiang, Q.; Zhu, L.; Zhang, M.; Jiang, J.; Xiong, M.; Yang, M.; Yang, J.; Shen, L.; et al. Protective effects of sodium butyrate on rotavirus inducing endoplasmic reticulum stress-mediated apoptosis via PERK-eIF2α signaling pathway in IPEC-J2 cells. J. Anim. Sci. Biotechnol. 2021, 12, 69. [Google Scholar] [CrossRef] [PubMed]
- Sakurazawa, T.; Ohkusa, T. Cytotoxicity of organic acids produced by anaerobic intestinal bacteria on cultured epithelial cells. J. Gastroenterol. 2005, 40, 600–609. [Google Scholar] [CrossRef] [PubMed]
- Rose, S.; Bennuri, S.C.; Murray, K.F.; Buie, T.; Winter, H.; Frye, R.E. Mitochondrial dysfunction in the gastrointestinal mucosa of children with autism: A blinded case-control study. PLoS ONE 2017, 12, e0186377. [Google Scholar] [CrossRef]
- Dzierzewicz, Z.; Orchel, A.; Weglarz, L.; Latocha, M.; Wilczok, T. Changes in the cellular behaviour of human colonic cell line Caco-2 in response to butyrate treatment. Acta Biochim. Pol. 2002, 49, 211–220. [Google Scholar] [CrossRef]
- Jahns, F.; Wilhelm, A.; Jablonowski, N.; Mothes, H.; Greulich, K.O.; Glei, M. Butyrate modulates antioxidant enzyme expression in malignant and non-malignant human colon tissues. Mol. Carcinog. 2015, 54, 249–260. [Google Scholar] [CrossRef]
- Halliwell, B. Oxidative stress in cell culture: An under-appreciated problem? FEBS Lett. 2003, 540, 3–6. [Google Scholar] [CrossRef]
- Ohata, A.; Usami, M.; Miyoshi, M. Short-chain fatty acids alter tight junction permeability in intestinal monolayer cells via lipoxygenase activation. Nutrition 2005, 21, 838–847. [Google Scholar] [CrossRef]
