Sublittoral Macrobenthic Communities of Storfjord (Eastern Svalbard) and Factors Influencing Their Distribution and Structure
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sample Collection and Analysis
2.3. Statistical Analysis
3. Results
3.1. Environmental Conditions
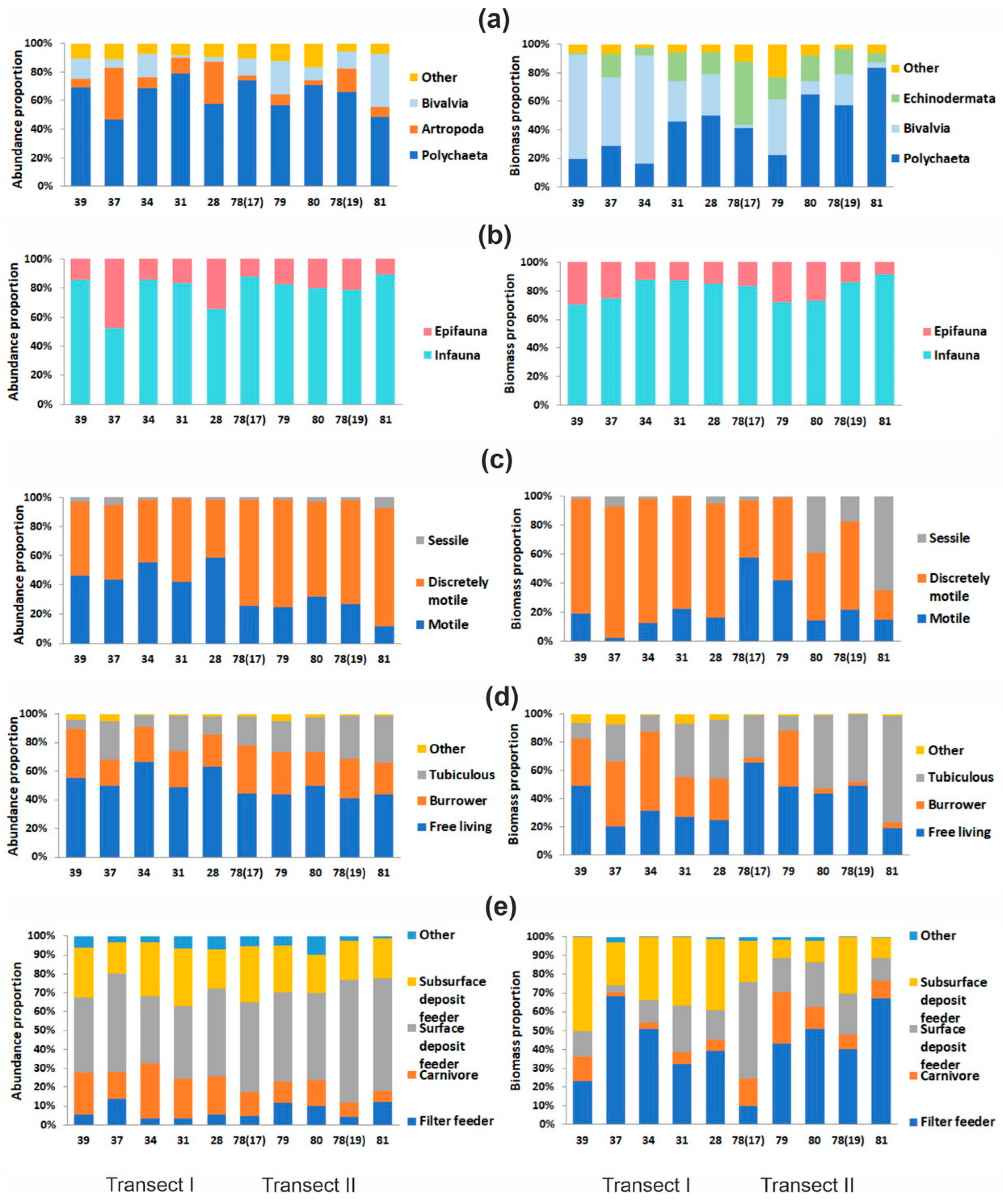
3.2. Benthic Diversity, Abundance, and Biomass
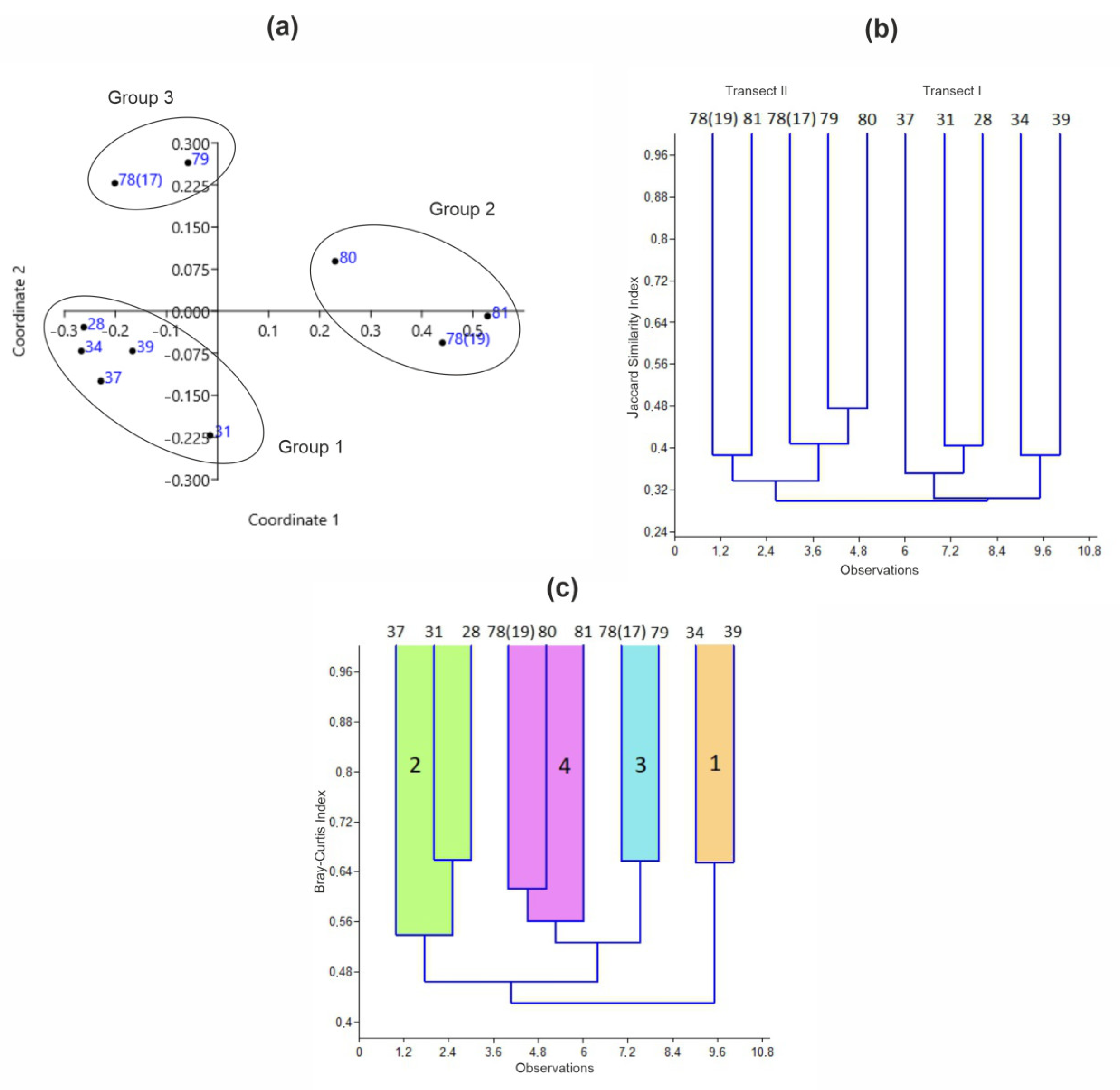
3.3. Benthic Communities
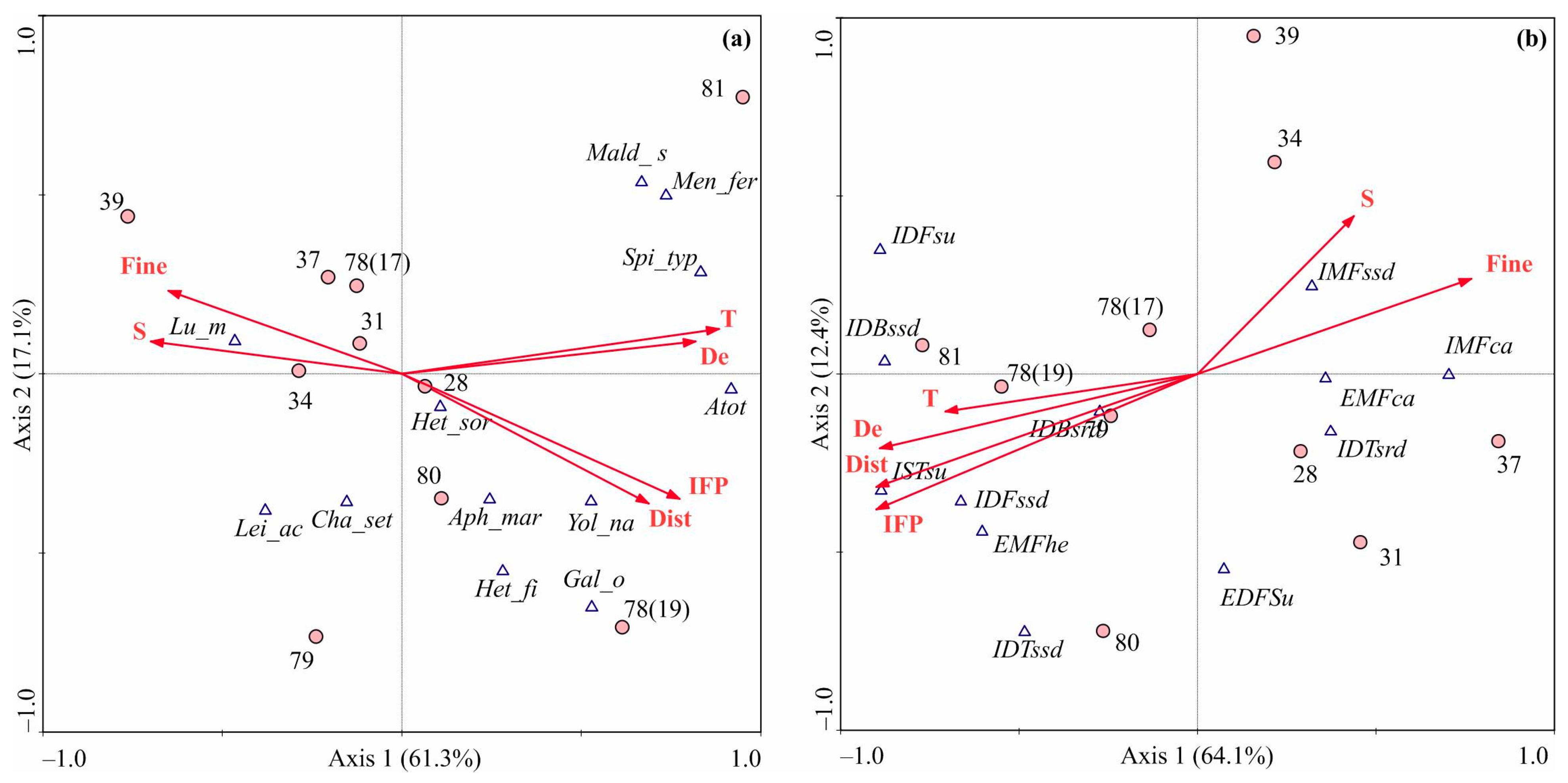
3.4. Environmental Factors Shaping Benthic Communities
4. Discussion
4.1. Environmental and Trophic Conditions in Storfjord
4.2. Current State of Soft-Bottom Benthic Communities
4.3. Distribution Patterns of Benthic Communities
4.4. Environmental Drivers of Macrozoobenthos
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Taxon | Station | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 39 | 37 | 34 | 31 | 28 | 78(17) | 79 | 80 | 78(19) | 81 | |
| Phylum Porifera | ||||||||||
| Porifera g.sp. | + | + | + | |||||||
| Phylum Cnidaria | ||||||||||
| Class Hydrozoa | ||||||||||
| Abietinaria pulchra (Nutting, 1904) | + | |||||||||
| Eudendrium sp. 1 | + | |||||||||
| Eudendrium sp. 2 | + | |||||||||
| Eudendrium sp. 3 | + | |||||||||
| Halecium curvicaule Lorenz, 1886 | + | |||||||||
| Lafoea fruticosa (M. Sars, 1850) | + | + | + | |||||||
| Ptychogena crocea Kramp and Damas, 1925 | + | |||||||||
| Rhizocaulus verticillatus (L., 1758) | + | |||||||||
| Sertularia albimaris Mereschkowsky, 1878 | + | + | ||||||||
| Symplectoscyphus tricuspidatus (Alder, 1856) | + | + | ||||||||
| Sertularella rugosa (L., 1758) | + | |||||||||
| Class Antozoa | ||||||||||
| Synarachnactis lloydii (Gosse, 1859) | + | |||||||||
| Gersemia rubiformis (Ehrenberg, 1834) | + | + | ||||||||
| Paraedwardsia arenaria Carlgren in Nordgaard, 1905 | + | + | + | + | + | |||||
| Phylum Nemertea | ||||||||||
| Nemertea g.sp. | + | + | + | + | + | + | + | + | + | + |
| Phylum Nematoda | ||||||||||
| Nematoda g.sp. | + | + | + | + | + | + | + | + | + | + |
| Phylum Annelida | ||||||||||
| Class Polychaeta | ||||||||||
| Aglaophamus malmgreni (Théel, 1879) | + | + | + | + | + | + | + | |||
| Amblyosyllis finmarchica (Malmgren, 1867) | + | |||||||||
| Ampharete finmarchica (M. Sars, 1865) | + | + | + | |||||||
| Ampharete lindstroemi Malmgren, 1867 sensu Hessle, 1917 | + | |||||||||
| Ampharetidae g.sp. | + | + | + | + | + | + | + | |||
| Amphicteis gunneri (M. Sars, 1835) | + | |||||||||
| Amphicteis ninonae Jirkov, 1985 | + | |||||||||
| Anobothrus gracilis (Malmgren, 1866) | + | + | ||||||||
| Aphelochaeta marioni (Saint-Joseph, 1894) | + | + | + | + | + | + | + | + | + | + |
| Aphelochaeta sp. 1 | + | + | + | + | + | + | + | + | + | + |
| Aphelochaeta sp. 2 | + | |||||||||
| Aphelochaeta sp. 3 | + | |||||||||
| Apistobranchus tullbergi (Théel, 1879) | + | + | + | |||||||
| Aricidea (Strelzovia) quadrilobata Webster and Benedict, 1887 | + | + | + | |||||||
| Aricidea hartmani Strelzov, 1968 | + | + | + | + | + | + | + | |||
| Artacama proboscidea Malmgren, 1866 | + | |||||||||
| Brada strelzovi Jirkov and Filippova in Jirkov, 2001 | + | |||||||||
| Bradabyssa villosa (Rathke, 1843) | + | + | + | + | + | |||||
| Bylgides elegans (Théel, 1879) | + | |||||||||
| Bylgides groenlandica (Malmgren, 1867) | + | |||||||||
| Bylgides sp. | + | + | ||||||||
| Capitella capitata (Fabricius, 1780) | + | + | + | + | ||||||
| Chaetozone setosa Malmgren, 1867 | + | + | + | + | + | + | + | + | + | + |
| Chirimia biceps biceps (Sars, 1861) | + | + | ||||||||
| Chone duneri Malmgren, 1867 | + | + | + | + | ||||||
| Chone murmanica Lukasch, 1910 | + | + | + | + | + | + | ||||
| Chone sp. | + | |||||||||
| Cirratullidae g.sp.1 | + | + | + | + | + | + | + | + | ||
| Cirratullidae g.sp. 2 | + | |||||||||
| Cirratulus cirratus (O.F. Müller, 1776) | + | + | + | + | + | |||||
| Cirrophorus branchiatus Ehlers, 1908 | + | |||||||||
| Cistenides hyperborea (Malmgren, 1866) | + | + | + | + | + | + | ||||
| Cossura longocirrata Webster and Benedict, 1887 | + | + | + | + | + | |||||
| Diplocirrus glaucus (Malmgren, 1867) | + | |||||||||
| Dipolydora caulleryi Mesnil, 1897 | + | |||||||||
| Dipolydora coeca (Öersted, 1843) | + | + | + | |||||||
| Dipolydora socialis (Schmarda, 1861) | + | |||||||||
| Dipolydora sp. | + | + | + | + | + | + | ||||
| Enipo torelli (Malmgren, 1865) | + | + | + | + | + | |||||
| Eteone flava (Fabricius, 1780) | + | + | + | + | + | + | + | + | + | + |
| Eteone spetsbergensis Malmgren, 1865 | + | |||||||||
| Euchone analis (Kröyer, 1856) | + | + | + | + | ||||||
| Exogoninae g.sp. | + | |||||||||
| Galathowenia oculata Zachs, 1923 | + | + | + | + | + | + | + | + | + | + |
| Gattyana amondseni (Malmgren, 1867) | + | |||||||||
| Gattyana cirrhosa (Pallas, 1766) | + | + | + | |||||||
| Glyphanostomum pallescens (Théel, 1879) | + | + | + | |||||||
| Harmothoe imbricata (Linnaeus, 1767) | + | |||||||||
| Heteromastus filiformis (Claparède, 1864) | + | + | + | + | + | + | + | + | + | + |
| Lanassa venusta venusta (Malm, 1874) | + | + | ||||||||
| Laonice cirrata (M. Sars, 1851) | + | + | ||||||||
| Leaena ebranchiata (M. Sars, 1865) | + | + | + | + | ||||||
| Laphania boecki Malmgren, 1865 | + | |||||||||
| Leitoscoloplos acutus (Verrill, 1873) | + | + | + | + | + | + | + | + | + | + |
| Levinsenia gracilis (Tauber, 1879) | + | + | + | + | + | + | + | + | + | + |
| Lumbriclymene minor Arvidsson, 1906 | + | + | + | + | + | + | ||||
| Lumbrineris mixochaeta Oug, 1998 | + | + | + | + | + | + | + | + | + | + |
| Lysippe labiata Malmgren, 1865 | + | + | + | + | + | + | + | + | + | |
| Maldane arctica Detinova, 1985 | + | |||||||||
| Maldane sarsi Malmgren, 1865 | + | + | + | + | + | + | + | + | + | + |
| Maldanidae g.sp. | + | |||||||||
| Melinna elisabethae McIntosh, 1914 | + | + | + | + | + | |||||
| Myriochele heeri Malmgren, 1867 | + | + | + | + | ||||||
| Nepftys ciliata (Müller, 1779) | + | + | + | + | + | + | ||||
| Nepftys paradoxa Malm, 1874 | + | + | + | |||||||
| Nephtyidae g.sp. | + | |||||||||
| Nothria hyperborea (Hansen, 1878) | + | + | + | + | + | |||||
| Ophelina cylindricaudata (Hansen, 1879) | + | + | ||||||||
| Oweniidae g.sp. | + | + | ||||||||
| Paradexiospira (Spirorbides) cancellata (Fabricius, 1780) | + | |||||||||
| Paradoneis lyra (Southern, 1914) | + | + | ||||||||
| Pholoe assimilis Örsted, 1845 | + | + | + | + | + | + | + | + | ||
| Pholoe sp. | + | + | ||||||||
| Phyllodoce groenlandica Örsted, 1842 | + | + | + | + | + | + | + | + | ||
| Polycirrus arcticus Sars, 1865 | + | + | + | + | + | + | + | + | ||
| Polycirrus medusa Grube, 1850 | + | |||||||||
| Polynoidae g.sp. | + | + | + | + | + | |||||
| Praxillella gracilis (M. Sars, 1861) | + | + | + | + | + | + | + | + | + | |
| Praxillella praetermissa (Malmgren, 1865) | + | + | + | |||||||
| Prionospio cirrifera (Wirén, 1883) | + | + | + | + | ||||||
| Pseudoscalibregma parvum (Hansen, 1879) | + | |||||||||
| Rhodine gracilior Tauber, 1879 | + | + | + | + | + | + | + | |||
| Saphobranchia hirsuta (Hansen, 1878) | + | |||||||||
| Saphobranchia longisetosa (Marenzeller, 1890) | + | |||||||||
| Scalibregma inflatum Rathke, 1843 | + | + | + | + | + | |||||
| Scoletoma fragilis (O.F. Müller, 1776) | + | + | ||||||||
| Sosane wireni Hessle, 1917 | + | |||||||||
| Sphaerodoridium kolchaki sp. n. | + | + | ||||||||
| Sphaerodoridium minutum (Webster & Benedict, 1887) | + | |||||||||
| Spio limicola Verrill, 1879 | + | + | + | + | ||||||
| Spiochaetopterus typicus M. Sars, 1856 | + | + | + | + | + | + | + | + | ||
| Spiophanes kroyeri Grube, 1860 | + | + | + | + | + | |||||
| Spirorbinae g.sp. | + | + | ||||||||
| Syllidae g.sp. | + | + | + | + | + | + | + | + | ||
| Terebellidae g.sp. | + | + | + | + | ||||||
| Terebellides gracilis Malm, 1874 | + | + | ||||||||
| Terebellides sp. | + | |||||||||
| Terebellides stroemii Sars, 1835 | + | + | + | + | + | + | + | + | + | + |
| Tharyx sp. 1 | + | + | + | + | + | + | + | + | ||
| Tharyx sp. 2 | + | + | + | + | + | + | + | |||
| Phylum Priapulida | ||||||||||
| Priapulus caudatus Lamarck, 1816 | + | + | + | + | + | + | + | |||
| Phylum Sipuncula | ||||||||||
| Class Sipunculidea | ||||||||||
| Golfingia (G.) elongata (Keferstein, 1863) | + | + | + | + | + | |||||
| Golfingia (G.) margaritacea (M. Sars, 1851) | + | |||||||||
| Nephasoma (N.) diaphanes diaphanes (Gerould, 1913) | + | + | + | + | ||||||
| Nephasoma (N.) eremita (M. Sars, 1851) | + | + | ||||||||
| Nephasoma (N.) lilljeborgi (Danielssen & Koren, 1880) | + | |||||||||
| Nephasoma (N.) abyssorum (Koren & Danielssen, 1876) | + | + | + | + | ||||||
| Phascolion strombus strombus (Montagu, 1804) | + | + | + | + | + | + | ||||
