Parabacteroides goldsteinii Alleviates Intestinal Inflammation in Dextran Sulfate Sodium-Treated Pigs
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of P. goldsteinii
2.2. Pigs and Experimental Design
2.3. Detection of IL-1β, IL-6, IL-8, and IL-10 in the Serum of Pigs
2.4. 16S rRNA Sequencing
2.5. Statistical Analysis
3. Results
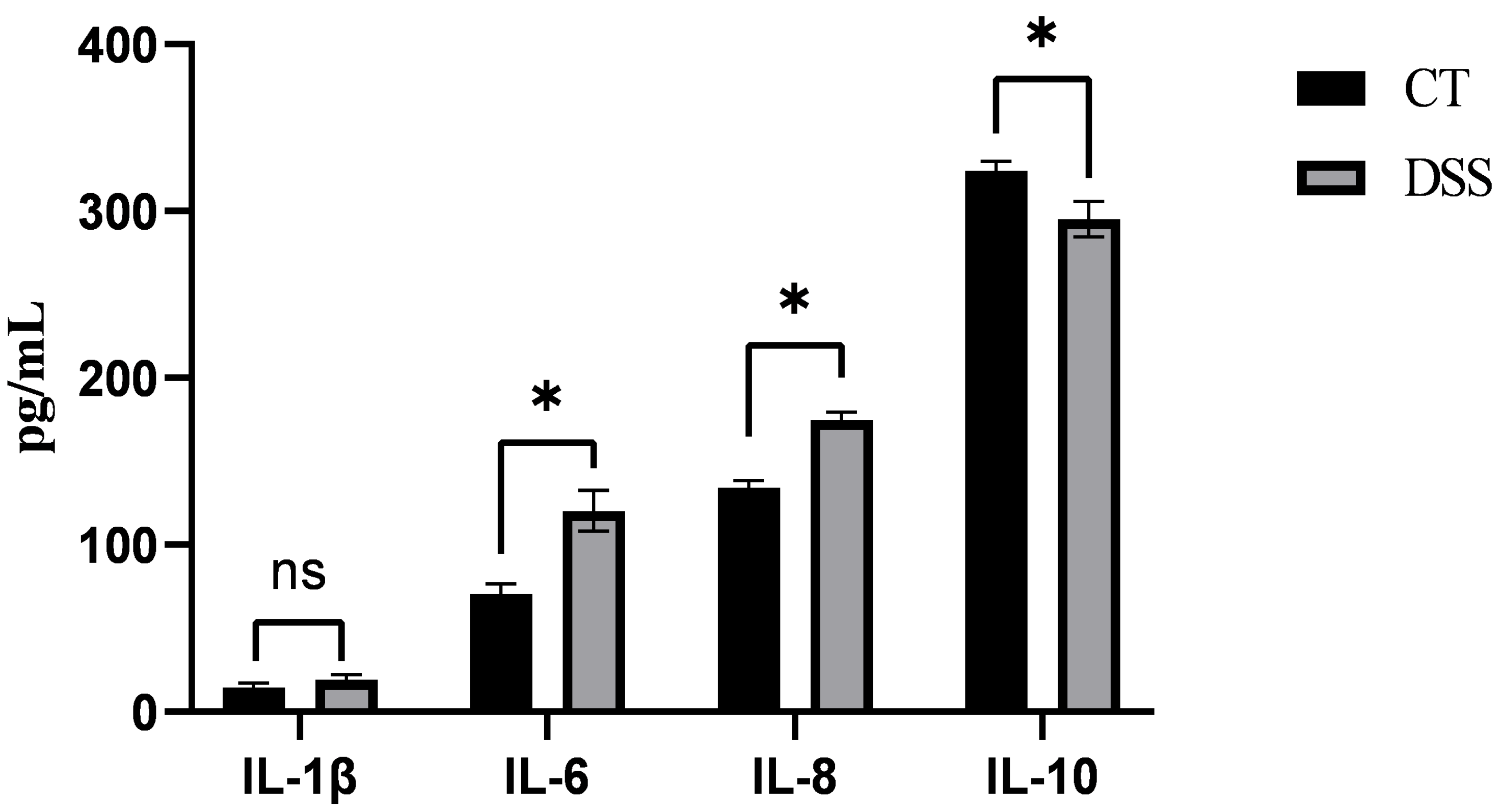
3.1. Effect of DSS on the Serum Levels of Inflammatory Factors in Piglets
3.2. Effect of PG on the Serum Levels of Inflammatory Factors in DSS-Induced Inflammatory Piglets
3.3. OTU Clustering Analysis
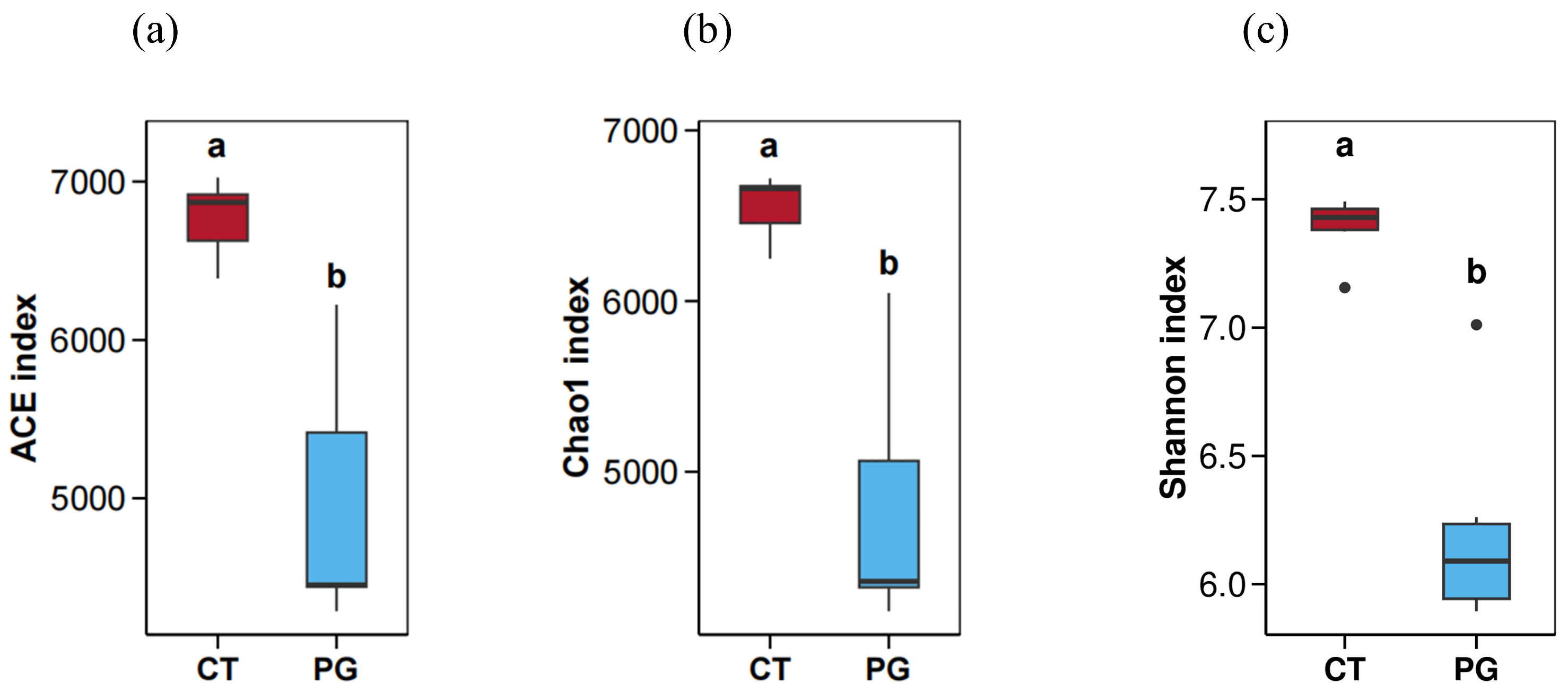
3.4. Alpha Diversity Analysis
3.5. Microbial Species Abundance and Taxonomic Statistics
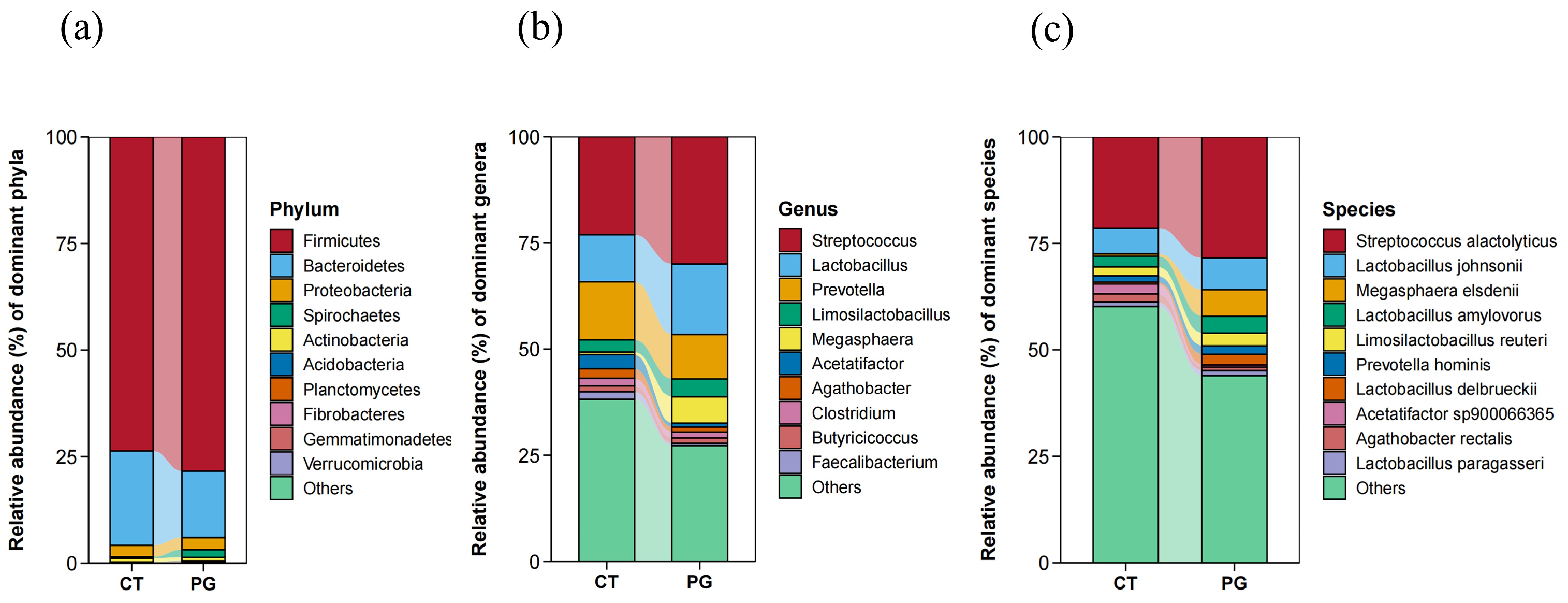
3.5.1. Phylum-Level Species Composition
3.5.2. Genus-Level Microbiota Composition
3.5.3. Species-Level Microbiota Composition
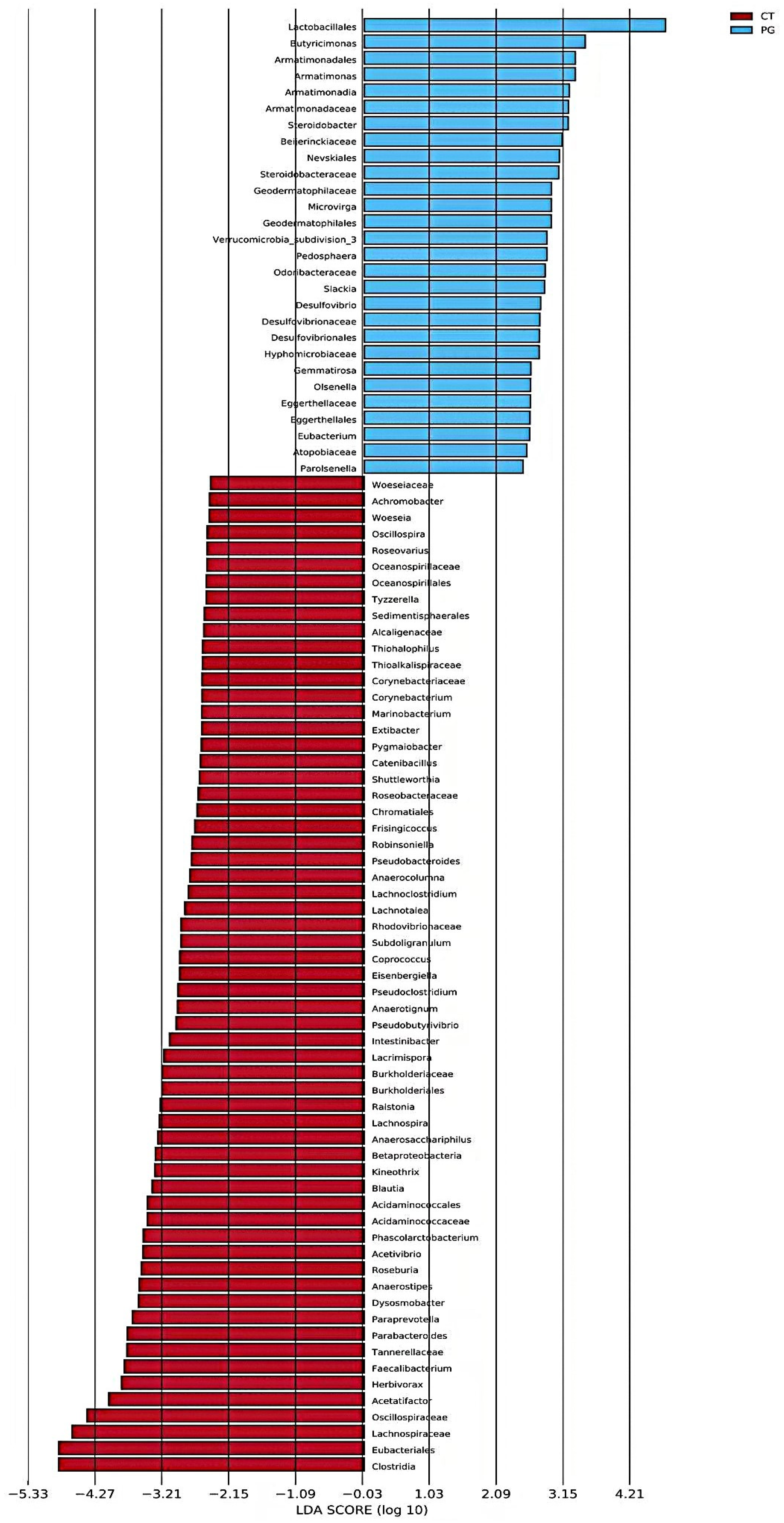
3.6. LEfSe Analysis
3.7. KEGG Pathway Functional Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fakhoury, M.; Negrulj, R.; Mooranian, A.; Al-Salami, H. Inflammatory bowel disease: Clinical aspects and treatments. J. Inflamm. Res. 2014, 7, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Kou, Y.; Li, J.; Zhu, Y.; Liu, J.; Ren, R.; Jiang, Y.; Wang, Y.; Qiu, C.; Zhou, J.; Yang, Z.; et al. Human Amniotic Epithelial Stem Cells Promote Colonic Recovery in Experimental Colitis via Exosomal MiR-23a-TNFR1-NF-κB Signaling. Adv. Sci. 2024, 11, e2401429. [Google Scholar] [CrossRef]
- Chu, H.; Khosravi, A.; Kusumawardhani, I.P.; Kwon, A.H.; Vasconcelos, A.C.; Cunha, L.D.; Mayer, A.E.; Shen, Y.; Wu, W.L.; Kambal, A.; et al. Gene-microbiota interactions contribute to the pathogenesis of inflammatory bowel disease. Science 2016, 352, 1116–1120. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Gong, T.; Jiang, Z.; Lu, Z.; Wang, Y. The Role of Probiotics in Alleviating Postweaning Diarrhea in Piglets from the Perspective of Intestinal Barriers. Front. Cell Infect. Microbiol. 2022, 12, 883107. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Qian, K.; Wang, C.; Wu, Y. Roles of Probiotic Lactobacilli Inclusion in Helping Piglets Establish Healthy Intestinal Inter-environment for Pathogen Defense. Probiotics Antimicrob. Proteins 2018, 10, 243–250. [Google Scholar] [CrossRef]
- Heo, J.M.; Opapeju, F.O.; Pluske, J.R.; Kim, J.C.; Hampson, D.J.; Nyachoti, C.M. Gastrointestinal health and function in weaned pigs: A review of feeding strategies to control post-weaning diarrhoea without using in-feed antimicrobial compounds. J. Anim. Physiol. Anim. Nutr. 2013, 97, 207–237. [Google Scholar] [CrossRef]
- Upadhaya, S.D.; Kim, I.H. Maintenance of gut microbiome stability for optimum intestinal health in pigs—A review. J. Anim. Sci. Biotechnol. 2022, 13, 140. [Google Scholar] [CrossRef]
- Nechitailo, К.S.; Sizova, E.A.; Lebedev, S.V.; Ryazantseva, K.V. Causes, mechanisms of development and manifestations of antibiotic resistance in poultry farming, consequences and methods of overcoming (review). World. Poult. Sci. J. 2024, 80, 453–479. [Google Scholar] [CrossRef]
- Li, S.; Zhang, C.; Li, F.; Hua, T.; Zhou, Q.; Ho, S.H. Technologies towards antibiotic resistance genes (ARGs) removal from aquatic environment: A critical review. J. Hazard. Mater. 2021, 411, 125148. [Google Scholar] [CrossRef]
- Galli, G.M.; Andretta, I.; Levesque, C.; Stefanello, T.; Carvalho, C.L.; Perez Pelencia, J.Y.; Bueno Martins, G.; Souza de Lima Cony, B.; Romeiro de Oliveira, C.; Franceschi, C.H.; et al. Using probiotics to improve nutrient digestibility and gut-health of weaned pigs: A comparison of maternal and nursery supplementation strategies. Front. Vet. Sci. 2024, 11, 1356455. [Google Scholar] [CrossRef]