- Peng, L.; He, Z.; Chen, W.; Holzman, I.R.; Lin, J. Effects of Butyrate on Intestinal Barrier Function in a Caco-2 Cell Monolayer Model of Intestinal Barrier. Pediatr. Res. 2007, 61, 37–41. [Google Scholar] [CrossRef]
- Ma, X.X.; Fan, P.; Li, L.S.; Qiao, S.Y.; Zhang, G.L.; Li, D.F. Butyrate promotes the recovering of intestinal wound healing through its positive effect on the tight junctions. J. Anim. Sci. 2012, 90 (Suppl. S4), 266–268. [Google Scholar] [CrossRef]
- Wang, H.B.; Wang, P.Y.; Wang, X.; Wan, Y.L.; Liu, Y.C. Butyrate Enhances Intestinal Epithelial Barrier Function via Up-Regulation of Tight Junction Protein Claudin-1 Transcription. Dig. Dis. Sci. 2012, 57, 3126–3135. [Google Scholar] [CrossRef] [PubMed]
- Zakrzewski, S.S.; Fromm, M.; Schulzke, J.D.; Günzel, D. Zinc strengthens the jejunal barrier by reversibly tightening the paracellular route. Am. J. Physiol-Gastrointest. Liver Physiol. 2017, 313, G537–G548. [Google Scholar] [CrossRef] [PubMed]
- Keely, S.J.; Barrett, K.E. Intestinal secretory mechanisms and diarrhea. Am. J. Physiol-Gastrointest. Liver Physiol. 2022, 322, G405–G420. [Google Scholar] [CrossRef] [PubMed]
- Boffa, L.C.; Vidali, G.; Mann, R.S.; Allfrey, V.G. Suppression of histone deacetylation in vivo and in vitro by sodium butyrate. J. Biol. Chem. 1978, 253, 3364–3366. [Google Scholar] [CrossRef]