| Golfingia (G.) vulgaris vulgaris (de Blainville, 1827) | + | + | ||||||||
| Phylum Arthropoda | ||||||||||
| Class Malacostraca | ||||||||||
| Order Amphipoda | ||||||||||
| Aceroides latipes (G.O. Sars, 1882) | + | + | + | + | ||||||
| Ampelisca eschrichti Krøyer, 1842 | + | + | + | |||||||
| Amphipoda g.sp. | + | |||||||||
| Anonyx nugax (Phipps, 1774) | + | + | + | |||||||
| Anonyx sp. | + | |||||||||
| Argissa hamatipes (Norman, 1869) | + | |||||||||
| Arrhis phyllonyx (M. Sars, 1858) | + | + | ||||||||
| Bathymedon obtusifrons (Hansen, 1883) | + | |||||||||
| Byblis sp. | + | |||||||||
| Centromedon productus (Goёs, 1866) | + | + | + | + | + | + | ||||
| Centromedon pumilus (Liljeborg, 1865) | + | + | ||||||||
| Ericthonius megalops (G.O. Sars, 1879) | + | |||||||||
| Gammaropsis melanops G.O. Sars, 1882 | + | + | ||||||||
| Gronella groenlandica (Hansen, 1888) | + | |||||||||
| Halirages sp. | + | |||||||||
| Haploops setosa Boeck, 1871 | + | + | + | + | ||||||
| Haploops sp. | + | |||||||||
| Haploops tubicola Liljeborg, 1855 | + | + | + | + | + | + | + | + | + | |
| Hippomedon propinqvus G.O. Sars, 1890 | + | |||||||||
| Idunella aequicornis (G.O. Sars, 1876) | + | + | + | |||||||
| Ischyrocerus megacheir (Boesk, 1871) | + | |||||||||
| Ischyrocerus megalops G.O. Sars, 1894 | + | + | + | |||||||
| Lepidepecreum umbo (Goёs,1866) | + | + | ||||||||
| Menigrates obtusifrons (Boeck, 1861) | + | + | + | |||||||
| Metopa boeckii G.O. Sars, 1892 | + | |||||||||
| Metopa sp. | + | |||||||||
| Neohela monstrosa (Boeck, 1861) | + | |||||||||
| Onisimus derjugini Gurjanova, 1929 | + | + | ||||||||
| Onisimus litoralis (Krøyer, 1845) | + | |||||||||
| Onisimus normani (G.O. Sars, 1895) | + | |||||||||
| Onisimus sp. | + | + | ||||||||
| Orchomene sp. | + | |||||||||
| Paradulichia typica Boeck, 1870 | + | + | ||||||||
| Paraphoxus oculatus (G.O. Sars, 1879) | + | |||||||||
| Photis tenuicornis G.O. Sars, 1882 | + | + | + | + | ||||||
| Quasimelita formosa Murdoch, 1865 | + | |||||||||
| Tmetonyx cicada (Fabricius, 1780) | + | + | ||||||||
| Tryphosella spitzbergensis Chevreux, 1926 | + | |||||||||
| Unciola leucopis (Krøyer, 1845) | + | + | + | + | + | |||||
| Order Cumacea | ||||||||||
| Diastylis goodsiri (Bell, 1855) | + | + | + | + | + | + | + | + | + | |
| Diastylis lepechini Zimmer, 1926 | + | + | + | + | + | + | ||||
| Diastylis rathkei (Krøyer, 1841) | + | |||||||||
| Diastylis spinulosa Heller, 1875 | + | + | + | |||||||
| Ektonodiastylis nimia (Hansen, 1920) | + | + | ||||||||
| Eudorella emarginata (Krøyer, 1846) | + | + | + | + | + | + | + | + | + | + |
| Eudorella spitzbergensis Zimmer, 1926 | + | |||||||||
| Leptostylis villosa G.O. Sars, 1869 | + | + | ||||||||
| Leucon acutirostris G.O. Sars, 1864 | + | + | + | + | + | + | ||||
| Leucon nasica (Krøyer, 1841) | + | + | + | + | ||||||
| Leucon nasicoides Lillijeborg, 1855 | + | |||||||||
| Leucon nathorsti Ohlin, 1901 | + | |||||||||
| Leucon pallidus G.O. Sars, 1864 | + | |||||||||
| Petalosarsia declivis (Sars, 1865) | + | |||||||||
| Order Decapoda | ||||||||||
| Pagurus pubescens (Krøyer, 1838) | + | |||||||||
| Order Isopoda | ||||||||||
| Caecognathia elongata (Krøyer, 1846) | + | + | + | + | + | + | + | + | + | |
| Calathura brachiata (Stimpson, 1854) | + | + | ||||||||
| Desmosoma strombergi Svavarsson, 1988 | + | |||||||||
| Desmosoma tetarta (Hessler, 1970) | + | |||||||||
| Desmosomatidae g.sp. | + | |||||||||
| Munna fabricii Krøyer, 1846 | + | + | ||||||||
| Munna hanseni Stappers, 1911 | + | |||||||||
| Order Tanaidacea | ||||||||||
| Akanthophoreus gracilis (Krøyer, 1847) | + | + | + | |||||||
| Pseudosphyrapus anomalus (G.O. Sars, 1899) | + | + | ||||||||
| Pseudotanais lilljeborgi G.O. Sars, 1882 | + | + | + | + | + | |||||
| Thorkelius latiremis (Hansen, 1913) | + | |||||||||
| Typhlotanais finmarchicus G.O. Sars, 1881 | + | + | ||||||||
| Class Thecostraca | ||||||||||
| Balanus sp. | + | |||||||||
| Class Ostracoda | ||||||||||
| Order Podocopida | ||||||||||
| Actinocythereis dunelmensis (Norman, 1865) | + | + | + | + | + | + | + | + | + | |
| Elofsonella concinna (Jones, 1857) | + | + | + | + | ||||||
| Heterocyprideis fascis (Brady & Norman, 1889) Hazel, 1968 | + | + | + | + | + | + | + | |||
| Heterocyprideis sorbyana (Jones, 1857) | + | + | + | + | + | + | + | |||
| Rabilimis mirabilis (Brady, 1868) | + | + | + | + | ||||||
| Roundstonia macchesneyi (Brady & Crosskey, 1871) | + | + | + | |||||||
| Sarsicytheridea bradii (Norman, 1865) | + | |||||||||
| Order Myodocopida | ||||||||||
| Philomedes globosus | + | + | + | + | + | + | + | + | ||
| Class Pycnogonida | ||||||||||
| Nymphon hirtipes Bell, 1855 | + | |||||||||
| Phylum Mollusca | ||||||||||
| Class Bivalvia | ||||||||||
| Bathyarca glacialis (Gray, 1824) | + | + | + | + | + | + | + | + | ||
| Ciliatocardium ciliatum (Fabricius, 1780) | + | + | + | + | ||||||
| Cuspidaria arctica (M. Sars, 1859) | + | + | + | |||||||
| Cuspidaria sp. | + | + | + | + | ||||||
| Dacrydium vitreum (Møller, 1842) | + | + | + | + | + | + | ||||
| Ennucula tenuis (Montagu, 1808) | + | + | + | + | + | + | + | + | + | + |
| Hiatella arctica (L., 1767) | + | + | ||||||||
| Kurtiella derjugini (Gorbunov, 1952) | + | + | + | |||||||
| Macoma calcarea (Gmelin, 1791) | + | + | + | |||||||
| Macoma moesta (Deshayes, 1855) | + | |||||||||
| Macoma sp. | + | + | ||||||||
| Macoma torelli (A. S. Jensen, 1905) | + | |||||||||
| Mendicula ferruginosa (Forbes, 1844) | + | + | + | + | + | |||||
| Musculus niger (J. E. Gray, 1824) | + | + | ||||||||
| Musculus sp. | + | + | + | |||||||
| Nuculana pernula (Müller, 1779) | + | + | + | + | + | + | + | |||
| Parathyasira equalis (Verrill et Bush, 1898) | + | + | + | + | ||||||
| Thracia myopsis Møller, 1842 | + | + | ||||||||
| Thyasira gouldi (Philippi, 1845) | + | + | + | |||||||
| Thyasiridae g.sp. | + | + | ||||||||
| Tridonta borealis Schumacher, 1817 | + | + | + | |||||||
| Tridonta montagui (Dillwyn, 1817) | + | + | + | |||||||
| Yoldia hyperborean (Gould, 1841) | + | + | + | + | ||||||
| Yoldiella frigida (Torell, 1859) | + | + | ||||||||
| Yoldiella lenticular (Møller, 1842) | + | + | + | + | + | |||||
| Yoldiella lucida (Lovén, 1846) | + | + | + | |||||||
| Yoldiella nana (M. Sars, 1865) | + | + | + | + | + | + | + | + | + | + |
| Bivalvia g.sp. | + | |||||||||
| Class Gastropoda | ||||||||||
| Admete viridula (Fabricius, 1780) | + | |||||||||
| Colus sabini (Gray, 1824) | + | |||||||||
| Cylichna alba (T. Brown, 1827) | + | + | + | |||||||
| Cylichnoides scalptus (Reeve, 1855) | + | + | ||||||||
| Euspira pallida (Broderip & G. B. Sowerby I, 1829) | + | |||||||||
| Frigidoalvania cruenta (Odhner, 1915) | + | |||||||||
| Frigidoalvania janmayeni (Friele, 1878) | + | + | + | + | + | + | + | + | ||
| Hermania scabra (O. F. Müller, 1784) | + | |||||||||
| Margarites groenlandicus (Gmelin, 1791) | + | + | + | |||||||
| Propebela rugulata (Reeve, 1846) | + | + | ||||||||
| Retusa obtusa (Montagu, 1803) | + | + | + | |||||||
| Siphonodentalium lobatum (G. B. Sowerby II, 1860) | + | |||||||||
| Turritellopsis stimpsoni Dall, 1919 | + | + | ||||||||
| Class Caudofoveata | ||||||||||
| Chaetoderma nitidulum Lovén, 1844 | + | + | + | |||||||
| Phylum Bryozoa | ||||||||||
| Class Gymnolaemata | ||||||||||
| Alcyonidium disciforme (Smitt, 1878) | + | + | + | |||||||
| Alcyonidium radicellatum Kluge, 1946 | + | + | + | |||||||
| Amphiblestrum solidum (Packard, 1863) | + | |||||||||
| Arctonula arctica (M. Sars, 1851) | + | |||||||||
| Bugulopsis peachii (Busk, 1851) | + | + | + | |||||||
| Callopora craticula (Alder, 1856) | + | + | + | + | + | + | ||||
| Callopora lineata (Linnaeus, 1767) | + | + | ||||||||
| Cauloramphus cymbaeformis (Hinks, 1877) | + | + | ||||||||
| Copidozoum smitti (Kluge, 1946) | + | + | ||||||||
| Celleporella hyalina (Linnaeus, 1767) | + | + | ||||||||
| Celleporina ventricosa (Lorenz, 1886) | + | |||||||||
| Cheilopora sincera (Smitt, 1868) | + | + | + | + | + | |||||
| Cribrilina spitzbergensis Norman, 1903 | + | |||||||||
| Cystisella saccata (Busk, 1856) | + | + | + | + | + | |||||
| Dendrobeania fruticosa (Packard, 1863) | + | |||||||||
| Einhornia arctica (Borg, 1931) | + | + | + | |||||||
| Escharella ventricosa (Hassall, 1842) | + | + | + | |||||||
| Escharopsis lobata (Lamouroux, 1821) | + | |||||||||
| Escharoides jacksonii (Waters, 1900) | + | |||||||||
| Eucratea loricata (Linnaeus, 1758) | + | + | + | + | + | + | + | + | + | + |
| Hemicyclopora emucronata (Smitt, 1872) | + | |||||||||
| Hincksipora spinulifera (Hincks, 1889) | + | |||||||||
| Hippoporina harmsworthi (Waters, 1900) | + | |||||||||
| Hippoporina reticulatopunctata (Hincks, 1877) | + | + | + | + | + | |||||