- Yaqoob, M.U.; Wang, G.; Wang, M. An updated review on probiotics as an alternative of antibiotics in poultry—A review. Anim. Biosci. 2022, 35, 1109–1120. [Google Scholar] [CrossRef]
- Ali, M.S.; Lee, E.B.; Hsu, W.H.; Suk, K.; Sayem, S.A.J.; Ullah, H.M.A.; Lee, S.J.; Park, S.C. Probiotics and Postbiotics as an Alternative to Antibiotics: An Emphasis on Pigs. Pathogens 2023, 12, 874. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, T.; Sequoia, J. Probiotics for Gastrointestinal Conditions: A Summary of the Evidence. Am. Fam. Physician 2017, 96, 170–178. [Google Scholar]
- Azad, M.A.K.; Sarker, M.; Li, T.; Yin, J. Probiotic Species in the Modulation of Gut Microbiota: An Overview. Biomed. Res. Int. 2018, 2018, 9478630. [Google Scholar] [CrossRef] [PubMed]
- Jakubczyk, D.; Leszczyńska, K.; Górska, S. The Effectiveness of Probiotics in the Treatment of Inflammatory Bowel Disease (IBD)-A Critical Review. Nutrients 2020, 12, 1973. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, L.; Wang, X.; Yi, Y.; Shan, Y.; Liu, B.; Zhou, Y.; Lü, X. Roles of intestinal Parabacteroides in human health and diseases. FEMS Microbiol. Lett. 2022, 369, fnac072. [Google Scholar] [CrossRef]
- Bakir, M.A.; Sakamoto, M.; Kitahara, M.; Matsumoto, M.; Benno, Y. Bacteroides dorei sp. nov., isolated from human faeces. Int. J. Syst. Evol. Microbiol. 2006, 56, 1639–1643. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.C.; Lin, T.L.; Chen, T.W.; Kuo, Y.L.; Chang, C.J.; Wu, T.R.; Shu, C.C.; Tsai, Y.H.; Swift, S.; Lu, C.C. Gut microbiota modulates COPD pathogenesis: Role of anti-inflammatory Parabacteroides goldsteinii lipopolysaccharide. Gut 2022, 71, 309–321. [Google Scholar] [CrossRef]