- Candido, E.P.M.; Reeves, R.; Davie, J.R. Sodium butyrate inhibits histone deacetylation in cultured cells. Cell 1978, 14, 105–113. [Google Scholar] [CrossRef]
- Whitlock, J.P.; Augustine, R.; Schulman, H. Calcium-dependent phosphorylation of histone H3 in butyrate-treated HeLa cells. Nature 1980, 287, 74–76. [Google Scholar] [CrossRef]
- Della Ragione, F.; Criniti, V.; Della Pietra, V.; Borriello, A.; Oliva, A.; Indaco, S.; Yamamoto, T.; Zappia, V. Genes modulated by histone acetylation as new effectors of butyrate activity. FEBS Lett. 2001, 499, 199–204. [Google Scholar] [CrossRef]
- van der Hee, B.; Wells, J.M. Microbial Regulation of Host Physiology by Short-chain Fatty Acids. Trends Microbiol. 2021, 29, 700–712. [Google Scholar] [CrossRef]
- Mariadason, J.M.; Corner, G.A.; Augenlicht, L.H. Genetic Reprogramming in Pathways of Colonic Cell Maturation Induced by Short Chain Fatty Acids: Comparison with Trichostatin A, Sulindac, and Curcumin and Implications for Chemoprevention of Colon Cancer. Cancer Res. 2000, 60, 4561–4572. [Google Scholar]
- Tian, C.; Guo, J.; Wang, G.; Sun, B.; Na, K.; Zhang, X.; Xu, Z.; Cheng, M.; He, Z.; Sun, J. Efficient Intestinal Digestion and On Site Tumor-Bioactivation are the Two Important Determinants for Chylomicron-Mediated Lymph-Targeting Triglyceride-Mimetic Docetaxel Oral Prodrugs. Adv. Sci. 2019, 6, 1901810. [Google Scholar] [CrossRef]