| Hippothoa divaricata arctica Kluge, 1906 | + | |||||||||
| Leieschara subgracilis (d’Orbigny, 1853) | + | + | + | + | + | |||||
| Microporella arctica Norman, 1903 | + | |||||||||
| Myriozoella crustacea (Smitt, 1868) | + | + | + | + | + | |||||
| Palmiskenea skenei (Ellis & Solander, 1786) | + | |||||||||
| Parasmittina jeffreysi (Norman, 1876) | + | |||||||||
| Porella acutirostris Smitt, 1868 | + | |||||||||
| Porella belli (Dawson, 1859) | + | |||||||||
| Porella fragilis Levinsen, 1914 | + | + | + | |||||||
| Pseudoflustra solida (Stimpson, 1854) | + | + | ||||||||
| Ragionula rosacea (Busk, 1856) | + | |||||||||
| Rhamphostomella hincksi Nordgaard, 1906 | + | |||||||||
| Rhamphostomella scabra (O. Fabricius, 1824) | + | |||||||||
| Schizoporella costata Kluge, 1962 | + | |||||||||
| Stomachetosella magniporata (Nordgaard, 1906) | + | |||||||||
| Stomacrustula cruenta (Busk, 1854) | + | + | ||||||||
| Tegella armifera (Hincks, 1880) | + | |||||||||
| Tricellaria gracilis (Van Beneden, 1848) | + | + | + | |||||||
| Tricellaria ternata (Ellis & Solander, 1786) | + | |||||||||
| Turbicellepora canaliculata (Busk, 1881) | + | |||||||||
| Turbicellepora incrassata (Lamarck, 1816) | + | + | ||||||||
| Class Stenolaemata | ||||||||||
| Crisia eburneodenticulata Smitt ms in Busk, 1875 | + | + | ||||||||
| Exidmonea atlantica (Forbes in Johnston, 1847) | + | + | + | |||||||
| Oncousoecia diastoporides (Norman, 1869) | + | |||||||||
| Patinella verrucaria (Linnaeus, 1758) | + | + | + | |||||||
| Pleuronea fenestra (Busk, 1859) | + | |||||||||
| Tubulipora flabellaris (O. Fabricius, 1780) | + | |||||||||
| Tubulipora sp. | + | + | + | |||||||
| Tubulipora soluta Kluge, 1946 | + | + | + | |||||||
| Bryozoa g.sp. | + | + | ||||||||
| Phylum Brachiopoda | ||||||||||
| Novocrania anomala (O. F. Müller, 1776) | + | + | + | + | ||||||
| Phylum Echinodermata | ||||||||||
| Class Asteroidea | ||||||||||
| Ctenodiscus crispatus (Retzius, 1805) | + | + | + | + | + | + | + | + | ||
| Class Holothuroidea | ||||||||||
| Ekmania barthi (Troschel, 1846) | + | |||||||||
| Eupyrgus scaber Lütken, 1857 | + | + | + | + | + | |||||
| Myriotrochus rinkii Steenstrup, 1851 | + | + | + | + | + | |||||
| Psolus phantapus Strussenfelt, 1765 | + | |||||||||
| Class Ophiuroidea | ||||||||||
| Amphiura sundevalli (Müller & Troschel, 1842) | + | + | + | + | + | + | + | + | + | |
| Ophiacantha bidentata (Retzius, 1805) | + | + | + | + | ||||||
| Ophiocten sericeum (Forbes, 1852) | + | + | + | + | + | + | + | + | + | + |
| Ophiura sarsi Lütken, 1855 | + | + | + | + | ||||||
| Ophiuroidea g.sp. juv. | + | + | + | + | ||||||
| Phylum Hemichordata | ||||||||||
| Enteropneusta g.sp. | + | + | ||||||||
| Phylum Chordata | ||||||||||
| Class Ascidiacea | ||||||||||
| Cnemidocarpa rhizopus (Redikorzev, 1907) | + | |||||||||
References
- Jakobsen, T.; Ozhigin, V.K. (Eds.) The Barents Sea: Ecosystem, Resources, Management: Half a Century of Russian-Norwegian Co-operation; Tapir Academic Press: Trondheim, Norway, 2011. [Google Scholar]
- Timmermans, M.L.; Marshall, J. Understanding Arctic Ocean circulation: A review of ocean dynamics in a changing climate. J. Geophys. Res. Oceans 2020, 125, e2018JC014378. [Google Scholar] [CrossRef]
- Dvoretsky, A.G.; Dvoretsky, V.G. Filling knowledge gaps in Arctic marine biodiversity: Environment, plankton, and benthos of Franz Josef Land, Barents Sea. Ocean Coast. Manag. 2024, 249, 106987. [Google Scholar]
- Dvoretsky, A.G.; Dvoretsky, V.G. Commercial fish and shellfish in the Barents Sea: Have introduced crab species affected the population trajectories of commercial fish? Rev. Fish Biol. Fish. 2015, 25, 297–322. [Google Scholar] [CrossRef]
- Dvoretsky, A.G.; Dvoretsky, V.G. Ecology and distribution of red king crab larvae in the Barents Sea: A review. Water 2022, 14, 2328. [Google Scholar] [CrossRef]
- Dvoretsky, V.G.; Dvoretsky, A.G. Effects of water temperature on zooplankton abundance and biomass in the southwestern Barents Sea: Implications for Arctic monitoring and management. Ocean Coast. Manag. 2025, 261, 107506. [Google Scholar] [CrossRef]
- Dvoretsky, A.G.; Dvoretsky, V.G. New echinoderm-crab epibiotic associations from the coastal Barents Sea. Animals 2021, 11, 917. [Google Scholar] [CrossRef]
- Dvoretsky, A.G.; Dvoretsky, V.G. Epibionts of an introduced king crab in the Barents Sea: A second five-year study. Diversity 2023, 15, 29. [Google Scholar] [CrossRef]
- Dvoretsky, V.G.; Dvoretsky, A.G. Summer zooplankton assemblages in the Barents Sea: Spatial variations and effects of environmental conditions as revealed from in situ and satellite data. Prog. Oceanogr. 2025, 231, 103417. [Google Scholar]
- Dvoretsky, A.G.; Dvoretsky, V.G. Stock dynamics of female red king crab in a small bay of the Barents Sea in relation to environmental factors. Animals 2025, 15, 99. [Google Scholar] [CrossRef]
- Dvoretsky, A.G.; Dvoretsky, V.G. Aquaculture of green sea urchin in the Barents Sea: A brief review of Russian studies. Rev. Aquacult. 2020, 12, 1280–1290. [Google Scholar] [CrossRef]
- Dvoretsky, A.G.; Dvoretsky, V.G. Cucumaria in Russian waters of the Barents Sea: Biological aspects and aquaculture potential. Front. Mar. Sci. 2021, 8, 613453. [Google Scholar] [CrossRef]
- Dvoretsky, A.G.; Dvoretsky, V.G. 2024b. Distribution patterns and biological aspects of Strongylocentrotus droebachiensis (Echinoidea: Echinoida) in Russian waters of the Barents Sea: Implications for commercial exploration. Rev. Fish Biol. Fish. 2024, 34, 1215–1229. [Google Scholar] [CrossRef]
- Filgueira, R.; Grant, J.; Strand, Ø. Implementation of marine spatial planning in shellfish aquaculture management: Modeling studies in a Norwegian fjord. Ecol. Appl. 2014, 24, 832–843. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, F.; Kleiven, A.R.; Ottesen, M.V.; Søvik, G. Inclusion of recreational fishing in data-limited stocks: A case study on Norway lobster (Nephrops norvegicus) in Norway. Can. J. Fish. Aquat. Sci. 2022, 79, 969–978. [Google Scholar] [CrossRef]
- Bianchi, T.S.; Arndt, S.; Austinc, W.E.N.; Benn, D.I.; Bertrand, S.; Cui, X.; Faust, J.C.; Koziorowska-Makuch, K.; Moy, C.M.; Savage, C.; et al. Fjords as aquatic critical zones (ACZs). Earth-Sci. Rev. 2020, 203, 103145. [Google Scholar]
- De Rovere, F.; Langone, L.; Schroeder, K.; Miserocchi, S.; Giglio, F.; Aliani, S.; Chiggiato, J. Water masses variability in inner Kongsfjorden (Svalbard) during 2010–2020. Front. Mar. Sci. 2022, 9, 741075. [Google Scholar] [CrossRef]
- Skogseth, R.; Fer, I.; Haugan, P.M. Dense-water production and overflow from an Arctic coastal polynya in Storfjorden. Geophys. Monogr. Ser. 2005, 158, 73–88. [Google Scholar]
- Skogseth, R.; Olivier, L.L.A.; Nilsen, F.; Falck, E.; Fraser, N.; Tverberg, V.; Ledang, A.B.; Vader, A.; Jonassen, M.O.; Søreide, J.; et al. Variability and decadal trends in the Isfjorden (Svalbard) ocean climate and circulation—An indicator for climate change in the European Arctic. Prog. Oceanogr. 2020, 187, 102394. [Google Scholar] [CrossRef]
- Pavlov, A.K.; Tverberg, V.; Ivanov, B.V.; Nilsen, F.; Falk-Petersen, S.; Granskog, M.A. Warming of Atlantic Water in two west Spitsbergen fjords over the last century (1912–2009). Polar Res. 2013, 32, 11206. [Google Scholar] [CrossRef]
- Tislenko, D.I.; Ivanov, B.V. Long-period variability of Atlantic water temperature in the fjords of West Svalbard Island during the first (1920–1940) and about modern warming in the Arctic. Probl. Arct. Antarct. 2015, 2, 93–100. (In Russian) [Google Scholar]
- Ingvaldsen, R.B.; Assmann, K.M.; Primicerio, R.; Fossheim, M.; Polyakov, I.V.; Dolgov, A.V. Physical manifestations and ecological implications of Arctic Atlantification. Nat. Rev. Earth Environ. 2021, 2, 874–889. [Google Scholar] [CrossRef]
- Bloshkina, E.V.; Pavlov, A.K.; Filchuk, K. Warming of Atlantic Water in three west Spitsbergen fjords: Recent patterns and century-long trends. Polar Res. 2021, 40, 5392. [Google Scholar] [CrossRef]
- Athanase, M.; Provost, C.; Perez-Hernandez, M.D.; Sennechael, N.; Bertosio, C.; Artana, C.; Garric, G.; Lellouche, J.-M. Atlantic water modification north of Svalbard in the Mercator physical system from 2007 to 2020. J. Geophys. Res. Oceans 2020, 125, e2020JC016463. [Google Scholar] [CrossRef]
- Dahlke, S.; Hughes, N.E.; Wagner, P.M.; Gerland, S.; Wawrzyniak, T.; Ivanov, B.; Maturilli, M. The observed recent surface air temperature development across Svalbard and concurring footprints in local sea ice cover. Int. J. Climatol. 2020, 40, 5246–5265. [Google Scholar] [CrossRef]