- Ahmed, S.; Busetti, A.; Fotiadou, P.; Vincy Jose, N.; Reid, S.; Georgieva, M.; Brown, S.; Dunbar, H.; Beurket-Ascencio, G.; Delday, M.I.; et al. In vitro Characterization of Gut Microbiota-Derived Bacterial Strains with Neuroprotective Properties. Front. Cell Neurosci. 2019, 13, 402. [Google Scholar] [CrossRef]
- Hiippala, K.; Kainulainen, V.; Suutarinen, M.; Heini, T.; Bowers, J.R.; Jasso-Selles, D.; Lemmer, D.; Valentine, M.; Barnes, R.; Engelthaler, D.M.; et al. Isolation of Anti-Inflammatory and Epithelium Reinforcing Bacteroides and Parabacteroides Spp. from A Healthy Fecal Donor. Nutrients 2020, 12, 935. [Google Scholar] [CrossRef]
- Wu, T.R.; Lin, C.S.; Chang, C.J.; Lin, T.L.; Martel, J.; Ko, Y.F.; Ojcius, D.M.; Lu, C.C.; Young, J.D.; Lai, H.C. Gut commensal Parabacteroides goldsteinii plays a predominant role in the anti-obesity effects of polysaccharides isolated from Hirsutella sinensis. Gut 2019, 68, 248–262. [Google Scholar] [CrossRef] [PubMed]
- Ishioka, M.; Miura, K.; Minami, S.; Shimura, Y.; Ohnishi, H. Altered Gut Microbiota Composition and Immune Response in Experimental Steatohepatitis Mouse Models. Dig. Dis. Sci. 2017, 62, 396–406. [Google Scholar] [CrossRef]
- National Research Council. Nutrient Requirements of Swine: Eleventh Revised Edition; The National Academies Press: Washington, DC, USA, 2012. [Google Scholar]
- Zhao, X.; Jiang, L.; Fang, X.; Guo, Z.; Wang, X.; Shi, B.; Meng, Q. Host-microbiota interaction-mediated resistance to inflammatory bowel disease in pigs. Microbiome 2022, 10, 115. [Google Scholar] [CrossRef]
- Hu, H.; Huang, Y.; Li, A.; Mi, Q.; Wang, K.; Chen, L.; Zhao, Z.; Zhang, Q.; Bai, X.; Pan, H. Effects of different energy levels in low-protein diet on liver lipid metabolism in the late-phase laying hens through the gut-liver axis. J. Anim. Sci. Biotechnol. 2024, 15, 98. [Google Scholar] [CrossRef] [PubMed]
- Warren, J.S. Interleukins and tumor necrosis factor in inflammation. Crit. Rev. Clin. Lab. Sci. 1990, 28, 37–59. [Google Scholar] [CrossRef]