- Chriett, S.; Dąbek, A.; Wojtala, M.; Vidal, H.; Balcerczyk, A.; Pirola, L. Prominent action of butyrate over β-hydroxybutyrate as histone deacetylase inhibitor, transcriptional modulator and anti-inflammatory molecule. Sci. Rep. 2019, 9, 742. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Liu, Y.; Dong, W.; Zhu, G.q.; Wu, S.; Bao, W. CD14 in the TLRs signaling pathway is associated with the resistance to E. coli F18 in Chinese domestic weaned piglets. Sci. Rep. 2016, 6, 24611. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Zheng, J.; Chen, Y.; Wang, T.; Zhang, Z.; Shan, Y.; Xu, J.; Yue, M.; Fang, W.; Li, X. Utility Evaluation of Porcine Enteroids as PDCoV Infection Model in vitro. Front. Microbiol. 2020, 11, 821. [Google Scholar] [CrossRef] [PubMed]
- Segain, J.P. Butyrate inhibits inflammatory responses through NFkappa B inhibition: Implications for Crohn’s disease. Gut 2000, 47, 397–403. [Google Scholar] [CrossRef]
- Bode, K.A.; Schroder, K.; Hume, D.A.; Ravasi, T.; Heeg, K.; Sweet, M.J.; Dalpke, A.H. Histone deacetylase inhibitors decrease Toll-like receptor-mediated activation of proinflammatory gene expression by impairing transcription factor recruitment. Immunology 2007, 122, 596–606. [Google Scholar] [CrossRef]
- Lima, M.S.R.; de Lima, V.C.O.; Piuvezam, G.; de Azevedo, K.P.M.; Maciel, B.L.L.; de Morais, A.A.H. Mechanisms of action of molecules with anti-TNF-alpha activity on intestinal barrier inflammation: A systematic review protocol. Medicine 2019, 98, e17285. [Google Scholar] [CrossRef]
- Kalliolias, G.D.; Ivashkiv, L.B. TNF biology, pathogenic mechanisms and emerging therapeutic strategies. Nat. Rev. Rheumatol. 2016, 12, 49–62. [Google Scholar] [CrossRef]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef]
