Spatiotemporal Changes in Catch Composition of Marine Species Across Seawater Temperature Shift Points in Korean Water
Simple Summary
Abstract
1. Introduction
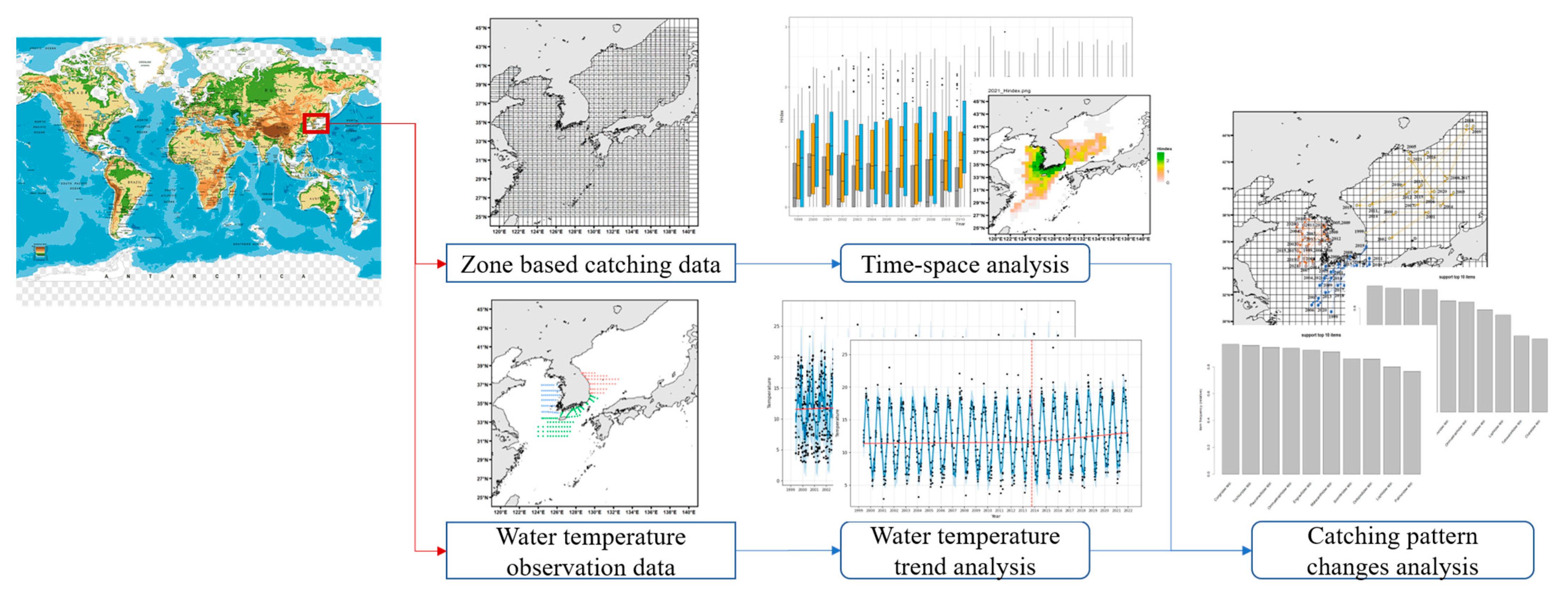
2. Materials and Methods