- Kaur, S.; Bansal, Y.; Kumar, R.; Bansal, G. A panoramic review of IL-6: Structure, pathophysiological roles and inhibitors. Bioorg. Med. Chem. 2020, 28, 115327. [Google Scholar] [CrossRef] [PubMed]
- Baggiolini, M.; Clark-Lewis, I. Interleukin-8, a chemotactic and inflammatory cytokine. FEBS Lett. 1992, 307, 97–101. [Google Scholar] [CrossRef]
- Ouyang, W.; Rutz, S.; Crellin, N.K.; Valdez, P.A.; Hymowitz, S.G. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu. Rev. Immunol. 2011, 29, 71–109. [Google Scholar] [CrossRef]
- Yuan, S.N.; Wang, M.X.; Han, J.L.; Feng, C.Y.; Wang, M.; Wang, M.; Sun, J.Y.; Li, N.Y.; Simal-Gandara, J.; Liu, C. Improved colonic inflammation by nervonic acid via inhibition of NF-κB signaling pathway of DSS-induced colitis mice. Phytomedicine 2023, 112, 154702. [Google Scholar] [CrossRef]
- Xavier, R.J.; Podolsky, D.K. Unravelling the pathogenesis of inflammatory bowel disease. Nature 2007, 448, 427–434. [Google Scholar] [CrossRef]
- Halloran, K.; Underwood, M.A. Probiotic mechanisms of action. Early Hum. Dev. 2019, 135, 58–65. [Google Scholar] [CrossRef]
- Ashraf, R.; Shah, N.P. Immune system stimulation by probiotic microorganisms. Crit. Rev. Food Sci. Nutr. 2014, 54, 938–956. [Google Scholar] [CrossRef] [PubMed]
- Kverka, M.; Zakostelska, Z.; Klimesova, K.; Sokol, D.; Hudcovic, T.; Hrncir, T.; Rossmann, P.; Mrazek, J.; Kopecny, J.; Verdu, E.F.; et al. Oral administration of Parabacteroides distasonis antigens attenuates experimental murine colitis through modulation of immunity and microbiota composition. Clin. Exp. Immunol. 2011, 163, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Xu, Y.; Wang, X.; Rao, L.; Yan, X.; Gao, R.; Shen, T.; Zhou, Y.; Kong, C.; Zhou, L. Probiotic Cocktail Alleviates Intestinal Inflammation Through Improving Gut Microbiota and Metabolites in Colitis Mice. Front. Cell. Infect. Microbiol. 2022, 12, 886061. [Google Scholar] [CrossRef]