- Lentschat, A.; El-Samalouti, V.T.; Schletter, J.; Kusumoto, S.; Brade, L.; Rietschel, E.T.; Gerdes, J.; Ernst, M.; Flad, H.-D.; Ulmer, A.J. The Internalization Time Course of a Given Lipopolysaccharide Chemotype Does Not Correspond to Its Activation Kinetics in Monocytes. Infect. Immun. 1999, 67, 2515–2521. [Google Scholar] [CrossRef]
- Ding, N.; Wei, B.; Fu, X.; Wang, C.; Wu, Y. Natural Products that Target the NLRP3 Inflammasome to Treat Fibrosis. Front. Pharmacol. 2020, 11, 591393. [Google Scholar] [CrossRef]
- Brown, A.J.; Goldsworthy, S.M.; Barnes, A.A.; Eilert, M.M.; Tcheang, L.; Daniels, D.; Muir, A.I.; Wigglesworth, M.J.; Kinghorn, I.; Fraser, N.J.; et al. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J. Biol. Chem. 2003, 278, 11312–11319. [Google Scholar] [CrossRef]
- Wu, J.; Zhou, Z.; Hu, Y.; Dong, S. Butyrate-induced GPR41 Activation Inhibits Histone Acetylation and Cell Growth. J. Genet. Genomics. 2012, 39, 375–384. [Google Scholar] [CrossRef]
- Kovanda, L.L. Short Chain Fatty Acids: Impacts on Intestinal Physiology and Disease Resistance to Enterotoxigenic Escherichia coli in Pigs. Ph.D. Thesis, University of California, Berkeley, CA, USA, 2024. Available online: https://escholarship.org/uc/item/7m1786m6 (accessed on 15 January 2020).
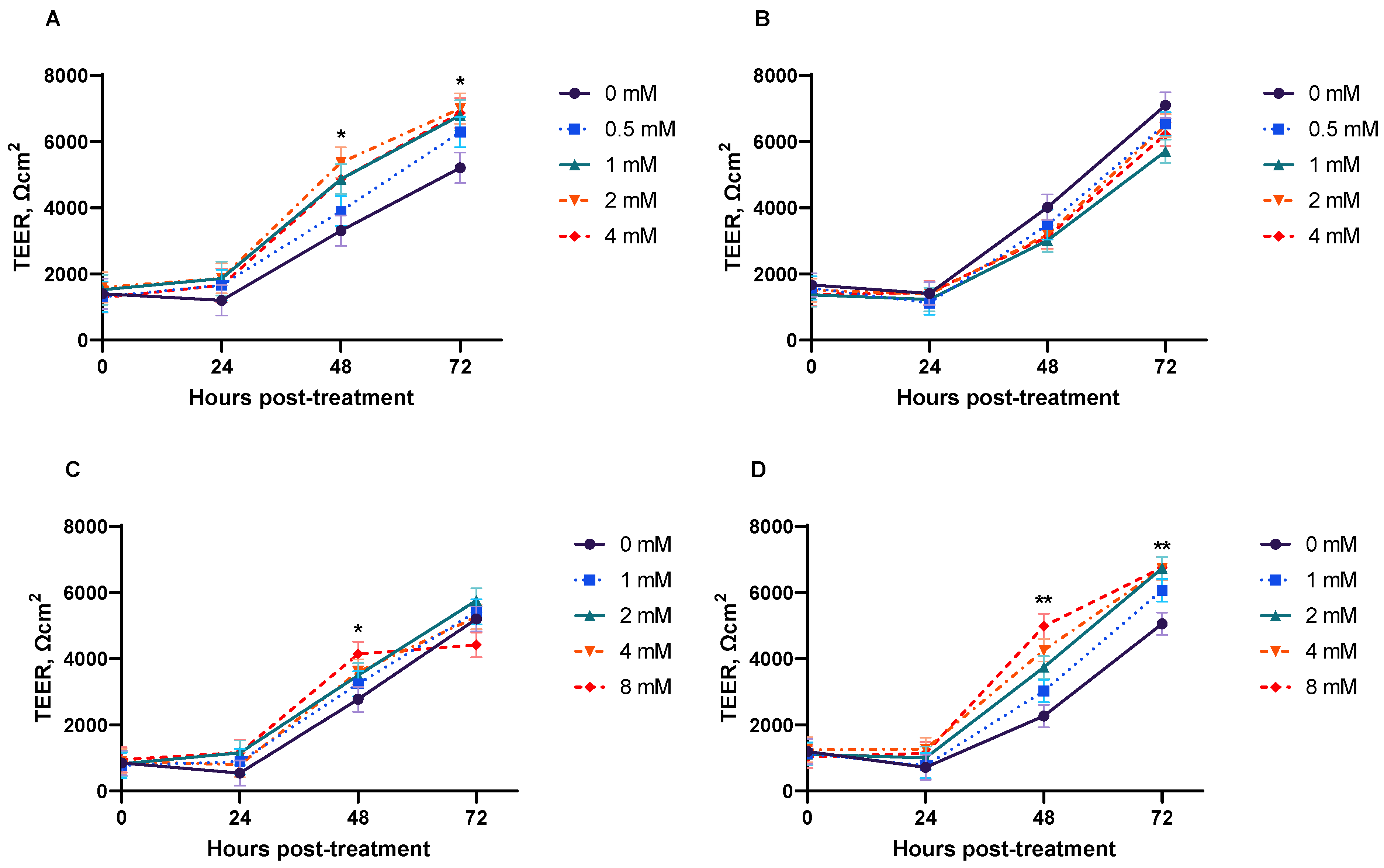
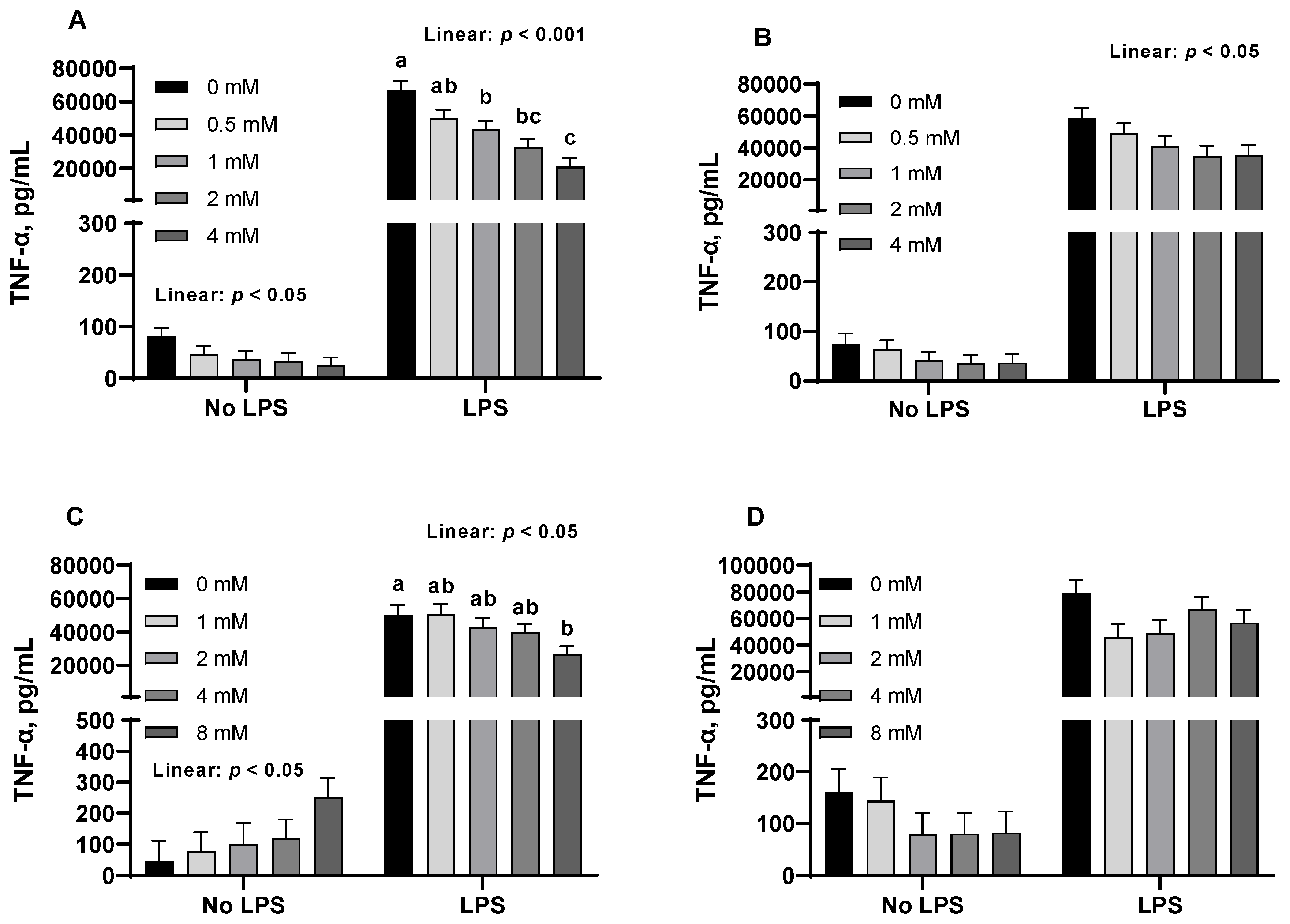
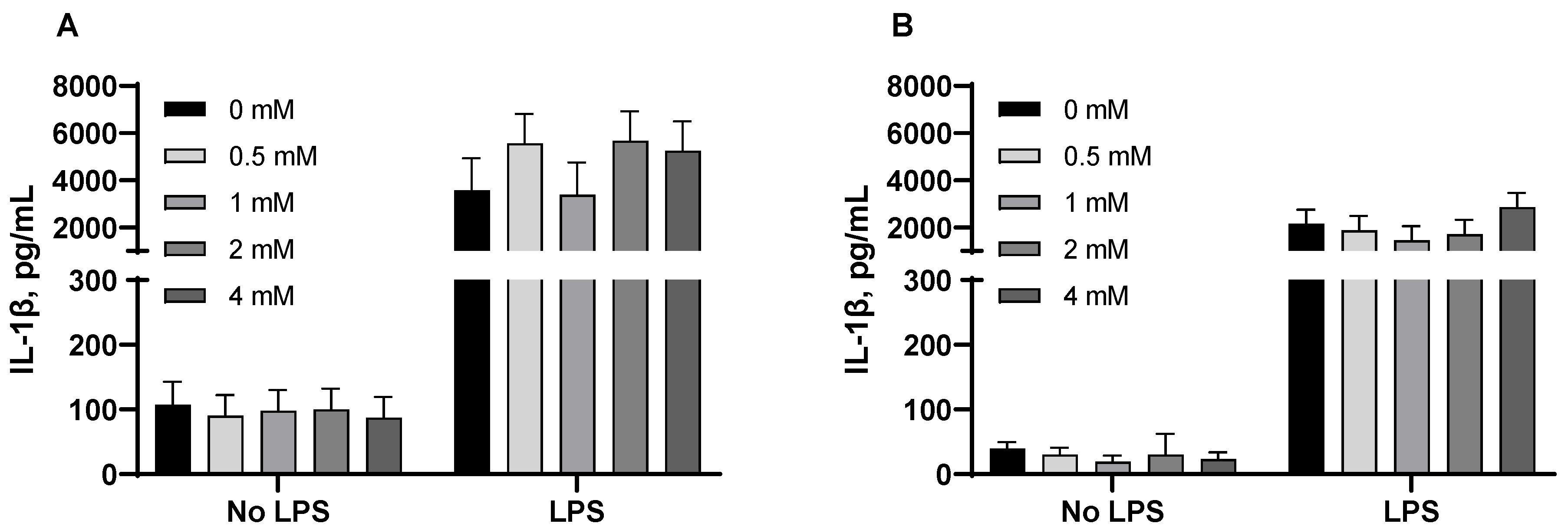