2.1. Analysis Data
2.2. Analysis Method
3. Results
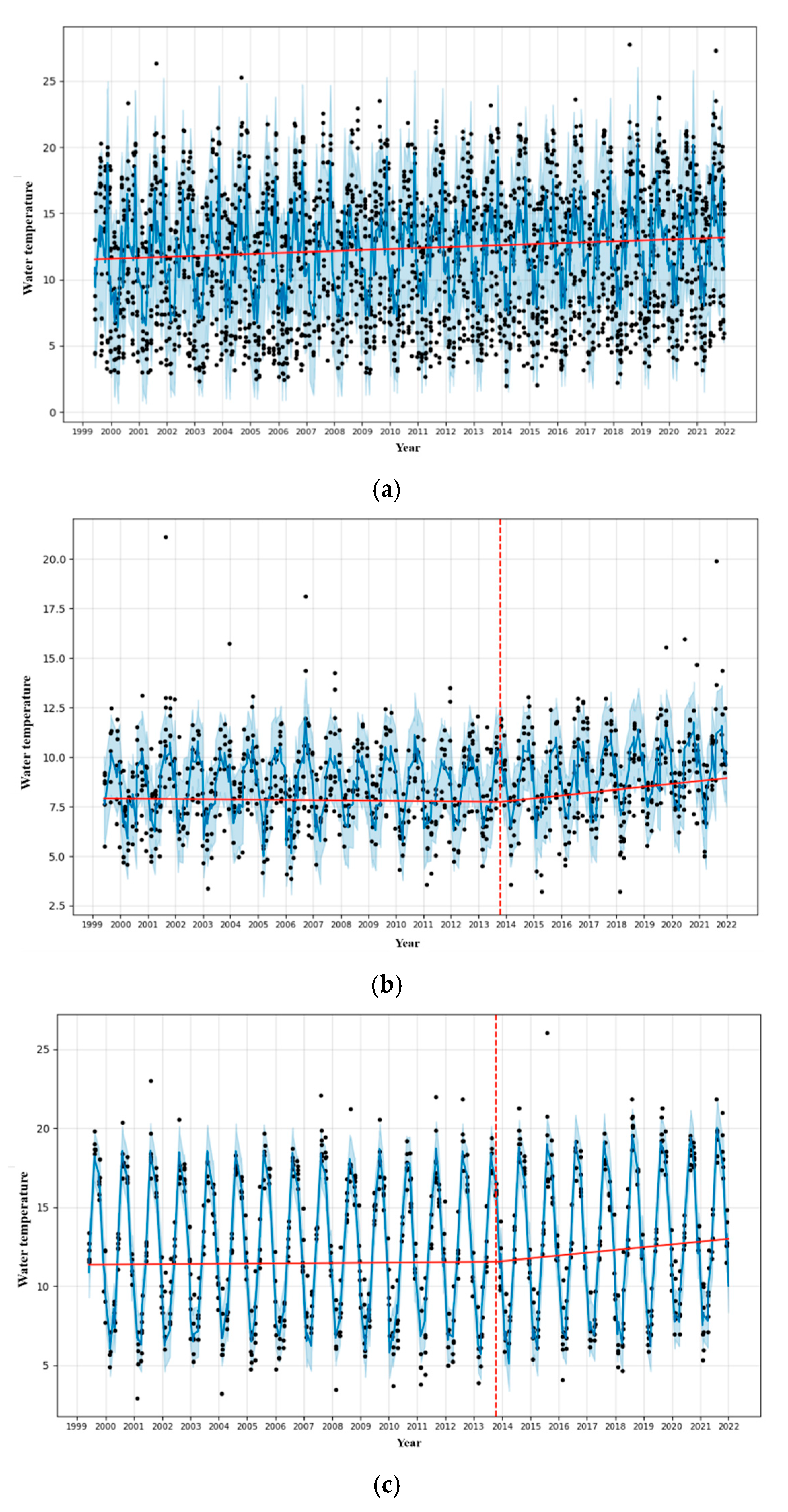
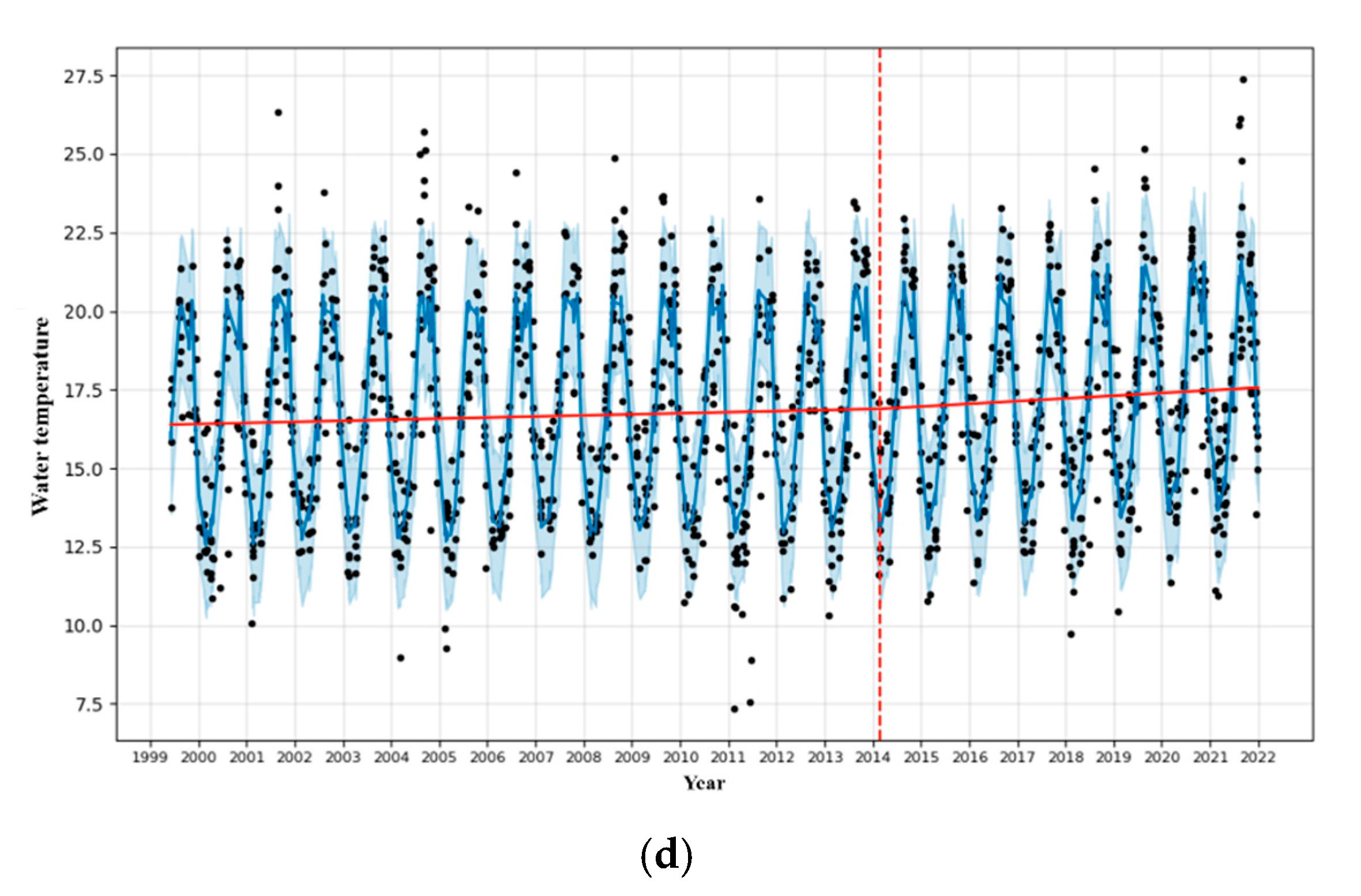