- Roy, V.; Iken, K.; Archambault, P. Environmental drivers of the Canadian Arctic megabenthic communities. PLoS ONE 2014, 9, e100900. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.L.; Piepenburg, D.; Pantiukhin, D.; Kraan, C. Unraveling the effects of environmental drivers and spatial structure on benthic species distribution patterns in Eurasian-Arctic seas (Barents, Kara and Laptev Seas). Polar Biol. 2020, 43, 1693–1705. [Google Scholar] [CrossRef]
- Carroll, M.L.; Denisenko, S.G.; Renaud, P.E.; Ambrose, W.G., Jr. Benthic infauna of the seasonally ice-covered western Barents Sea: Patterns and relationships to environmental forcing. Deep-Sea Res. II 2008, 55, 2340–2351. [Google Scholar] [CrossRef]
- Cochrane, S.K.J.; Pearson, T.H.; Greenacre, M.; Costelloe, J.; Ellingsen, I.H.; Dahle, S.; Gulliksen, B. Benthic fauna and functional traits along a Polar Front transect in the Barents Sea—Advancing tools for ecosystem-scale assessments. J. Mar. Syst. 2012, 94, 204–217. [Google Scholar] [CrossRef]
- Evseeva, O.Y.; Ishkulova, T.G.; Dvoretsky, A.G. Environmental drivers of an intertidal bryozoan community in the Barents Sea: A case study. Animals 2022, 12, 552. [Google Scholar] [CrossRef]
- Kortsch, S.; Primicerio, R.; Beuchel, F.; Renaud, P.E.; Rodrigues, J.; Lønne, O.J.; Gulliksen, B. Climate-driven regime shifts in Arctic marine benthos. Proc. Natl. Acad. Sci. USA 2012, 109, 14052–14057. [Google Scholar] [CrossRef]
- Pantiukhin, D.; Piepenburg, D.; Hansen, M.L.; Kraan, C. Data-driven bioregionalization: A seascape-scale study of macrobenthic communities in the Eurasian Arctic. J. Biogeogr. 2021, 48, 2877–2890. [Google Scholar] [CrossRef]
- Olsen, G.H.; Carroll, M.L.; Renaud, P.E.; Ambrose, W.G.; Olssøn, R.; Carroll, J. Benthic community response to petroleum-associated components in Arctic versus temperate marine sediments. Mar. Biol. 2007, 151, 2167–2176. [Google Scholar] [CrossRef]
- Konovalov, D.; Renaud, P.E.; Berge, J.; Voronkov, A.Y.; Cochrane, S.K.J. Contaminants, benthic communities, and bioturbation: Potential for PAH mobilization in Arctic sediments. Chem. Ecol. 2010, 26, 197–208. [Google Scholar] [CrossRef]
- Caridi, F.; Sabbatini, A.; Morigi, C.; Dell’Anno, A.; Negri, A.; Lucchi, R.G. Patterns and environmental drivers of diversity and community composition of macrofauna in the Kveithola Trough (NW Barents Sea). J. Sea Res. 2019, 153, 101780. [Google Scholar] [CrossRef]
- Pavlova, L.V.; Zuyev, Y.A.; Dvoretsky, A.G. Shallow-water benthic communities on soft bottoms of a sub-Arctic fjord (Southern Barents Sea, Russia) along a gradient of ecological factors. Diversity 2023, 15, 84. [Google Scholar] [CrossRef]
- Dvoretsky, A.G.; Dvoretsky, V.G. Red king crab (Paralithodes camtschaticus) fisheries in Russian waters: Historical review and present status. Rev. Fish Biol. Fish. 2018, 28, 331–353. [Google Scholar]
- Syvitski, J.P.M.; Farrow, G.E.; Atkinson, R.J.A.; Moore, P.G.; Andrews, J.T. Baffin Island fjord macrobenthos: Bottom communities and environmental significance. Arctic 1989, 42, 232–247. [Google Scholar] [CrossRef]
- Holte, B.; Gulliksen, B. Common macrofaunal dominant species in the sediments of some north Norwegian and Svalbard glacial fjords. Polar Biol. 1998, 19, 375–382. [Google Scholar] [CrossRef]
- Włodarska-Kowalczuk, M.; Węsławski, J.M.; Kotwicki, L. Spitsbergen glacial bays macrobenthos—A comparative study. Polar Biol. 1998, 20, 66–73. [Google Scholar] [CrossRef]
- Włodarska-Kowalczuk, M.; Pearson, T.H.; Kendall, M.A. Benthic response to chronic natural physical disturbance by glacial sedimentation in an Arctic fjord. Mar. Ecol. Prog. Ser. 2005, 303, 31–41. [Google Scholar] [CrossRef]
- Lyubina, O.S.; Zimina, O.L.; Frolova, E.A.; Lyubin, P.A.; Frolov, A.A.; Dikaeva, D.R.; Akhmetchina, O.Y.; Garbul, E.A. Features of the benthic communities distribution in the fjords of West Spitsbergen. Probl. Arct. Antarct. 2011, 1, 28–40. (In Russian) [Google Scholar]
- Meyer, K.S.; Sweetman, A.K.; Young, C.M.; Renaud, P.E. Environmental factors structuring Arctic megabenthos—A case study from a shelf and two fjords. Front. Mar. Sci. 2015, 2, 22. [Google Scholar] [CrossRef]
- Jorda-Molina, E.; Renaud, P.E.; Silberberger, M.J.; Sen, A.; Bluhm, B.A.; Carroll, M.L.; Ambrose, W.G., Jr.; Cottier, F.; Reiss, H. Seafloor warm water temperature anomalies impact benthic macrofauna communities of a high-Arctic cold-water fjord. Mar. Environ. Res. 2023, 189, 106046. [Google Scholar] [PubMed]
- Hop, H.; Wiencke, C. (Eds.) The Ecosystem of Kongsfjorden, Svalbard; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Udalov, A.; Chikina, M.; Chava, A.; Vedenin, A.; Shchuka, S.; Mokievsky, V. Patterns of benthic communities in Arctic fjords (Novaya Zemlya archipelago, Kara Sea): Resilience vs. fragility. Front. Ecol. Evol. 2021, 9, 777006. [Google Scholar]
- Lundesgaard, Ø.; Sundfjord, A.; Lind, S.; Nilsen, F.; Renner, A.H.H. Import of Atlantic Water and sea ice controls the ocean environment in the northern Barents Sea. Ocean Sci. 2022, 18, 1389–1418. [Google Scholar] [CrossRef]
- Włodarska-Kowalczuk, M.; Górska, B.; Deja, K.; Morata, N. Do benthic meiofaunal and macrofaunal communities respond to seasonality in pelagic processes in an Arctic fjord (Kongsfjorden, Spitsbergen)? Polar Biol. 2016, 39, 2115–2129. [Google Scholar] [CrossRef]
- Hop, H.; Pearson, T.; Hegseth, E.N.; Kovacs, K.M.; Wiencke, C.; Kwasniewski, S.; Eiane, K.; Mehlum, F.; Gulliksen, B.; Wlodarska-Kowalczuk, M.; et al. The marine ecosystem of Kongsfjorden, Svalbard. Polar Res. 2002, 21, 167–208. [Google Scholar]
- Kendall, M.A.; Widdicombe, S.; Weslawski, J.M. A multi-scale assessment of the biodiversity of benthic infauna of the high-latitude Kongsfjord, Svalbard. Polar Biol. 2003, 26, 283–388. [Google Scholar] [CrossRef]
- Weslawski, J.M.; Wlodarska-Kowalczuk, M.; Legezynska, J. Occurrence of soft bottom macrofauna along the depth gradient in the High Arctic, 79° N. Polar Res. 2003, 24, 73–88. [Google Scholar]
- Kaczmarek, H.; Włodarska-Kowalczuk, M.; Legezynska, J.; Zajaczkowski, M. Shallow sublittoral macrozoobenthos in Kongsfjord, West Spitsbergen, Svalbard. Pol. Polar Res. 2005, 26, 137–155. [Google Scholar]
- Laudien, J.; Herrmann, M.; Arntz, W.E. Soft bottom species richness and diversity as a function of depth and iceberg scour in Arctic glacial Kongsfjorden (Svalbard). Polar Biol. 2007, 30, 1035–1046. [Google Scholar] [CrossRef]
- Włodarska-Kowalczuk, M.; Pearson, T. Soft-bottom faunal associations and factors affecting species distributions in an Arctic glacial fjord (Kongsfjorden, Spitsbergen). Polar Biol. 2004, 27, 155–167. [Google Scholar] [CrossRef]
- Frolova, E.A.; Dikaeva, D.R.; Lyubina, O.S.; Frolov, A.A.; Garbul, E.A.; Artyukh, O.L.; Akhmetchina, O.Y.; Pavlova, L.V. Benthos composition and distribution in Hornsund (Spitsbergen). In The Nature of the Arctic Shelves and Archipelagos; GEOS: Moscow, Russia, 2008; Volume 8, pp. 363–366. (In Russian) [Google Scholar]
- Ronowicz, M.; Włodarska-Kowalczuk, M.; Kuklijski, P. Patterns of hydroid (Cnidaria, Hydrozoa) species richness and distribution in an Arctic glaciated fjord. Polar Biol. 2011, 34, 1437–1445. [Google Scholar] [CrossRef][Green Version]
- Kędra, M.; Pabis, K.; Gromisz, S.; Wesławski, J.M. Distribution patterns of polychaete fauna in an Arctic fjord (Hornsund, Spitsbergen). Polar Biol. 2013, 36, 1463–1472. [Google Scholar] [CrossRef]
- Dikaeva, D.R.; Frolova, E.A. Modern distribution of Polychaeta communities in fjords of West Spitsbergen. Vestn. MGTU 2014, 17, 118–127. (In Russian) [Google Scholar]
- Zaborska, A.; Włodarska-Kowalczuk, M.; Legezynska, J.; Jankowska, E.; Winogradow, A.; Deja, K. Sedimentary organic matter sources, benthic consumption and burial in west Spitsbergen fjords—Signs of maturation of Arctic fjordic systems? J. Mar. Syst. 2016, 180, 112–123. [Google Scholar] [CrossRef]
- Drewnik, A.; Lacka, M.; Promińska, A.; Zaborska, A.; Gluchowska, M. From the worm’s point of view. I: Environmental settings of benthic ecosystems in Arctic fjord (Hornsund, Spitsbergen). Polar Biol. 2016, 39, 1411–1424. [Google Scholar] [CrossRef]
- Legeżyńska, J.; Włodarska-Kowalczuk, M.; Gluchowska, M.; Ormańczyk, M.; Kędra, M.; Węsławski, J.M. The malacostracan fauna of two Arctic fjords (west Spitsbergen): The diversity and distribution patterns of its pelagic and benthic components. Oceanologia 2017, 59, 541–564. [Google Scholar] [CrossRef]
- Dunlop, K.; Renaud, P.E.; Berge, J.; Jones, D.O.B.; Harbour, R.P.; Tandberg, A.H.S.; Sweetman, A.K. Benthic scavenger community composition and carrion removal in Arctic and Subarctic fjords. Polar Biol. 2021, 44, 31–43. [Google Scholar] [CrossRef]