- Kim, H.B.; Isaacson, R.E. The pig gut microbial diversity: Understanding the pig gut microbial ecology through the next generation high throughput sequencing. Vet. Microbiol. 2015, 177, 242–251. [Google Scholar] [CrossRef]
- Li, P.; Chang, M. Roles of PRR-Mediated Signaling Pathways in the Regulation of Oxidative Stress and Inflammatory Diseases. Int. J. Mol. Sci. 2021, 22, 7688. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Peng, K.; Xiao, S.; Long, Y.; Yu, Q. The role of Lactobacillus in inflammatory bowel disease: From actualities to prospects. Cell Death Discov. 2023, 9, 361. [Google Scholar] [CrossRef]
- Wang, M.X.; Lin, L.; Chen, Y.D.; Zhong, Y.P.; Lin, Y.X.; Li, P.; Tian, X.; Han, B.; Xie, Z.Y.; Liao, Q.F. Evodiamine has therapeutic efficacy in ulcerative colitis by increasing Lactobacillus acidophilus levels and acetate production. Pharmacol. Res. 2020, 159, 104978. [Google Scholar] [CrossRef]
- El-Baz, A.M.; Khodir, A.E.; Adel El-Sokkary, M.M.; Shata, A. The protective effect of Lactobacillus versus 5-aminosalicylic acid in ulcerative colitis model by modulation of gut microbiota and Nrf2/Ho-1 pathway. Life Sci. 2020, 256, 117927. [Google Scholar] [CrossRef]
- Jang, Y.J.; Kim, W.K.; Han, D.H.; Lee, K.; Ko, G. Lactobacillus fermentum species ameliorate dextran sulfate sodium-induced colitis by regulating the immune response and altering gut microbiota. Gut Microbes 2019, 10, 696–711. [Google Scholar] [CrossRef]
- Corr, S.C.; Li, Y.; Riedel, C.U.; O’Toole, P.W.; Hill, C.; Gahan, C.G. Bacteriocin production as a mechanism for the antiinfective activity of Lactobacillus salivarius UCC118. Proc. Natl. Acad. Sci. USA 2007, 104, 7617–7621. [Google Scholar] [CrossRef] [PubMed]