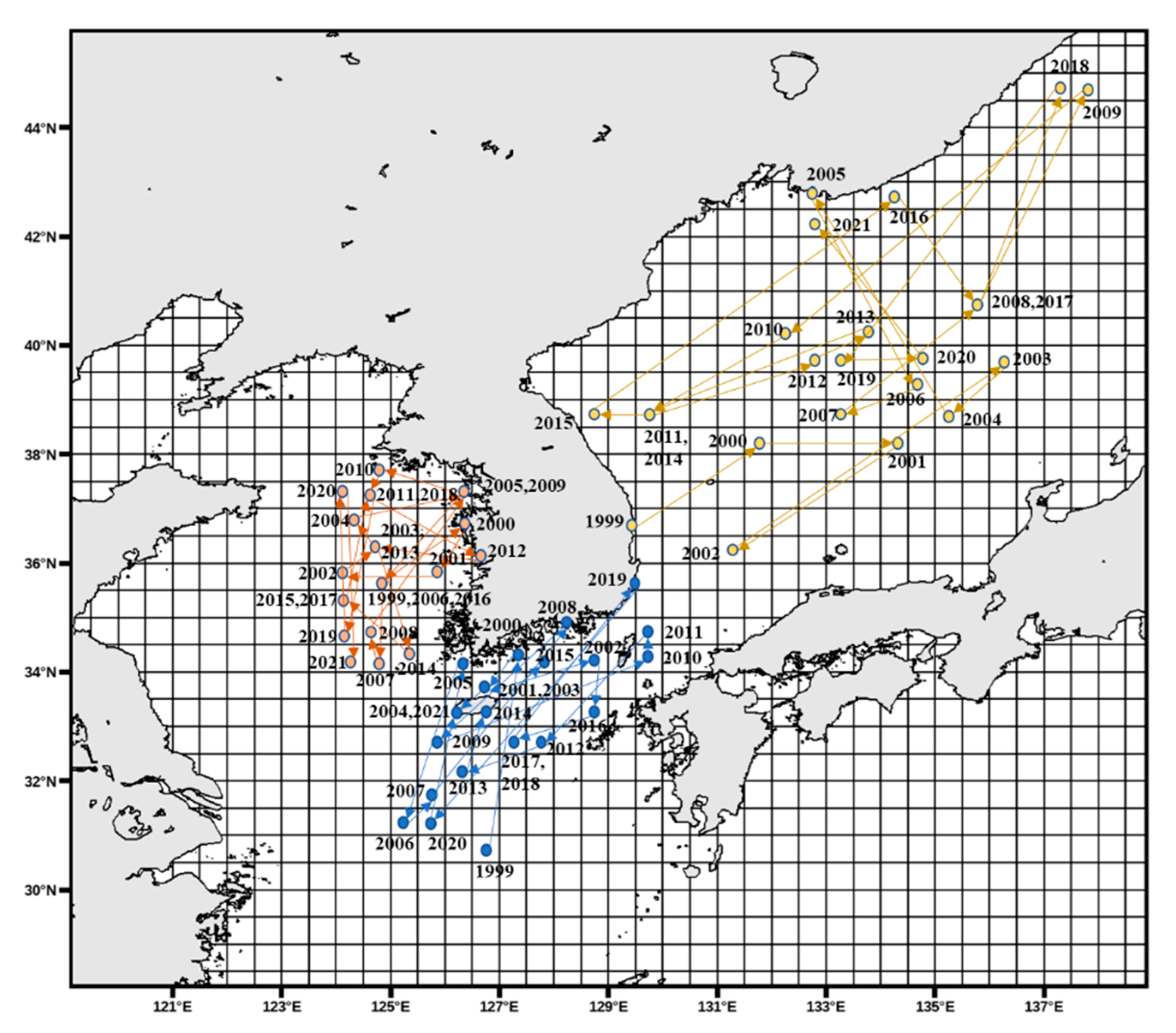
3.1. Analysis of Fishing Spatiotemporal Characteristics