- Noskovich, A.E.; Dvoretsky, A.G. Spatial distribution and growth patterns of a common bivalve mollusk (Macoma calcarea) in Svalbard fjords in relation to environmental factors. Animals 2024, 14, 3352. [Google Scholar] [CrossRef]
- Carroll, M.L.; Ambrose, W.G., Jr. Benthic infaunal community variability on the northern Svalbard shelf. Polar Biol. 2012, 35, 1259–1272. [Google Scholar]
- Węsławski, J.M.; Kendall, M.A.; Włodarska-Kowalczuk, M.; Iken, K.; Kędra, M.; Legezynska, J.; Sejr, M.K. Climate change effects on Arctic fjord and coastal macrobenthic diversity—Observations and predictions. Mar. Biodiv. 2011, 41, 71–85. [Google Scholar] [CrossRef]
- Al-Habahbeh, A.K.; Kortsch, S.; Bluhm, B.A.; Beuchel, F.; Gulliksen, B.; Ballantine, C.; Cristini, D.; Primicerio, R. Arctic coastal benthos long–term responses to perturbations under climate warming. Philos. Trans. R. Soc. A 2020, 378, 20190355. [Google Scholar] [CrossRef]
- Skogseth, R.; McPhee, M.G.; Nilsen, F.; Smedsrud, L.H. Creation and tidal advection of a cold salinity front in Storfjorden: 1. Polynya dynamics. J. Geophys. Res. Oceans 2013, 118, 3278–3291. [Google Scholar] [CrossRef]
- Barton, B.I.; Lenn, Y.D.; Lique, C. Observed Atlantification of the Barents Sea causes the polar front to limit the expansion of winter sea ice. J. Phys. Oceanogr. 2018, 48, 1849–1866. [Google Scholar] [CrossRef]
- Ivanov, B.V.; Pavlov, A.K.; Andreev, O.M.; Zhuravskiy, D.M.; Svyashchennikov, P.N. Investigation of snow and ice cover in Gronfjorden (Spitsbergen): Historical data, in situ observations and modeling. Probl. Arctic Antarct. 2012, 2, 43–54. [Google Scholar]
- Brotzky, V.A. Materials for the quantitative evaluation of the bottom fauna of the Storfjord (East Spitzbergen). Ber. Wiss. Meeresinst. 1930, 4, 47–61. [Google Scholar]
- Kendall, M.A. Are Arctic soft sediment macrobenthic communities impoverished? Polar Biol. 1996, 16, 393–399. [Google Scholar] [CrossRef]
- Gulliksen, B.; Palerud, R.; Brattegard, T.; Sneli, J. Distribution of Marine Benthic Macro-Organisms at Svalbard (Including Bear Island) and Jan Mayen; Research Report for DN 1999-4; Directorate for Nature Management: Trondheim, Norway, 1999; 148p. [Google Scholar]
- Anisimova, N.A.; Jørgensen, L.L.; Lyubin, P.A.; Manushin, I.E. Mapping and Monitoring of Benthos in the Barents Sea and Svalbard Waters: Results from the Joint Russian—Norwegian Benthic Programme 2006–2008; IMR-PINRO Joint Report Series 1–2010; IMR: Bergen, Norway, 2010. [Google Scholar]
- Schauer, U. The release of brine-enriched shelf water from Storfjord into the Norwegian Sea. J. Geophys. Res. Oceans 1995, 100, 16015–16028. [Google Scholar] [CrossRef]
- Loeng, H. Features of the physical oceanographic conditions of the Barents Sea. Polar Res. 1991, 10, 5–18. [Google Scholar] [CrossRef]
- Skogseth, R.; Haugan, P.M.; Jakobsson, M. Watermass transformations in Storfjorden. Cont. Shelf Res. 2005, 25, 667–695. [Google Scholar] [CrossRef]
- Quadfasel, D.; Rudels, B.; Kurz, K. Outflow of dense water from a Svalbard fjord into the Fram Strait. Deep-Sea Res. 1988, 35, 1143–1150. [Google Scholar] [CrossRef]
- Smedsrud, L.H.; Budgell, W.P.; Jenkins, A.D.; Ådlandsvik, B. Fine-scale sea-ice modeling of the Storfjorden polynya, Svalbard. Ann. Glaciol. 2006, 44, 73–79. [Google Scholar] [CrossRef][Green Version]
- Owrid, G.; Socal, G.; Civitarese, G.; Luchetta, A.; Wiktor, J.; Nöthig, E.M.; Strass, V. Spatial variability of phytoplankton, nutrients and new production estimates in the waters around Svalbard. Polar Res. 2000, 19, 155–171. [Google Scholar] [CrossRef]
- Haarpaintner, J.; O’Dwyer, J.; Gascard, J.-C.; Haugan, P.M.; Schauer, U.; Østerhus, S. Seasonal transformation of water masses, circulation and brine formation observed in Storfjorden, Svalbard. Ann. Glaciol. 2001, 33, 437–443. [Google Scholar] [CrossRef]
- Skogseth, R.; Haugan, P.M.; Haarpaintner, J. Ice and brine production in Storfjorden from four winters of satellite and in situ observations and modeling. J. Geophys. Res. Oceans 2004, 109, 1–15. [Google Scholar] [CrossRef]
- Lydersen, C.; Assmy, P.; Falk-Petersen, S.; Kohler, J.; Kovacs, K.M.; Reigstad, M.; Steen, H.; Strøma, H.; Sundfjord, A.; Varpe, Ø.; et al. The importance of tidewater glaciers for marine mammals and seabirds in Svalbard, Norway. J. Mar. Syst. 2014, 129, 452–471. [Google Scholar]
- Schuler, T.V.; Kohler, J.; Elagina, N.; Hagen, J.O.M.; Hodson, A.J.; Jania, J.A.; Kääb, A.M.; Luks, B.; Malecki, J.; Moholdt, G.; et al. Reconciling Svalbard Glacier Mass Balance. Front. Earth Sci. 2020, 8, 156. [Google Scholar]
- Fossile, E.; Nardelli, M.P.; Jouini, A.; Lansard, B.; Pusceddu, A.; Moccia, D.; Michel, E.; Péron, O.; Howa, H.; Mojtahid, M. Benthic foraminifera as tracers of brine production in the Storfjorden “sea ice factory”. Biogeosciences 2020, 17, 1933–1953. [Google Scholar] [CrossRef]
- Meshcheryakov, N.I.; Usyagina, I.S.; Ilyin, G.V. Chronology of modern sedimentation in the Stur-Fjord Strait (Svalbard Archipelago). Geochemistry 2023, 68, 521–532. (In Russian) [Google Scholar]
- Glud, R.N.; Holby, O.; Hoffmann, F.; Canfield, D.E. Benthic mineralization and exchange in Arctic sediments (Svalbard, Norway). Mar. Ecol. Prog. Ser. 1998, 173, 237–251. [Google Scholar] [CrossRef]
- Winkelmann, D.; Knies, J. Recent distribution and accumulation of organic carbon on the continental margin west off Spitsbergen. Geochem. Geophys. Geosyst. 2005, 6, Q09012. [Google Scholar] [CrossRef]
- Istoshin, Y.V. Marine Hydrometry; Hydrometeorological Publishing House: Leningrad, USSR, 1967; 407p. (In Russian) [Google Scholar]
- Grebmeier, J.M.; Barry, J.P. The influence of oceanographic processes on pelagic-benthic coupling in polar regions: A benthic perspective. J. Mar. Syst. 1991, 2, 495–518. [Google Scholar] [CrossRef]
- Denisenko, S.G.; Barbashova, M.A.; Skvortsov, V.V.; Belyakov, V.P.; Kurashov, E.A. The results of assessment of the ecological state of zoobenthos communities according to the “Difference of Evenness” Index (DE). Inland Water Biol. 2013, 6, 39–47. [Google Scholar] [CrossRef]
- Golikov, A.N.; Averincev, V.G. Biocoenoses of the upper regions of the shelf of Franz Josef Land archipelago and some regularities of their distribution. In Biocoenoses of the Shelf of Franz Josef Land and the Fauna of Adjacent Water; Explorations of the Fauna of the Seas; Golikov, A.N., Ed.; Nauka: Moscow, Russia, 1990; Volume 14, pp. 5–54. (In Russian) [Google Scholar]
- Clarke, K.R.; Warwick, R.M. Changes in Marine Communities: An Approach to Statistical Analysis and Interpretation, 2nd ed.; PRIMER-E: Plymouth, UK, 2001. [Google Scholar]
- Macdonald, T.A.; Burd, B.J.; Macdonald, V.I.; van Roodselaar, A. Taxonomic and feeding guild classification for the marine benthic macroinvertebrates of the Strait of Georgia, British Columbia. Can. Tech. Rep. Fish. Aquat. Sci. 2010, 2874, 1–63. [Google Scholar]
- Jumars, P.A.; Dorgan, K.M.; Lindsay, S.M. Diet of Worms Emended: An Update of Polychaete Feeding Guilds. Annu. Rev. Mar. Sci. 2015, 7, 497–520. [Google Scholar] [CrossRef] [PubMed]
- Gagaev, S.Y. Polychaetes (Annelida: Polychaeta) of the Kara Sea. Proc. Zool. Inst. RAS 2021, 325, 183–196. [Google Scholar] [CrossRef]
- ter Braak, C.J.F.; Smilauer, P. CANOCO Reference Manual and CanoDraw for Windows User’s Guide: Software for Canonical Community Ordination (Version 4.5); Microcomputer Power: Ithaca, NY, USA, 2002. [Google Scholar]
- Lockwood, C. Reconstruction of Ice Stream Retreat and Palaeoceanographic Development During the Deglaciation and Holocene in the Storfjorden Trough, Svalbard Based on Geophysical Data, Benthic Foraminiferal and Sedimentological Records. Master’s Thesis, UiT—The Arctic University of Norway, Tromsø, Norway, 2016; 136p. [Google Scholar]
- Vivier, F.; Lourenco, A.; Michel, E.; Skogseth, R.; Rousset, C.; Lansard, B.; Bouruet-Aubertot, P.; Boutin, J.; Bombled, B.; Cuypers, Y.; et al. Summer hydrography and circulation in Storfjorden, Svalbard, following a record low winter sea-ice extent in the Barents Sea. J. Geophys. Res. Oceans 2023, 128, e2022JC018648. [Google Scholar] [CrossRef]
- Promińska, A.; Cizek, M.; Walczowski, W. Kongsfjorden and Hornsund hydrography—Comparative study based on a multiyear survey in fjords of West Spitsbergen. Oceanologia 2017, 59, 397–412. [Google Scholar] [CrossRef]
- Svendsen, H.; Beszczynska-Møller, A.; Hagen, J.O.; Lefauconnier, B.; Tverberg, V.; Gerland, S.; Ørbæk, J.B.; Bischof, K.; Papucci, C.; Zajaczkowski, M.; et al. The physical environment of Kongsfjorden—Krossfjorden, an Arctic fjord system in Svalbard. Polar Res. 2002, 21, 133–166. [Google Scholar]
- Anderson, L.G.; Falck, E.; Jones, E.P.; Jutterström, S.; Swift, J.H. Enhanced uptake of atmospheric CO2 during freezing of seawater: A field study in Storfjorden, Svalbard. J. Geophys. Res. 2004, 109, C06004. [Google Scholar] [CrossRef]