- Brownlie, E.J.E.; Chaharlangi, D.; Wong, E.O.; Kim, D.; Navarre, W.W. Acids produced by lactobacilli inhibit the growth of commensal Lachnospiraceae and S24-7 bacteria. Gut Microbes 2022, 14, 2046452. [Google Scholar] [CrossRef] [PubMed]
- Meimandipour, A.; Shuhaimi, M.; Soleimani, A.F.; Azhar, K.; Hair-Bejo, M.; Kabeir, B.M.; Javanmard, A.; Anas, O.M.; Yazid, A.M. Selected microbial groups and short-chain fatty acids profile in a simulated chicken cecum supplemented with two strains of Lactobacillus. Poult. Sci. 2010, 89, 470–476. [Google Scholar] [CrossRef]
- Walker, A.W.; Duncan, S.H.; McWilliam Leitch, E.C.; Child, M.W.; Flint, H.J. pH and peptide supply can radically alter bacterial populations and short-chain fatty acid ratios within microbial communities from the human colon. Appl. Environ. Microbiol. 2005, 71, 3692–3700. [Google Scholar] [CrossRef]
- Sakamoto, M.; Takagaki, A.; Matsumoto, K.; Kato, Y.; Goto, K.; Benno, Y. Butyricimonas synergistica gen. nov., sp. nov. and Butyricimonas virosa sp. nov., butyric acid-producing bacteria in the family ‘Porphyromonadaceae’ isolated from rat faeces. Int. J. Syst. Evol. Microbiol. 2009, 59, 1748–1753. [Google Scholar] [CrossRef]
- Sun, M.; Wu, W.; Liu, Z.; Cong, Y. Microbiota metabolite short chain fatty acids, GPCR, and inflammatory bowel diseases. J. Gastroenterol. 2017, 52, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lavelle, A.; Sokol, H. Gut microbiota-derived metabolites as key actors in inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 223–237. [Google Scholar] [CrossRef]
- Lee, H.; An, J.; Kim, J.; Choi, D.; Song, Y.; Lee, C.K.; Kong, H.; Kim, S.B.; Kim, K. A Novel Bacterium, Butyricimonas virosa, Preventing HFD-Induced Diabetes and Metabolic Disorders in Mice via GLP-1 Receptor. Front. Microbiol. 2022, 13, 858192. [Google Scholar] [CrossRef]