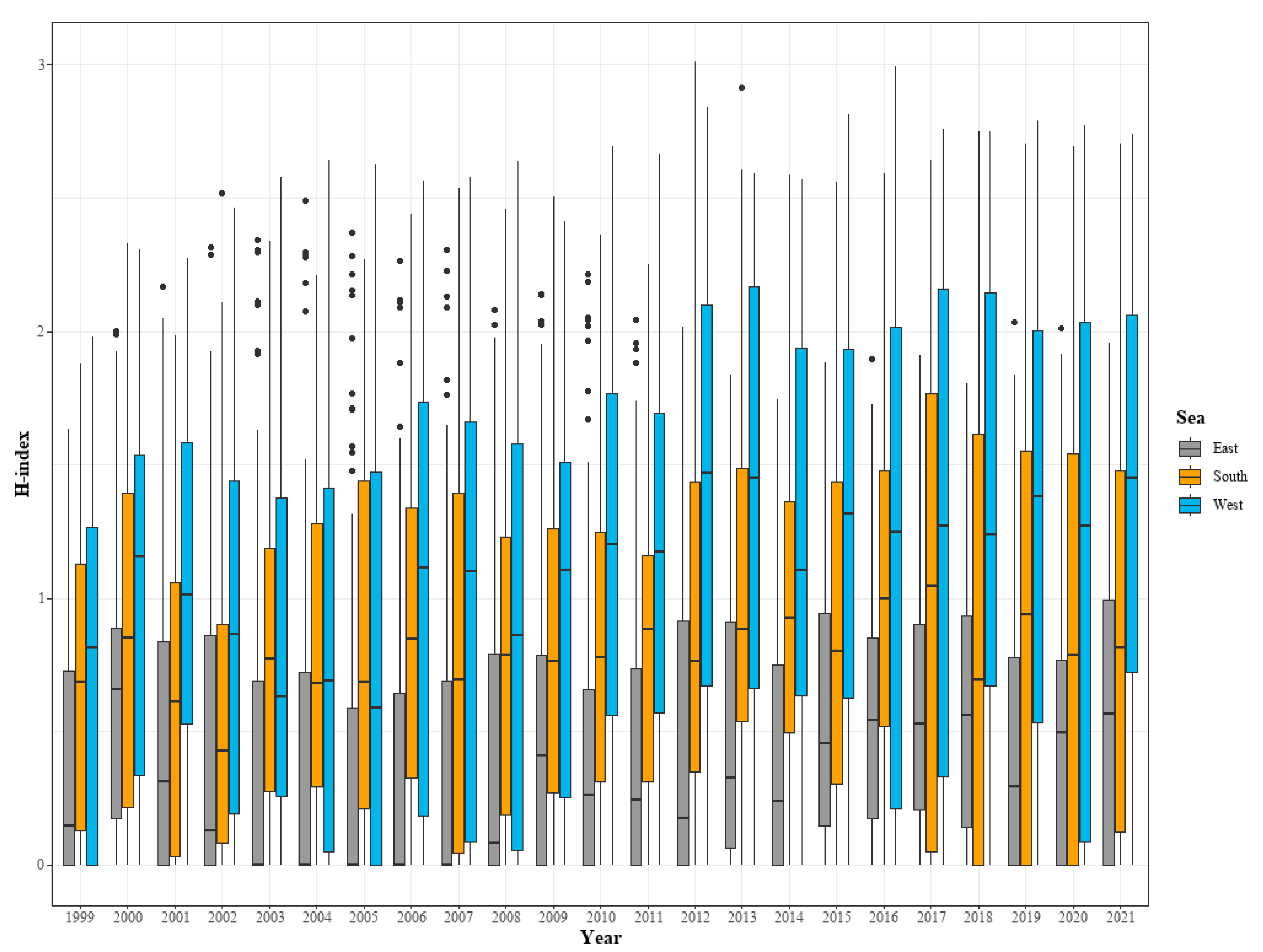
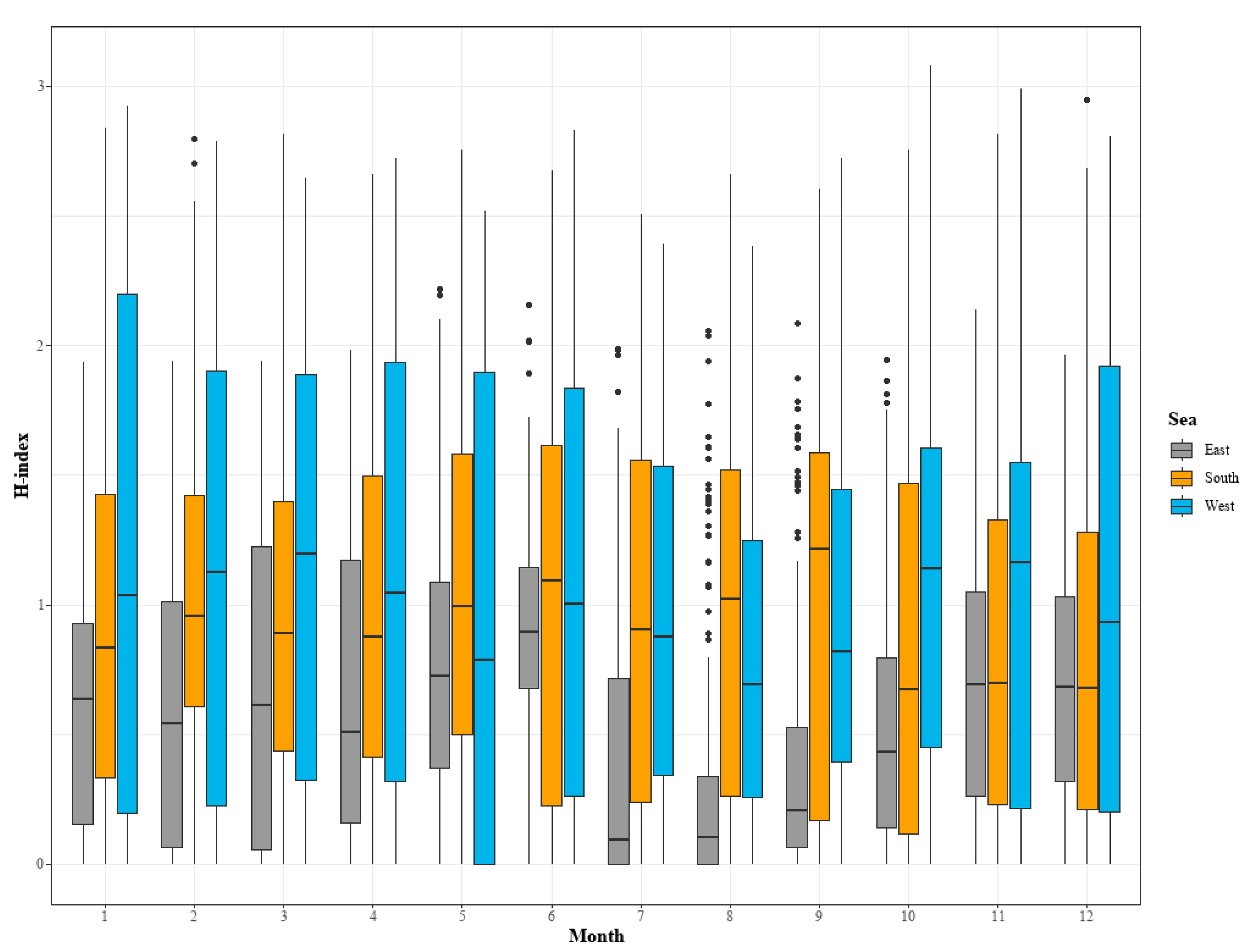
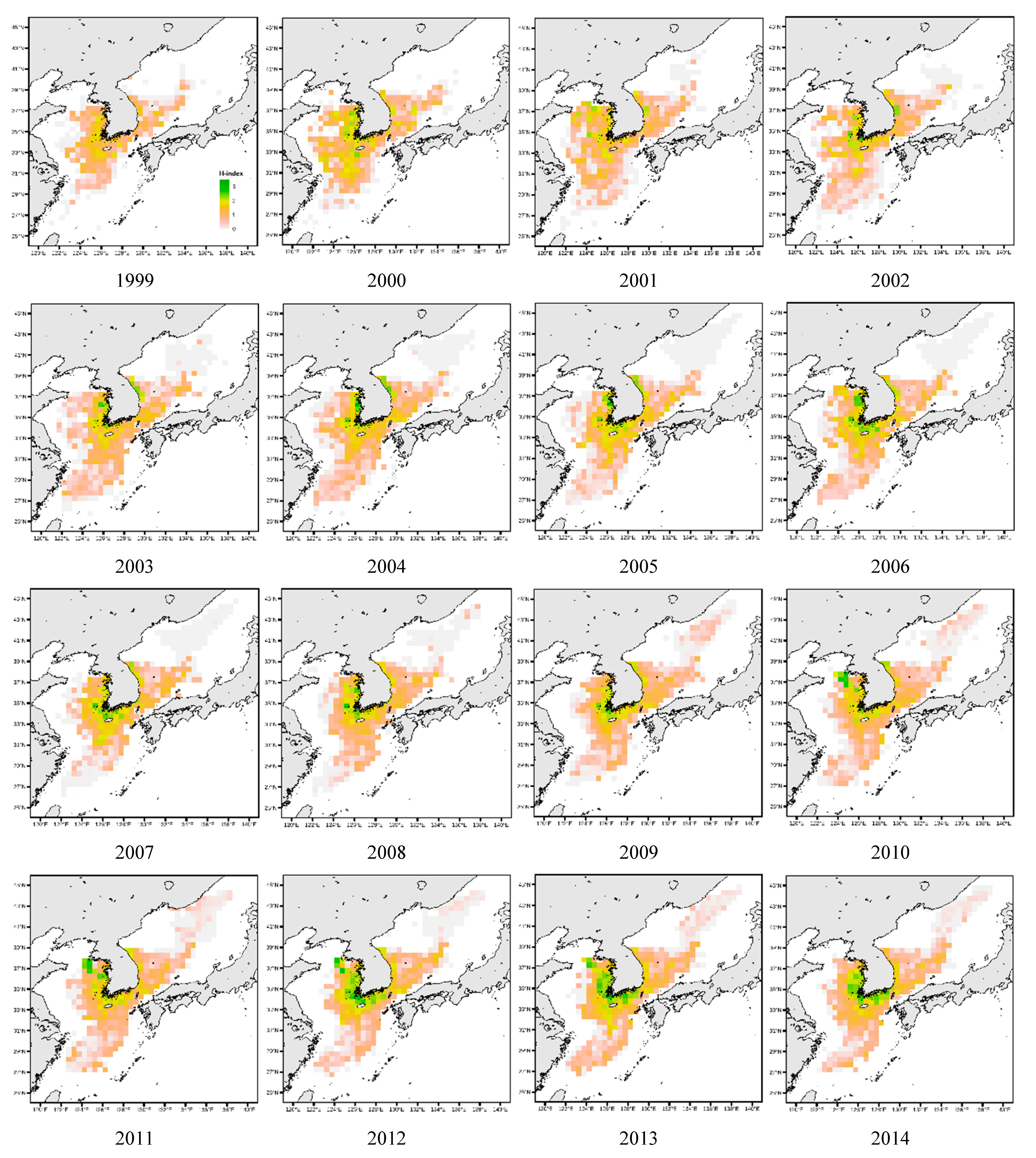