- Moiseev, D.V.; Ionov, V.V. Some results of oceanographic investigations in fjords and bays of West Spitsbergen Island in summer of 2001 and 2002. In Complex Investigations of Spitsbergen Nature; Matishov, G.G., Ed.; KSC RAS: Apatity, Russia, 2006; pp. 261–270. (In Russian) [Google Scholar]
- Drewnik, A.; Wesławski, J.M.; Włodarska-Kowalczuk, M. Benthic crustacea and mollusca distribution in Arctic fjord—Case study of patterns in Hornsund, Svalbard. Oceanologia 2017, 59, 565–575. [Google Scholar] [CrossRef]
- Geyer, F.; Fer, I.; Eldevik, T. Dense overflow from an Arctic fjord: Mean seasonal cycle, variability and wind influence. Cont. Shelf Res. 2009, 29, 2110–2121. [Google Scholar] [CrossRef]
- Zajaczkowski, M.; Nygård, H.; Jørgen, E.N.; Berge, H. Vertical flux of particulate matter in an Arctic fjord: The case of lack of sea-ice cover in Adventfjorden 2006–2007. Polar Biol. 2010, 33, 223–239. [Google Scholar] [CrossRef]
- Petit, T.; Hamre, B.; Sandven, H.; Röttgers, R.; Kowalczuk, P.; Zablocka, M.; Granskog, M.A. Inherent optical properties of dissolved and particulate matter in an Arctic fjord (Storfjorden, Svalbard) in early summer. Ocean Sci. 2022, 18, 455–468. [Google Scholar] [CrossRef]
- Zaborska, A.; Pempkowiak, J.; Papucci, C. Some sediment characteristics and sedimentation rates in an Arctic fjord (Kongsfjorden, Svalbard). Annu. Environ. Prot. 2006, 8, 79–96. [Google Scholar]
- Gjelten, H.M.; Nordli, Ø.; Isaksen, K.; Førland, E.J.; Sviashchennikov, P.N.; Wyszynski, P.; Prokhorova, U.V.; Przybylak, R.; Ivanov, B.V.; Urazgildeeva, A.V. Air temperature variations and gradients along the coast and fjords of western Spitsbergen. Polar Res. 2016, 35, 29878. [Google Scholar] [CrossRef]
- Nilsen, F.; Skogseth, R.; Vaardal-Lunde, J.; Inall, M. A Simple Shelf Circulation Model: Intrusion of Atlantic Water on the West Spitsbergen Shelf. J. Phys. Oceanogr. 2016, 46, 1209–1224. [Google Scholar] [CrossRef]
- Matishov, G.G.; Berdnikov, S.V.; Zhichkin, A.P.; Dzhenyuk, S.L.; Smolyar, I.V.; Kulygin, V.V.; Yaitskaya, N.A.; Povazhniy, V.V.; Sheverdyaev, I.V.; Kumpan, S.V.; et al. Atlas of Climatic Changes in Nine Large Marine Ecosystems of the Northern Hemisphere (1827–2013). In NOAA Atlas NESDIS 78; Matishov, G.G., Sherman, K., Levitus, S., Eds.; NOAA: Silver Spring, MD, USA, 2014; 131p. Available online: https://repository.library.noaa.gov/view/noaa/1302/noaa_1302_DS1.pdf (accessed on 10 March 2025). (In Russian)
- Syvitski, J.P.M.; Burrell, D.C.; Skei, J.M. Fjords: Processes and Products; Springer: New York, NY, USA, 1987; 379p. [Google Scholar]
- Li, W.K.W.; McLaughlin, F.A.; Lovejoy, C.; Carmack, E.C. Smallest algae thrive as the Arctic Ocean freshens. Science 2009, 326, 539. [Google Scholar] [CrossRef]
- Arrigo, K.R.; van Dijken, G.L. Continued increases in Arctic Ocean primary production. Progr. Oceanogr. 2015, 136, 60–70. [Google Scholar] [CrossRef]
- Lewis, K.M.; van Dijken, G.L.; Arrigo, K.R. Changes in phytoplankton concentration now drive increased Arctic Ocean primary production. Science 2020, 369, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Dvoretsky, V.G.; Vodopianova, V.V.; Bulavina, A.S. Effects of climate change on chlorophyll a in the Barents Sea: A long-term assessment. Biology 2023, 12, 119. [Google Scholar] [CrossRef] [PubMed]
- Piwosz, K.; Walkusz, W.; Hapter, R.; Wieczorek, P.; Hop, H.; Wiktor, J. Comparison of productivity and phytoplankton in a warm (Kongsfjorden) and a cold (Hornsund) Spitsbergen fjord in mid-summer 2002. Polar Biol. 2009, 32, 549–559. [Google Scholar] [CrossRef]
- Wiktor, J.; Głuchowska, M.; Błachowiak-Samołyk, K.; Piwosz, K.; Kwaśniewski, S.; Jankowska, K.; Dmoch, K.; Węsławski, J.M. Arctic fjord during warming: Planktonic point of view. Ukr. Antarct. J. 2022, 20, 67–84. [Google Scholar] [CrossRef]
- Bodur, Y.V.; Renaud, P.E.; Lins, L.; Da Costa Monteiro, L.; Ambrose, W.G., Jr.; Felden, J.; Krumpen, T.; Wenzhofer, F.; Włodarska-Kowalczuk, M.; Braeckman, U. Weakened pelagic-benthic coupling on an Arctic outflow shelf (Northeast Greenland) suggested by benthic ecosystem changes. Elem. Sci. Anth. 2024, 12, 00005. [Google Scholar]
- Piepenburg, D.; Ambrose, W.G., Jr.; Brandt, A.; Renaud, P.E.; Ahrens, M.J.; Jensen, P. Benthic community patterns reflect water column processes in the Northeast Water Polynya (Greenland). J. Mar. Syst. 1997, 10, 467–482. [Google Scholar] [CrossRef]
- Zimina, O.L.; Frolova, E.A.; Dikaeva, D.R.; Akhmetchina, O.Y.; Garbul, E.A.; Frolov, A.A.; Nekhaev, I.O. Fauna and distribution of abundance and biomass of zoobenthos in the northern Barents Sea in April and May 2016. Trans. Kola Sci. Cent. Oceanol. 2017, 4, 66–80. (In Russian) [Google Scholar]
- Pavlova, L.V.; Dvoretsky, A.G.; Frolov, A.A.; Zimina, O.L.; Evseeva, O.Y.; Dikaeva, D.R.; Rumyantseva, Z.Y.; Panteleeva, N.N. The impact of sea ice loss on benthic communities of the Makarov Strait (Northeastern Barents Sea). Animals 2023, 13, 2320. [Google Scholar] [CrossRef]
- Włodarska-Kowalczuk, M.; Renaud, P.E.; Węsławski, J.M.; Cochrane, S.K.; Denisenko, S.G. Species diversity, functional complexity and rarity in Arctic fjordic versus open shelf benthic systems. Mar. Ecol. Prog. Ser. 2012, 463, 73–87. [Google Scholar] [CrossRef]
- Holte, B.; Dahle, S.; Naes, K.; Gulliksen, B. Some macrofaunal effects of local pollution and glacier-induced sedimentation, with indicative chemical analyses, in the sediments of two arctic fjords. Polar Biol. 1996, 14, 917–927. [Google Scholar] [CrossRef]
- Kędra, M.; Gromisz, S.; Jaskula, R.; Legezynska, J.; Maciejewska, B.; Malec, E.; Opanowski, A.; Ostrowska, K.; Włodarska-Kowalczuk, M.; Wesławski, J.M. Soft bottom macrofauna of an All Taxa Biodiversity Site: Hornsund (77° N, Svalbard). Pol. Polar Res. 2010, 31, 309–326. [Google Scholar]
- Węsławski, J.M.; Włodarska-Kowalczuk, M.; Kędra, M.; Legezynska, J.; Kotwicki, L. Eight species that rule today’s European Arctic fjord benthos. Polar Polar Res. 2012, 33, 225–238. [Google Scholar] [CrossRef]
- Oug, E. Soft-bottom macrofauna in the high-latitude ecosystem of Balsfjord, northern Norway: Species composition, community structure and temporal variability. Sarsia 2000, 85, 1–13. [Google Scholar] [CrossRef]
- McGovern, M.; Poste, A.E.; Oug, E.; Renaud, P.E.; Trannum, H.C. Riverine impacts on benthic biodiversity and functional traits: A comparison of two sub-Arctic fjords. Estuar. Coast. Shelf Sci. 2020, 240, 106774. [Google Scholar] [CrossRef]
- Pavlova, L.V.; Akhmetchina, O.Y.; Garbul, E.A.; Dikaeva, D.R.; Zimina, O.L.; Noskovich, A.E.; Frolov, A.A.; Frolova, E.A. New data on the benthos condition of Kola Bay (Barents Sea). Trans. Kola Sci. Cent. RAS 2019, 6, 35–75. (In Russian) [Google Scholar]
- Fetzer, I.; Arntz, W.E. Reproductive strategies of benthic invertebrates in the Kara Sea (Russian Arctic): Adaptation of reproduction modes to cold water. Mar. Ecol. Prog. Ser. 2008, 356, 189–202. [Google Scholar] [CrossRef]
- Smith, C.R.; Mincks, S.L.; DeMaster, D.J. The FOODBANCS Project: Introduction and sinking flux of organic carbon, chlorophyll-a, and phytodetritus on the western Antarctic Peninsula continental shelf. Deep-Sea Res. II Top. Stud. Oceanogr. 2008, 55, 2404–2414. [Google Scholar] [CrossRef]
- Renaud, P.E.; Włodarska-Kowalczuk, M.; Trannum, H.; Holte, B.; Weslawski, J.M.; Cochrane, S.; Dahle, S.; Gulliksen, B. Multidecadal stability of benthic community structure in a high-Arctic fjord (van Mijenfjord, Spitsbergen). Polar Biol. 2007, 30, 295–305. [Google Scholar]
- Lyubin, P.A.; Anisimova, N.A.; Manushin, I.E.; Zhuravleva, N.E. Additional catch of macrozoobenthos in the ichthyological over-trawling as a mark of trawling. Bull. Murm. State Tech. Univ. 2010, 13, 641–646. (In Russian) [Google Scholar]
- Jørgensen, L.L.; Planque, B.; Thangstad, T.H.; Certain, G. Vulnerability of megabenthic species to trawling in the Barents Sea. ICES J. Mar. Sci. 2016, 73 (Suppl. 1), 84–97. [Google Scholar] [CrossRef]
- Misund, O.A.; Heggland, K.; Skogseth, R.; Falck, E.; Gjøsæter, H.; Sundet, J.; Watne, J.; Lønne, O.J. Norwegian fisheries in the Svalbard zone since 1980: Regulations, profitability, and warming waters affect landings. Polar Sci. 2016, 10, 312–322. [Google Scholar] [CrossRef]
- Born, M. The Demersal Fish Community on the West Spitsbergen Shelf: Biodiversity, Species Composition, Distribution, and Temporal Change in Relation to Climate. Master’s Thesis, UiT—The Arctic University of Norway, Tromsø, Norway, 2020; 85p. Available online: https://munin.uit.no/bitstream/handle/10037/19099/thesis.pdf (accessed on 5 March 2025).