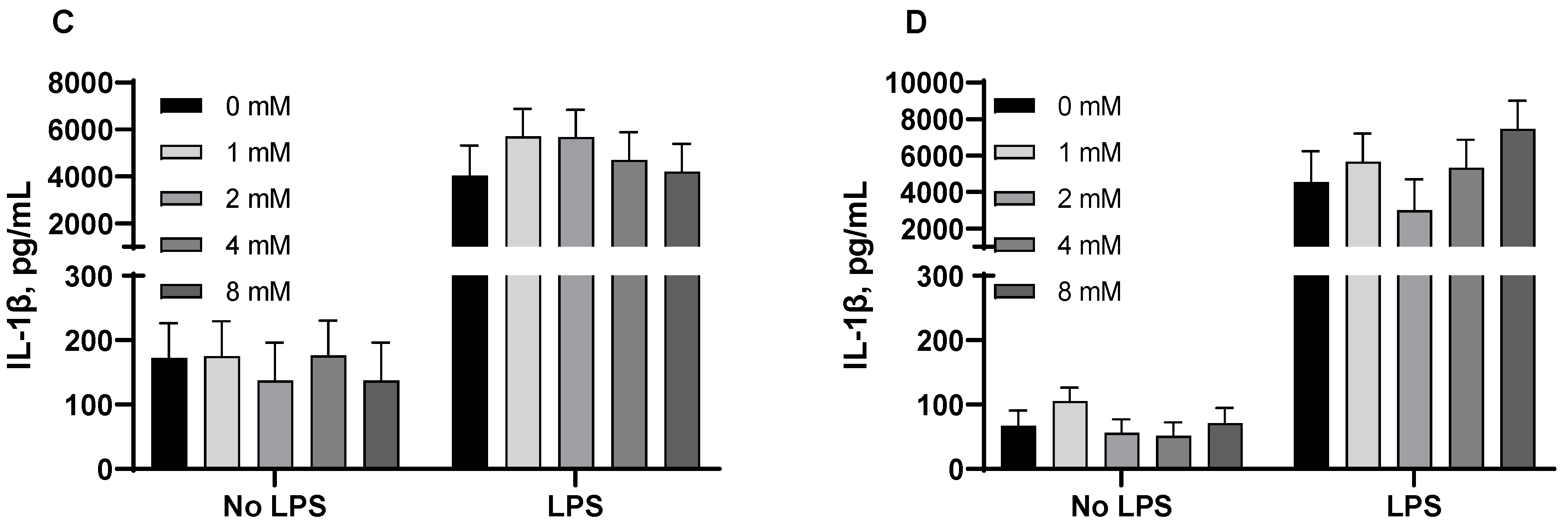
| Dose (mM) | 0 | 0.5 | 1 | 2 | 4 | SEM | p-Value |
|---|---|---|---|---|---|---|---|
| Butyric acid | 100.0 | 102.6 | 95.7 | 111.2 | 92.2 | 17.42 | 0.960 |
| Tributyrin | 100.0 | 142.2 | 125.6 | 146.1 | 91.7 | 17.24 | 0.061 |
| Dose (mM) | 0 | 2 | 4 | 8 | 16 | ||
| Sodium butyrate | 100.0 b | 131.1 a | 129.1 a | 115.1 ab | 107.8 ab | 7.31 | 0.015 |
| Monobutyrin | 100.0 b | 129.2 a | 104.5 ab | 111.2 ab | 100.6 ab | 6.87 | 0.031 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovanda, L.; Hejna, M.; Du, T.; Liu, Y. Butyrate Derivatives Exhibited Anti-Inflammatory Effects and Enhanced Intestinal Barrier Integrity in Porcine Cell Culture Models. Animals 2025, 15, 1289. https://doi.org/10.3390/ani15091289
Kovanda L, Hejna M, Du T, Liu Y. Butyrate Derivatives Exhibited Anti-Inflammatory Effects and Enhanced Intestinal Barrier Integrity in Porcine Cell Culture Models. Animals. 2025; 15(9):1289. https://doi.org/10.3390/ani15091289
Chicago/Turabian StyleKovanda, Lauren, Monika Hejna, Tina Du, and Yanhong Liu. 2025. "Butyrate Derivatives Exhibited Anti-Inflammatory Effects and Enhanced Intestinal Barrier Integrity in Porcine Cell Culture Models" Animals 15, no. 9: 1289. https://doi.org/10.3390/ani15091289
APA StyleKovanda, L., Hejna, M., Du, T., & Liu, Y. (2025). Butyrate Derivatives Exhibited Anti-Inflammatory Effects and Enhanced Intestinal Barrier Integrity in Porcine Cell Culture Models. Animals, 15(9), 1289. https://doi.org/10.3390/ani15091289