3.2. Analysis of Changes in Species Diversity
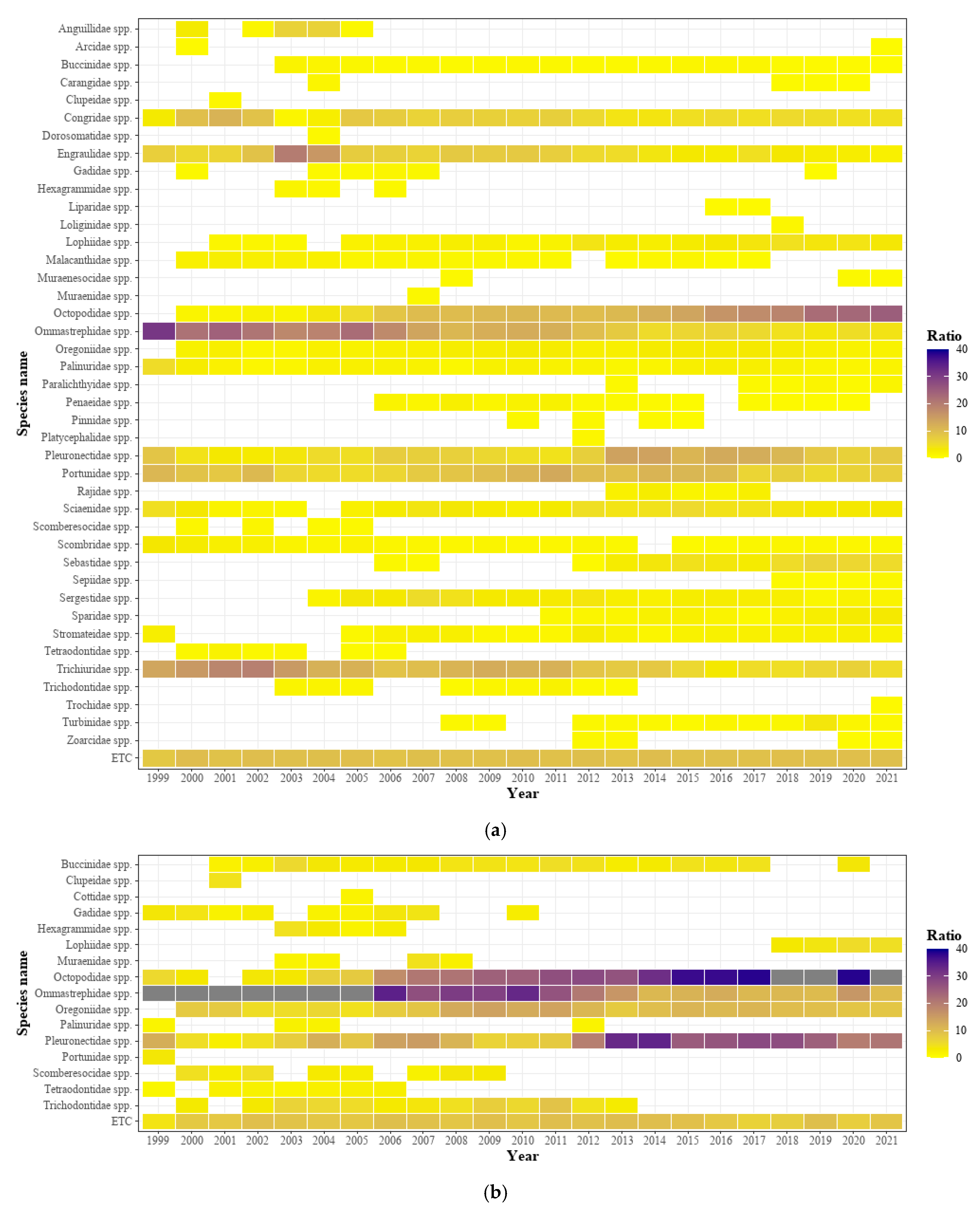
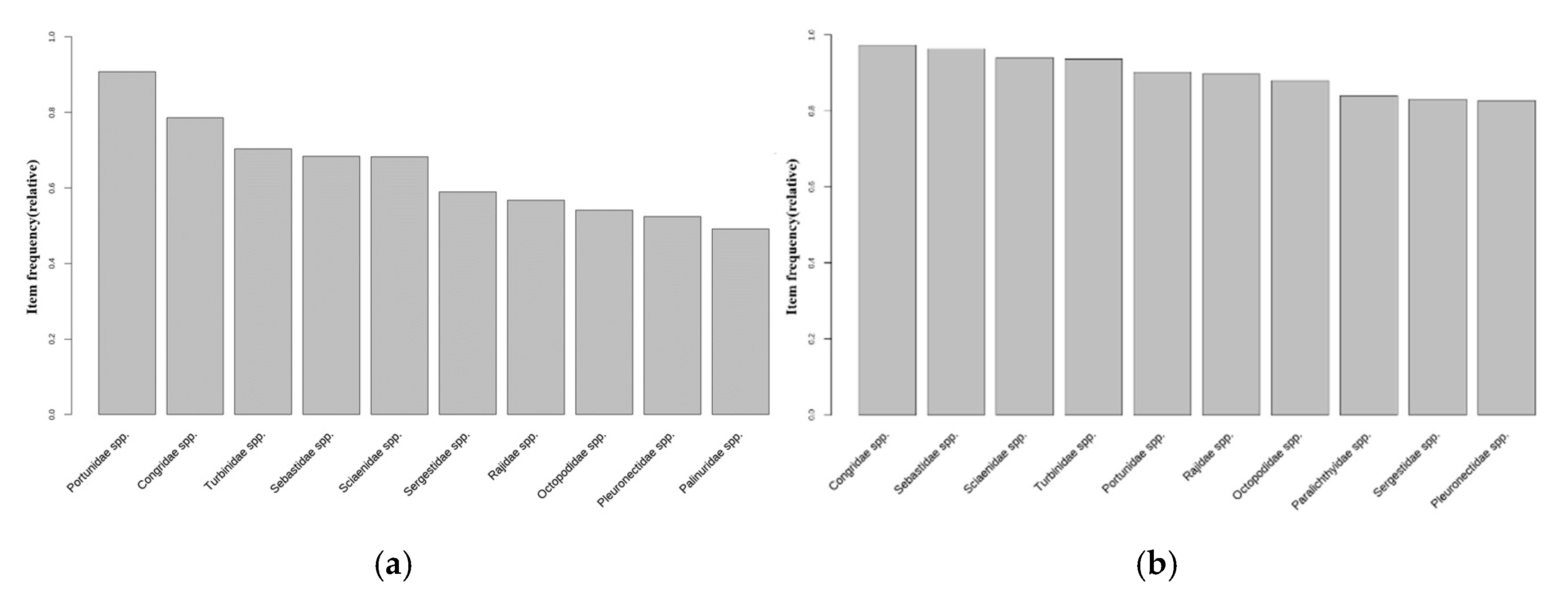