- Hamilton, C.D.; Lydersen, C.; Aars, J.; Biuw, M.; Boltunov, A.N.; Born, E.W.; Dietz, R.; Folkow, L.P.; Glazow, D.M.; Haug, T.; et al. Marine mammal hotspots in the Greenland and Barents Seas. Mar. Ecol. Prog. Ser. 2021, 659, 3–28. [Google Scholar] [CrossRef]
- Stead, R.A.; Thompson, R.J. The influence of an intermittent food supply on the feeding behaviour of Yoldia hyperborea (Bivalvia: Nuculanidae). J. Exp. Mar. Biol. Ecol. 2006, 332, 37–48. [Google Scholar] [CrossRef]
- Filatova, Z.A.; Barsanova, N.G. The communities of bottom fauna of the western part of the Bering Sea. Tr. Inst. Okeanol. 1964, 69, 6–97. (In Russian) [Google Scholar]
- Włodarska-Kowalczuk, M. Molluscs in Kongsfjorden (Spitsbergen, Svalbard): A species list and patterns of distribution and diversity. Polar Res. 2007, 26, 48–63. [Google Scholar] [CrossRef]
- Naumov, A.D. Clams of the White Sea: Ecological and Faunistic Analysis; Zoologicheskiy Institut: Saint Petersburg, Russia, 2006. (In Russian) [Google Scholar]
- Holte, B. The macrofauna and main functional interactions in the sill basin sediments of the pristine Holandsfjord, Northern Norway, with autecological reviews for some key species. Sarsia 1998, 83, 55–68. [Google Scholar] [CrossRef]
- Cochrane, S.K.J.; Denisenko, S.G.; Renaud, P.E.; Emblow, C.S.; Ambrose, W.G., Jr.; Ellingsen, I.H.; Skarðhamar, J. Benthic macrofauna and productivity regimes in the Barents Sea—Ecological implications in a changing Arctic. J. Sea Res. 2009, 61, 222–233. [Google Scholar] [CrossRef]
- Denisenko, S.G. Biodiversity and Bioresources of Macrozoobenthos of the Barents Sea: Structure and Long-Term Changes; Nauka: St. Petersburg, Russia, 2013. (In Russian) [Google Scholar]
- Lyubina, O.S.; Strelkova (Anisimova), N.A.; Lubin, P.A.; Frolova, E.A.; Dikaeva, D.R.; Zimina, O.L.; Akhmetchina, O.Y.; Manushin, I.E.; Nekhaev, I.O.; Frolov, A.A.; et al. Modern quantitative distribution of zoobenthos along the transect “Kola section”. Trans. Kola Sci. Centre Oceanol. 2016, 3, 65–91. (In Russian) [Google Scholar]
- Dikaeva, D.R.; Dvoretsky, A.G. Spatial patterns and environmental control of polychaete communities in the southwestern Barents Sea. Biology 2024, 13, 924. [Google Scholar] [CrossRef]
- Ambrose, W.G.; Renaud, P.E. Does a pulsed food supply to the benthos affect polychaete recruitment patterns in the Northeast Water Polynya? J. Mar. Syst. 1997, 10, 483–495. [Google Scholar] [CrossRef]
- Lovvorn, J.R.; Cooper, L.W.; Brooks, M.L.; De Ruyck, C.C.; Bump, J.K.; Grebmeier, J.M. Organic matter pathways to zooplankton and benthos under pack ice in late winter and open water in late summer in the north-central Bering Sea. Mar. Ecol. Prog. Ser. 2005, 291, 135–150. [Google Scholar] [CrossRef]
- Rhoads, D.C.; Young, D.K. The influence of deposit-feeding organisms on sediment stability and community trophic structure. J. Mar. Res. 1970, 28, 150–178. [Google Scholar] [CrossRef]
- Moore, P.G. Inorganic particulate suspensions in the sea and their effects on marine animals. Oceanogr. Mar. Biol. Annu. Rev. 1977, 15, 225–363. [Google Scholar]
- Fetzer, I.; Lønne, O.J.; Pearson, T. The distribution of juvenile benthic invertebrates in an Arctic glacial fjord. Polar Biol. 2002, 25, 303–315. [Google Scholar] [CrossRef]
- Mincks, S.L.; Smith, C.R.; DeMaster, D.J. Persistence of labile organic matter and microbial biomass in Antarctic shelf sediments: Evidence of a sediment “food bank”. Mar. Ecol. Prog. Ser. 2005, 300, 3–19. [Google Scholar] [CrossRef]
- Mincks, S.L. Benthic–Pelagic Coupling on the Antarctic Continental Shelf: Impacts of Seasonal Phytodetritus Deposition on the Benthic Community. Ph.D. Dissertation, University of Hawaii at Manoa, Honolulu, HI, USA, 2005; 202p. [Google Scholar]
- Arntz, W.E.; Brey, T.; Gallardo, V.A. Antarctic zoobenthos. Oceanogr. Mar. Biol. Ann. Rev. 1994, 32, 241–304. [Google Scholar]
- Glover, A.G.; Smith, C.R.; Mincks, S.L.; Sumida, P.Y.G.; Thurber, A.R. Macrofaunal abundance and composition on the West Antarctic Peninsula continental shelf: Evidence for a sediment “food bank” and similarities to deep-sea habitats. Deep-Sea Res. II 2008, 55, 2491–2501. [Google Scholar] [CrossRef]
- Kędra, M.; Kuliński, K.; Walkusz, W.; Legezyńska, J. The shallow benthic food web structure in the high Arctic does not follow seasonal changes in the surrounding environment. Estuar. Coast. Shelf Sci. 2012, 114, 183–191. [Google Scholar] [CrossRef]
- Gray, J.S.; Elliott, M. Ecology of Marine Sediments: Science to Management, 2nd ed.; Oxford University Press: Oxford, UK, 2009; p. 225. [Google Scholar]
- Wakeham, S.G.; Lee, C. Production, transport, and alteration of particulate organic matter in the marine water column. In Organic Geochemistry; Engel, M.H., Macko, S.A., Eds.; Plenum Press: New York, NY, USA, 1993; pp. 145–169. [Google Scholar]
- Roy, V.; Iken, K.; Gosselin, M.; Tremblay, J.-E.; Bélanger, S.; Archambault, P. Benthic faunal assimilation pathways and depth-related changes in food-web structure across the Canadian Arctic. Deep Sea Res. Part I Oceanogr. Res. Pap. 2015, 102, 55–71. [Google Scholar] [CrossRef]

| St. | CC | L | D | T | S | WM | IFP | Sediment | AC | PC | SC | LOI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Transect I | ||||||||||||
| 39 | 78°22.04′ N 19°38.78′ E | 14 | 89 | −1.71 | 35.28 | BSW | 6.2 ± 0.4 | Light brown mud, black and gray clay, gravel, pebbles | 52.8 | 40.1 | 88.7 | 9.4 |
| 37 | 78°11.71′ N 19°24.57′ E | 9 | 83 | −1.73 | 35.32 | BSW | 6.4 ± 0.4 | Light brown mud, gray clay, sand in the lower layers, pebbles | 40.3 | 43.1 | 81.0 | 8.2 |
| 34 | 77°59.97′ N 19°12.00′ E | 10 | 97 | −1.79 | 35.34 | BSW | 6.6 ± 0.5 | Light brown mud, black and gray plastic clay, a small pebbles | 54.8 | 37.4 | 88.6 | 9.1 |
| 31 | 77°47.95′ N 18°56.62′ E | 13 | 91 | −1.28 | 34.9 | MW | 6.6 ± 0.5 | Grayish-brown mud, black and gray soft clay, stones | 58.7 | 37.7 | 93.2 | 8.7 |
| 28 | 77°30.56′ N 18°40.06′ E | 11 | 115 | −1.8 | 35.21 | BSW | 7.0 ± 0.3 | Grayish-brown watered clay mud, gray and black clay, gravel | 41.2 | 54.0 | 90.2 | 8.8 |
| Transect II | ||||||||||||
| 78(17) | 77°20.16′ N 19°34.20′ E | 34 | 151 | −1.87 | 35.37 | BSW | 7.8 ± 0.8 | Brown mud, gray and black clay, shells | 56.4 a | 30.7 a | 80.5 a | – |
| 79 | 77°05.22′ N 19°36.48′ E | 58 | 130 | −1.83 | 35.29 | BSW | 8.6 ± 1.0 | Sandy mud, gray and black viscous clay | 66.3 a | 22.0 a | 73.1 a | – |
| 80 | 76°50.16′ N 19°37.08′ E | 65 | 157 | −1.25 | 35.07 | MW | 9.0 ± 0.9 | Brown mud, gray and black soft clay, shells, a small pebbles | 69.2 a | 20.0 a | 71.1 a | – |
| 78(19) | 76°46.44′ N 19°41.58′ E | 68 | 149 | 0.17 | 34.9 | PW | 9.2 ± 1.0 | Brown sandy mud, gray soft clay, a small fine stones | 70.4 | 18.9 | 72.1 b | 8.9 |
| 81 | 76°35.40′ N 19°36.54′ E | 69 | 218 | 0.77 | 34.94 | PW | 9.4 ± 0.9 | Sandy mud, black and gray soft clay, broken shells, a small stones | 70.8 a | 18.8 a | 68.0 c | – |
| St. | α | s | Н′ | J′ | DE | N ± SE | CVN | B ± SE | CVB | AIM | NF |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Shallow transect I | |||||||||||
| 39 | 91 | 60.0 | 2.9 | 0.65 | −0.24 | 3923 ± 263 | 11.5 | 265 ± 46 | 29.9 | 0.07 | 27 |
| 37 | 135 | 85.3 | 3.6 | 0.74 | −0.47 | 5540 ± 92 | 3.0 | 264 ± 102 | 66.6 | 0.05 | 29 |
| 34 | 73 | 46.7 | 2.8 | 0.66 | −0.22 | 3743 ± 248 | 11.5 | 192 ± 45 | 40.9 | 0.05 | 25 |
| 31 | 86 | 56.7 | 2.9 | 0.66 | −0.25 | 5470 ± 542 | 17.2 | 245 ± 47 | 33.1 | 0.04 | 28 |
| 28 | 108 | 69.0 | 3.0 | 0.65 | −0.31 | 7627 ± 1384 | 31.4 | 167 ± 8 | 8.4 | 0.02 | 31 |
| X ± SE | 98 ± 11 | 63.5 ± 6.5 | 3.0 ± 0.1 | 0.67 ± 0.02 | −0.30 ± 0.04 | 5260 ± 702 | 14.9 ± 4.7 | 226 ± 20 | 35.8 ± 9.4 | 0.05 ± 0.01 | 28 ± 1 |
| Deep transect II | |||||||||||
| 78(17) | 100 | 63.3 | 2.8 | 0.62 | −0.13 | 5660 ± 317 | 9.7 | 148 ± 13 | 14.9 | 0.03 | 29 |
| 79 | 109 | 69.3 | 3.3 | 0.71 | −0.34 | 5477 ± 427 | 13.5 | 182 ± 36 | 33.9 | 0.03 | 28 |
| 80 | 78 | 54.0 | 2.9 | 0.66 | −0.33 | 5970 ± 122 | 3.5 | 146 ± 14 | 16.7 | 0.02 | 28 |
| 78(19) | 102 | 67.7 | 2.9 | 0.64 | −0.18 | 8527 ± 264 | 5.4 | 167 ± 46 | 48.0 | 0.02 | 29 |
| 81 | 122 | 80.3 | 3.0 | 0.63 | −0.48 | 8977 ± 460 | 8.9 | 104 ± 12 | 19.9 | 0.01 | 32 |
| X ± SE | 102 ± 7 | 66.9 ± 4.3 | 3.0 ± 0.1 | 0.65 ± 0.01 | −0.29 ± 0.06 | 6922 ± 754 | 8.2 ± 1.7 | 149 ± 13 | 26.7 ± 6.2 | 0.02 ± 0.00 | 29 ± 1 |
| Taxa | FO | Stations | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Shallow Transect I | Deep Transect II | ||||||||||
| 39 | 37 | 34 | 31 | 28 | 78(17) | 79 | 80 | 78(19) | 81 | ||
| Lumbrineris mixochaeta (P) | 100 | 15.2/1.7 | 6.5/0.2 | 20.8/1.3 | 15.1/1.2 | 12.3/1.8 | 6.7/1.6 | 4.6/1.3 | 9.7/3.2 | 2.2/1.1 | 1.9/0.9 |
| Chaetozone setosa (P) | 100 | 5.7/0.3 | 2.4/0.1 | 8.1/0.4 | 10.3/0.5 | 9.2/0.7 | 6.4/0.9 | 5.1/0.6 | 10.3/1.4 | 3.2/0.6 | 0.4/0.2 |
| Galathowenia oculata (P) | 100 | 0.3/0.0 | 3.7/0.1 | 1.2/0.0 | 2.7/0.1 | 0.6/0.0 | 6.8/0.4 | 10.1/0.3 | 18.0/0.7 | 23.2/1.1 | 4.0/0.3 |
| Heteromastus filiformis (P) | 100 | 3.5/0.1 | 0.8/0.0 | 2.2/0.2 | 5.6/0.2 | 2.6/0.1 | 16.3/0.5 | 10.8/0.3 | 10.5/0.4 | 10.3/0.8 | 2.5/0.3 |
| Leitoscoloplos acutus (P) | 100 | 10.9/0.2 | 3.0/0.1 | 13.9/0.3 | 8.1/0.2 | 9.0/0.5 | 6.0/0.5 | 7.1/1.2 | 6.6/0.5 | 4.5/0.4 | 1.3/0.3 |
| Maldane sarsi (P) | 100 | 3.7/10.3 | 10.3/19.1 | 3.6/6.7 | 16.0/33.9 | 8.3/36.5 | 6.4/18.1 | 4.8/6.4 | 1.9/7.1 | 4.7/26.8 | 16.5/5.0 |
| Eteone flava (P) | 97 | 1.3/0.0 | 0.4/0.0 | 0.9/0.1 | 1.2/0.1 | 0.6/1.1 | 1.1/0.2 | 0.7/0.1 | 0.7/0.2 | 0.8/0.3 | 0.2/0.0 |
| Aphelochaeta marioni (P) | 97 | 6.8/0.3 | 1.3/0.1 | 6.3/0.2 | 3.5/0.2 | 2.4/0.2 | 8.8/0.9 | 2.7/0.2 | 4.3/0.3 | 7.7/0.6 | 1.7/0.2 |