- Zong, X.; Luo, S.; Liu, S.; Deehan, E.C.; Wang, Y.; Jin, M. Nondigestible carbohydrates and gut microbiota: A dynamic duo in host defense. Anim. Nutr. 2024, 1, e7. [Google Scholar] [CrossRef]
- Li, D.; Yu, S.; Long, Y.; Shi, A.; Deng, J.; Ma, Y.; Wen, J.; Li, X.; Liu, S.; Zhang, Y.; et al. Tryptophan metabolism: Mechanism-oriented therapy for neurological and psychiatric disorders. Front. Immunol. 2022, 13, 985378. [Google Scholar] [CrossRef]



| Component | Content (%) | Nutritional Level b | Content (%) |
|---|---|---|---|
| Corn | 62.00 | Digestible energy (MJ/kg) | 13.61 |
| soybean meal | 26.60 | Crude protein | 18.01 |
| wheat bran | 5.50 | Ca | 0.77 |
| Soybean oil | 1.25 | Total P | 0.62 |
| CaHPO4·2H2O | 1.30 | Lysine | 1.29 |
| Limestone | 1.00 | Met+Cys | 0.74 |
| lysine | 0.50 | ||
| methionine | 0.15 | ||
| NaCl | 0.70 | ||
| Premix a | 1.00 | ||
| Total | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, X.; Guo, T.; He, Y.; Gao, S.; Su, J.; Pan, H.; Li, A. Parabacteroides goldsteinii Alleviates Intestinal Inflammation in Dextran Sulfate Sodium-Treated Pigs. Animals 2025, 15, 1231. https://doi.org/10.3390/ani15091231
Deng X, Guo T, He Y, Gao S, Su J, Pan H, Li A. Parabacteroides goldsteinii Alleviates Intestinal Inflammation in Dextran Sulfate Sodium-Treated Pigs. Animals. 2025; 15(9):1231. https://doi.org/10.3390/ani15091231
Chicago/Turabian StyleDeng, Xu, Taozong Guo, Yang He, Shengnan Gao, Jirong Su, Hongbin Pan, and Anjian Li. 2025. "Parabacteroides goldsteinii Alleviates Intestinal Inflammation in Dextran Sulfate Sodium-Treated Pigs" Animals 15, no. 9: 1231. https://doi.org/10.3390/ani15091231
APA StyleDeng, X., Guo, T., He, Y., Gao, S., Su, J., Pan, H., & Li, A. (2025). Parabacteroides goldsteinii Alleviates Intestinal Inflammation in Dextran Sulfate Sodium-Treated Pigs. Animals, 15(9), 1231. https://doi.org/10.3390/ani15091231