3.3. Analysis of Dominant Species by Changes in Water Temperature
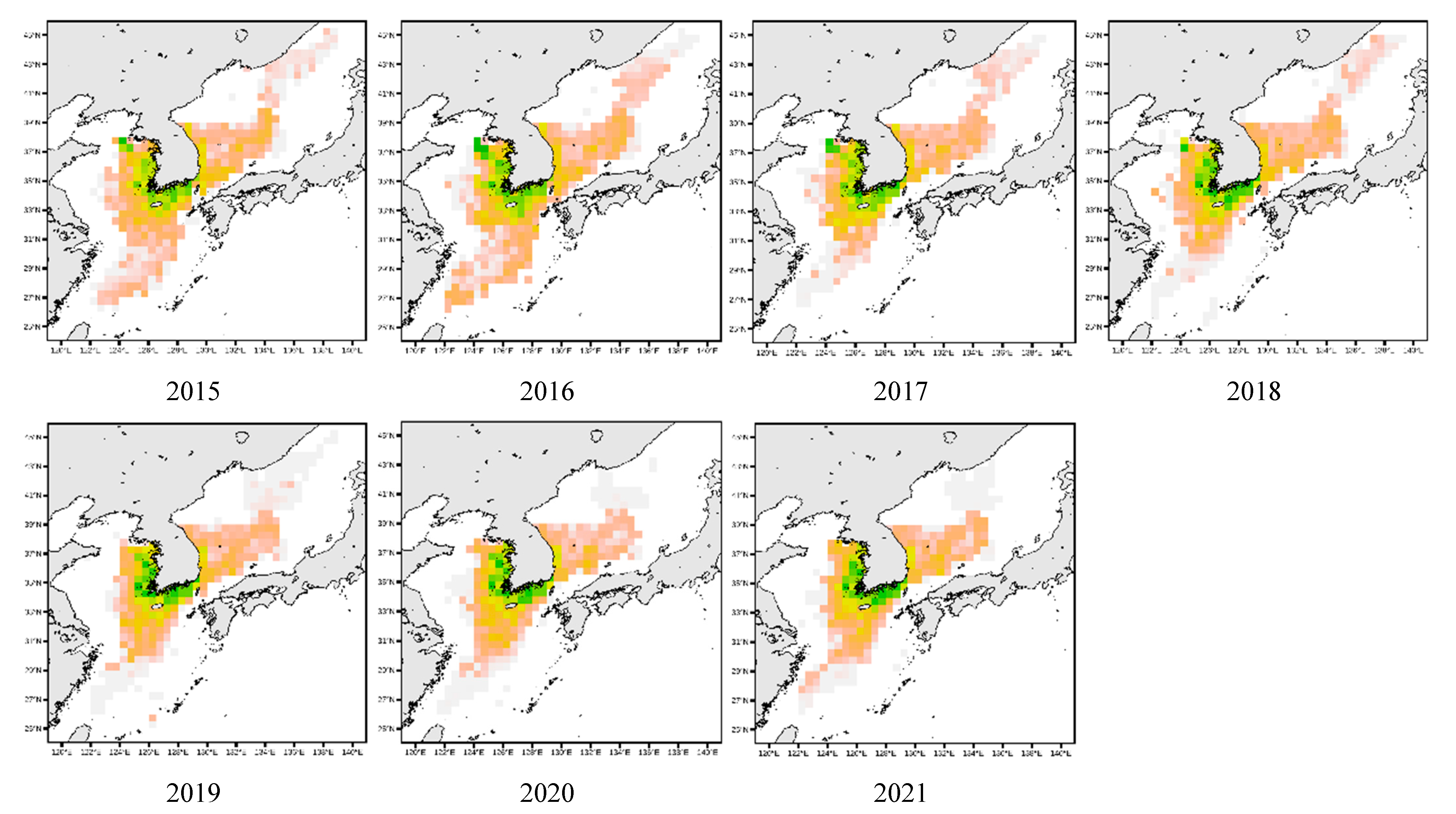