| Eudorella emarginata (C) | 93 | 1.2/0.0 | 0.3/0.0 | 1.1/0.1 | 1.0/0.0 | 0.4/0.1 | 0.6/0.1 | 0.4/0.0 | 0.7/0.1 | 0.2/0.0 | 0.0/0.0 |
| Levinsenia gracilis (P) | 93 | 2.4/0.0 | 3.5/0.0 | 1.2/0.0 | 5.7/0.1 | 3.8/0.1 | 0.1/0.0 | 2.1/0.0 | 0.8/0.0 | 0.7/0.0 | 0.9/0.0 |
| Ennucula tenuis (B) | 93 | 5.7/6.4 | 0.7/0.1 | 6.5/8.4 | 0.2/0.0 | 0.1/0.0 | 0.2/0.0 | 0.9/0.0 | 0.7/0.0 | 0.2/0.0 | 0.1/0.0 |
| Yoldiella nana (B) | 90 | 0.3/0.0 | 0.4/0.0 | 0.2/0.0 | 0.3/0.0 | 1.5/0.1 | 9.7/0.9 | 14.5/1.3 | 2.9/0.3 | 7.6/0.6 | 6.8/0.8 |
| Ophiocten sericeum (E) | 87 | 0.3/0.2 | 0.2/1.0 | 0.2/0.1 | 0.4/1.1 | 0.5/1.3 | 0.5/1.7 | 2.0/2.8 | 0.6/0.7 | 0.2/0.3 | 0.4/0.2 |
| Actinocythereis dunelmensis (C) | 83 | 2.0/0.0 | 3.0/0.0 | 4.0/0.0 | 1.4/0.0 | 2.3/0.0 | 0.1/0.0 | 0.0/0.0 | 0.2/0.0 | 2.5/0.0 | 0.5/0.0 |
| Terebellides stroemii (P) | 80 | 0.3/0.0 | 1.0/0.3 | 0.1/0.1 | 0.7/0.2 | 0.3/0.0 | 0.9/1.3 | 0.4/0.3 | 0.1/1.0 | 0.0/0.0 | 0.1/0.0 |
| Amphiura sundevalli (E) | 80 | 0.4/0.8 | 3.4/1.4 | 1.3/5.4 | 0.4/2.5 | 0.3/1.8 | 0.4/2.1 | 0.3/0.8 | 3.8/3.2 | 0.3/1.7 | - |
| Lysippe labiata (P) | 80 | 0.3/0.0 | 0.7/0.1 | 0.4/0.1 | 1.2/0.1 | 0.6/0.2 | 0.2/0.0 | 0.2/0.0 | - | 0.0/0.0 | 0.1/0.0 |
| Diastylis goodsiri (C) | 77 | 0.3/0.0 | 0.7/0.5 | 0.1/0.0 | 0.5/0.7 | 0.1/0.0 | 0.3/2.1 | 2.0/1.6 | 0.8/3.4 | 0.5/1.5 | - |
| Frigidoalvania janmayeni (G) | 77 | 0.5/0.0 | 0.5/0.0 | - | - | 0.2/0.0 | 3.9/1.3 | 3.5/0.8 | 7.1/1.8 | 1.8/0.1 | 0.9/0.4 |
| Polycirrus arcticus (P) | 70 | 0.6/0.2 | 0.1/0.1 | 1.4/1.0 | 0.7/0.7 | 0.1/0.1 | 2.7/2.4 | 1.7/1.3 | 1.2/2.2 | - | - |
| Caecognathia elongata (C) | 70 | 0.4/0.0 | 0.4/0.0 | 0.1/0.0 | 0.7/0.0 | 0.4/0.0 | - | 0.7/0.0 | 0.1/0.0 | 0.6/0.0 | 0.1/0.0 |
| Eucratea loricata (Br) | 70 | 0.2/0.0 | 0.1/0.0 | 0.1/0.0 | 0.1/0.0 | 0.1/0.7 | 0.1/0.0 | 0.1/0.0 | 0.1/0.0 | 0.1/0.0 | 0.1/0.1 |
| Pholoe assimilis (P) | 67 | 0.3/0.0 | 0.4/0.0 | 0.0/0.0 | 1.5/0.0 | 1.1/0.0 | 0.5/0.0 | 0.5/0.0 | 0.5/0.0 | - | 0.0/0.0 |
| Philomedes globosus (C) | 67 | - | 0.8/0.0 | 0.3/0.0 | 2.4/0.0 | 2.5/0.1 | - | 0.1/0.0 | 0.1/0.0 | 0.2/0.0 | 0.0/0.0 |
| Tharyx sp. (P) | 63 | 3.6/0.1 | 0.6/0.0 | 1.0/0.0 | 0.4/0.0 | 0.3/0.0 | 1.2/0.1 | 0.3/0.0 | - | - | 0.0/0.0 |
| Syllidae g.sp. (P) | 60 | 0.5/0.0 | 0.5/0.0 | 0.4/0.0 | 0.3/0.0 | 0.5/0.1 | 0.1/0.0 | - | - | 0.1/0.0 | 0.0/0.0 |
| Heterocyprideis sorbyana (C) | 57 | 0.7/0.0 | 13.8/0.0 | 0.4/0.0 | 1.3/0.0 | 10.9/0.1 | - | - | - | 5.6/0.1 | 0.0/0.0 |
| Cirratullidae g.sp. (P) | 57 | 0.3/0.0 | 0.1/0.0 | - | 0.5/0.0 | 2.8/0.1 | 0.5/0.0 | 0.5/0.0 | - | 0.5/0.0 | 0.0/0.0 |
| Ctenodiscus crispatus (E) | 57 | - | 0.1/0.0 | - | 0.2/16.0 | 0.1/9.8 | 0.7/40.2 | 0.5/11.3 | 0.4/0.3 | 0.2/13.6 | 0.2/4.7 |
| Phyllodoce groenlandica (P) | 53 | 0.2/0.0 | 0.1/0.0 | - | 0.2/0.0 | 0.3/1.2 | - | 0.1/0.0 | 0.2/1.3 | 0.3/1.6 | 0.1/0.4 |
| Heterocyprideis fascis (C) | 53 | 0.1/0.0 | 0.2/0.0 | 1.0/0.0 | 1.0/0.0 | 8.1/0.1 | 0.1/0.0 | - | - | 0.4/0.0 | - |
| Nuculana pernula (B) | 53 | 1.2/1.9 | 0.8/1.2 | 0.6/3.7 | 0.1/0.0 | 0.3/0.4 | 0.4/0.1 | 0.1/0.0 | - | - | - |
| Mendicula ferruginosa (B) | 50 | - | - | - | - | - | 0.3/0.0 | 1.8/0.0 | 1.1/0.1 | 2.5/0.1 | 27.3/1.5 |
| Spiochaetopterus typicus (P) | 50 | - | - | 0.2/1.0 | 0.1/0.0 | 0.0/1.3 | 0.1/1.9 | 0.1/0.6 | 2.2/39.2 | 1.1/17.5 | 3.3/64.0 |
| Cluster 1–Cluster 2 | Cluster 1–Cluster 3 | ||||||
| Taxon | Average Dissimilarity = 54.10% | Taxon | Average Dissimilarity = 52.66% | ||||
| Av. Diss | Contrib | Cum | Av. Diss | Contrib | Cum | ||
| Yoldia hyperborea | 3.98 | 7.40 | 7.35 | Yoldia hyperborea | 4.17 | 7.91 | 7.91 |
| Myriochele heeri | 3.35 | 6.20 | 13.55 | Ennucula tenuis | 3.02 | 5.73 | 13.64 |
| Ennucula tenuis | 3.11 | 5.70 | 19.28 | Nothria hyperborea | 2.30 | 4.36 | 18.00 |
| Bathyarca glacialis | 2.23 | 4.10 | 23.41 | Ctenodiscus crispatus | 2.28 | 4.33 | 22.33 |
| Tridonta montagui | 1.86 | 3.40 | 26.85 | Ciliatocardium ciliatum | 1.70 | 3.23 | 25.56 |
| Ciliatocardium ciliatum | 1.81 | 3.30 | 30.20 | Nematoda g.sp. | 1.68 | 3.18 | 28.75 |
| Nematoda g.sp. | 1.79 | 3.30 | 33.5 | Diastylis goodsiri | 1.66 | 3.142 | 31.89 |
| Cluster 1–Cluster 4 | Cluster 2–Cluster 3 | ||||||
| Taxon | Average Dissimilarity = 62.43% | Taxon | Average Dissimilarity = 49.96% | ||||
| Av. Diss | Contrib | Cum | Av. Diss | Contrib | Cum | ||
| Terebellidae g.sp. | 4.84 | 7.76 | 7.76 | Myriochele heeri | 2.83 | 5.66 | 5.66 |
| Yoldia hyperborea | 4.54 | 7.28 | 15.03 | Nematoda g.sp. | 2.41 | 4.82 | 10.48 |
| Ennucula tenuis | 3.25 | 5.20 | 20.24 | Tridonta montagui | 1.74 | 3.48 | 13.96 |
| Nematoda g.sp. | 1.94 | 3.11 | 23.35 | Ciliatocardium ciliatum | 1.51 | 3.01 | 16.97 |
| Actinocythereis dunelmensis | 1.89 | 3.02 | 26.37 | Phyllodoce groenlandica | 1.47 | 2.93 | 19.9 |
| Ciliatocardium ciliatum | 1.85 | 2.97 | 29.34 | Nothria hyperborea | 1.36 | 2.72 | 22.62 |
| Nuculana pernula | 1.76 | 2.83 | 32.17 | Tridonta borealis | 1.35 | 2.71 | 25.33 |
| Cluster 2–Cluster 4 | Cluster 3–Cluster 4 | ||||||
| Taxon | Average Dissimilarity = 55.83% | Taxon | Average Dissimilarity = 47.32% | ||||
| Av. Diss | Contrib | Cum | Av. Diss | Contrib | Cum | ||
| Terebellidae g.sp. | 4.88 | 8.74 | 8.74 | Terebellidae g.sp. | 4.12 | 8.70 | 8.70 |
| Myriochele heeri | 3.16 | 5.66 | 14.4 | Nematoda g.sp. | 2.56 | 5.41 | 14.12 |
| Actinocythereis dunelmensis | 1.85 | 3.32 | 17.72 | Actinocythereis dunelmensis | 1.85 | 3.92 | 18.03 |
| Tridonta montagui | 1.84 | 3.29 | 21.01 | Nothria hyperborea | 1.50 | 3.18 | 21.21 |
| Phyllodoce groenlandica | 1.62 | 2.89 | 23.91 | Ciliatocardium ciliatum | 1.49 | 3.15 | 24.36 |
| Tridonta borealis | 1.17 | 2.09 | 26.00 | Scoletoma fragilis | 1.32 | 2.78 | 27.14 |
| Pholoe assimilis | 1.11 | 1.99 | 27.99 | Ctenodiscus crispatus | 1.28 | 2.70 | 29.84 |
| Type 1: Yoldia hyperborea Stations 34 and 39 | Type 2: Maldane sarsi Stations 28, 31, and 37 | ||||||
| Dominant Species | R | N | B | Dominant species | R | N | B |
| Yoldia hyperborea | 175.9 | 76.7 | 57.1 | Maldane sarsi | 394.7 | 693.3 | 64.9 |
| Maldane sarsi | 107.7 | 138.3 | 19.9 | Lumbrineris mixochaeta | 99.8 | 706.7 | 2.1 |
| Lumbrineris mixochaeta | 99.8 | 685 | 3.3 | Nothria hyperborea | 38.4 | 20.0 | 3.0 |
| Ennucula tenuis | 93.3 | 233.3 | 16.5 | Tridonta borealis | 31.9 | 24.4 | 36.3 |
| Nemertini g.sp. | 69.6 | 18.3 | 7.2 | Tridonta montagui | 30.7 | 73.3 | 26.0 |
| Bathyarca glacialis | 61.3 | 11.7 | 25.1 | Aglaophamus malmgreni | 17.9 | 12.2 | 1.3 |
| Ciliatocardium ciliatum | 50.8 | 1.7 | 37.2 | Ctenodiscus crispatus | 17.6 | 7.8 | 18.6 |
| Scoletoma fragilis | 37.5 | 3.3 | 5.2 | Chaetozone setosa | 14.8 | 466.7 | 0.9 |
| Nuculana pernula | 24.5 | 35.0 | 6.1 | Eteone flava | 13.6 | 43.3 | 0.7 |
| Thyasira gouldi | 19.0 | 156.7 | 2.2 | Aphelochaeta marioni | 5.0 | 148.9 | 0.3 |
| Type 3: Maldane sarsi + Nemertini g.sp. Stations 78(17) and 79 | Type 4: Spiochaetopterus typicus Stations 78(19), 80, and 81 | ||||||
| Dominant Species | R | N | B | Dominant Species | R | N | B |
| Maldane sarsi | 125.2 | 311.7 | 19.2 | Spiochaetopterus typicus | 231.2 | 174.4 | 51.1 |
| Nemertini g.sp. | 137.4 | 20.0 | 15.6 | Maldane sarsi | 130.6 | 664.4 | 20.1 |
| Lumbrineris mixochaeta | 64.6 | 315.0 | 2.4 | Lumbrineris mixochaeta | 66.1 | 312.2 | 2.5 |
| Ctenodiscus crispatus | 47.4 | 33.3 | 40.0 | Diastylis goodsiri | 26.3 | 28.9 | 2.5 |
| Nepftys ciliata | 47.1 | 8.3 | 5.3 | Nepftys paradoxa | 21.7 | 2.2 | 2.9 |
| Ciliatocardium ciliatum | 44.2 | 6.7 | 21.6 | Phyllodoce groenlandica | 21.7 | 13.3 | 1.7 |
| Diastylis goodsiri | 34.3 | 63.3 | 3.0 | Thracia myopsis | 21.4 | 2.2 | 11.1 |
| Polycirrus arcticus | 24.7 | 123.3 | 2.9 | Galathowenia oculata | 20.8 | 1097.8 | 1.1 |
| Chaetozone setosa | 16.1 | 321.7 | 1.2 | Chaetozone setosa | 14.5 | 310.0 | 1.0 |
| Aphelochaeta marioni | 12.4 | 321.7 | 0.8 | Aphelochaeta marioni | 9.8 | 357.2 | 0.6 |
| Variable | EV | F | P | Variable | EV | F | P |
|---|---|---|---|---|---|---|---|
| Taxa Abundance | Abundance of Functional Groups | ||||||
| IFP | 59 | 11.5 | 0.001 | IFP | 54 | 9.35 | 0.001 |
| T | 10 | 2.35 | 0.094 | T | 7 | 1.32 | 0.248 |
| De | 6 | 1.5 | 0.232 | De | 3 | 0.93 | 0.447 |
| Dist | 8 | 2.19 | 0.073 | Dist | 10 | 3.63 | 0.047 |
| Fine | 4 | 1.21 | 0.317 | Fine | 8 | 1.88 | 0.181 |
| S | 1 | 0.33 | 0.816 | S | 9 | 1.69 | 0.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavlova, L.V.; Dvoretsky, A.G.; Frolov, A.A.; Zimina, O.L.; Evseeva, O.Y.; Dikaeva, D.R.; Rumyantseva, Z.Y.; Panteleeva, N.N.; Garbul, E.A. Sublittoral Macrobenthic Communities of Storfjord (Eastern Svalbard) and Factors Influencing Their Distribution and Structure. Animals 2025, 15, 1261. https://doi.org/10.3390/ani15091261
Pavlova LV, Dvoretsky AG, Frolov AA, Zimina OL, Evseeva OY, Dikaeva DR, Rumyantseva ZY, Panteleeva NN, Garbul EA. Sublittoral Macrobenthic Communities of Storfjord (Eastern Svalbard) and Factors Influencing Their Distribution and Structure. Animals. 2025; 15(9):1261. https://doi.org/10.3390/ani15091261
Chicago/Turabian StylePavlova, Lyudmila V., Alexander G. Dvoretsky, Alexander A. Frolov, Olga L. Zimina, Olga Yu. Evseeva, Dinara R. Dikaeva, Zinaida Yu. Rumyantseva, Ninel N. Panteleeva, and Evgeniy A. Garbul. 2025. "Sublittoral Macrobenthic Communities of Storfjord (Eastern Svalbard) and Factors Influencing Their Distribution and Structure" Animals 15, no. 9: 1261. https://doi.org/10.3390/ani15091261
APA StylePavlova, L. V., Dvoretsky, A. G., Frolov, A. A., Zimina, O. L., Evseeva, O. Y., Dikaeva, D. R., Rumyantseva, Z. Y., Panteleeva, N. N., & Garbul, E. A. (2025). Sublittoral Macrobenthic Communities of Storfjord (Eastern Svalbard) and Factors Influencing Their Distribution and Structure. Animals, 15(9), 1261. https://doi.org/10.3390/ani15091261