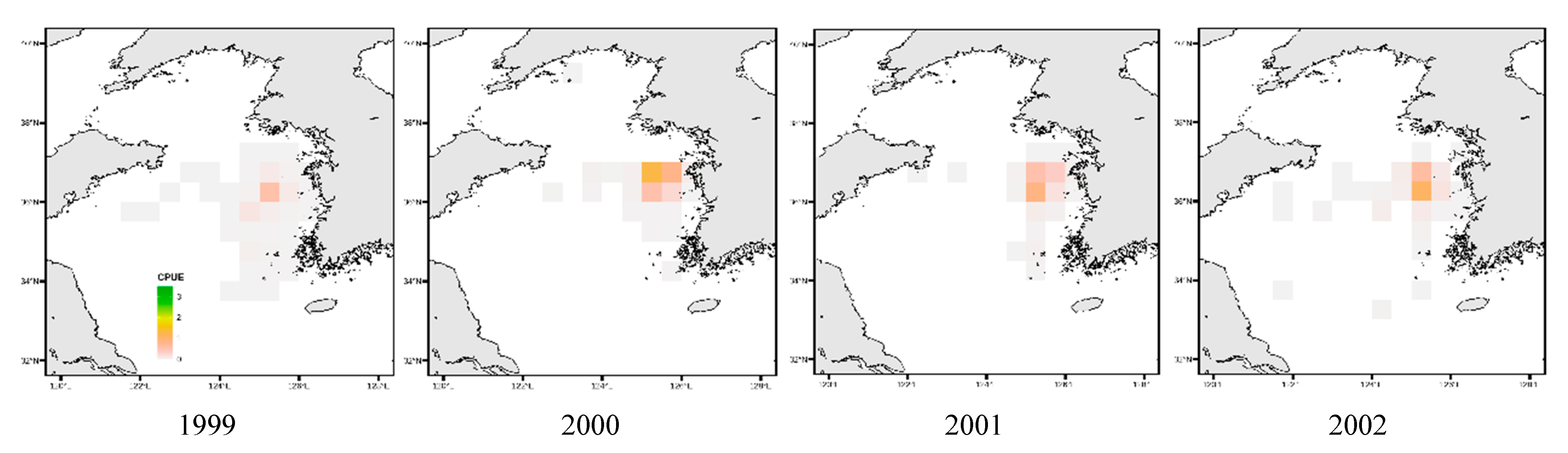
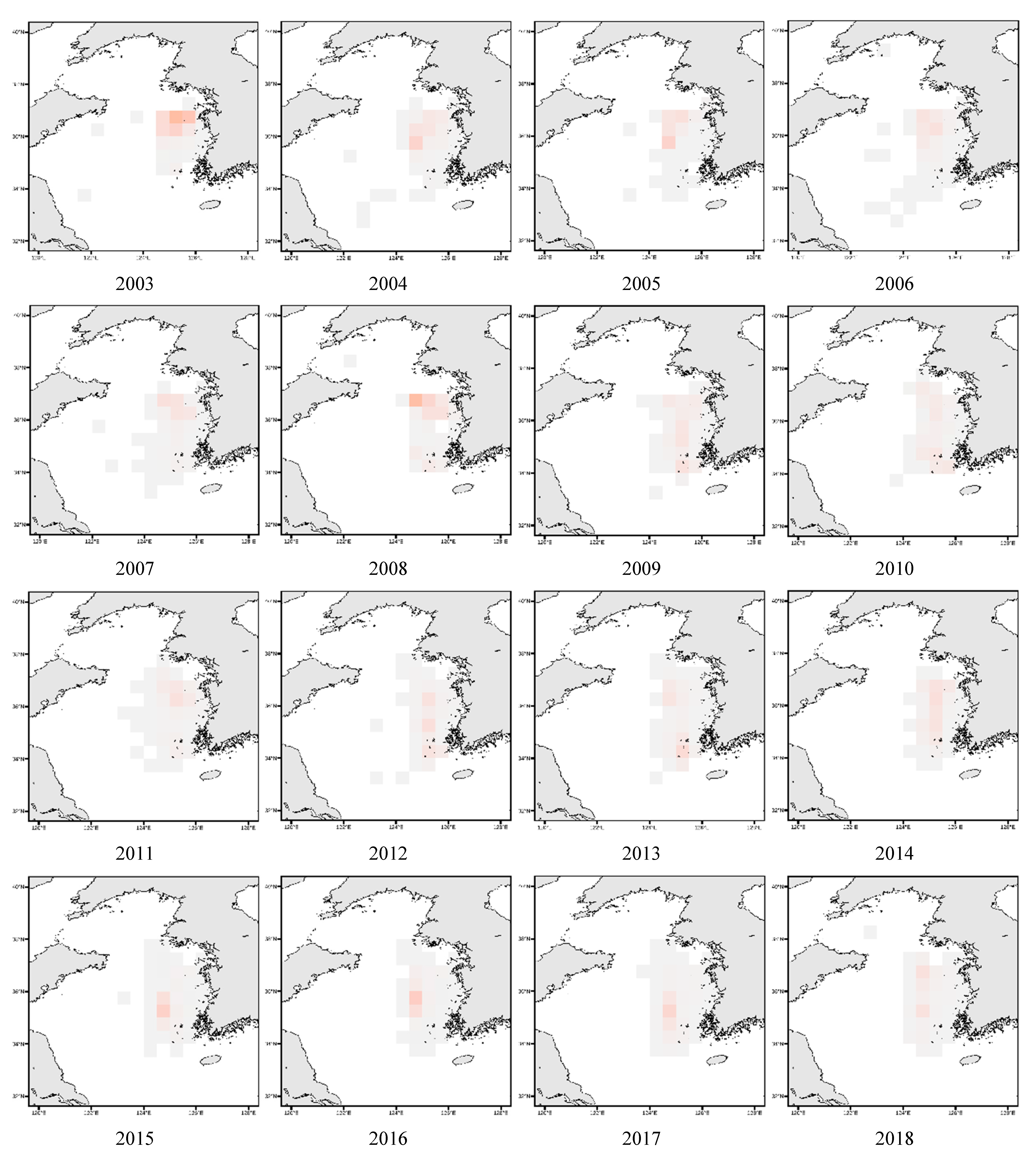
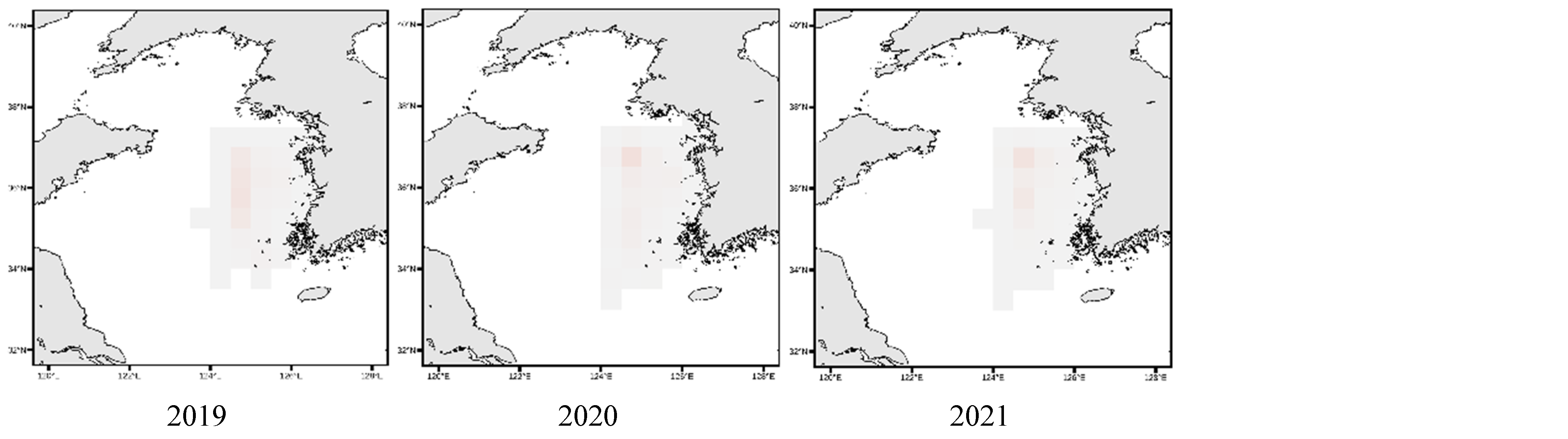
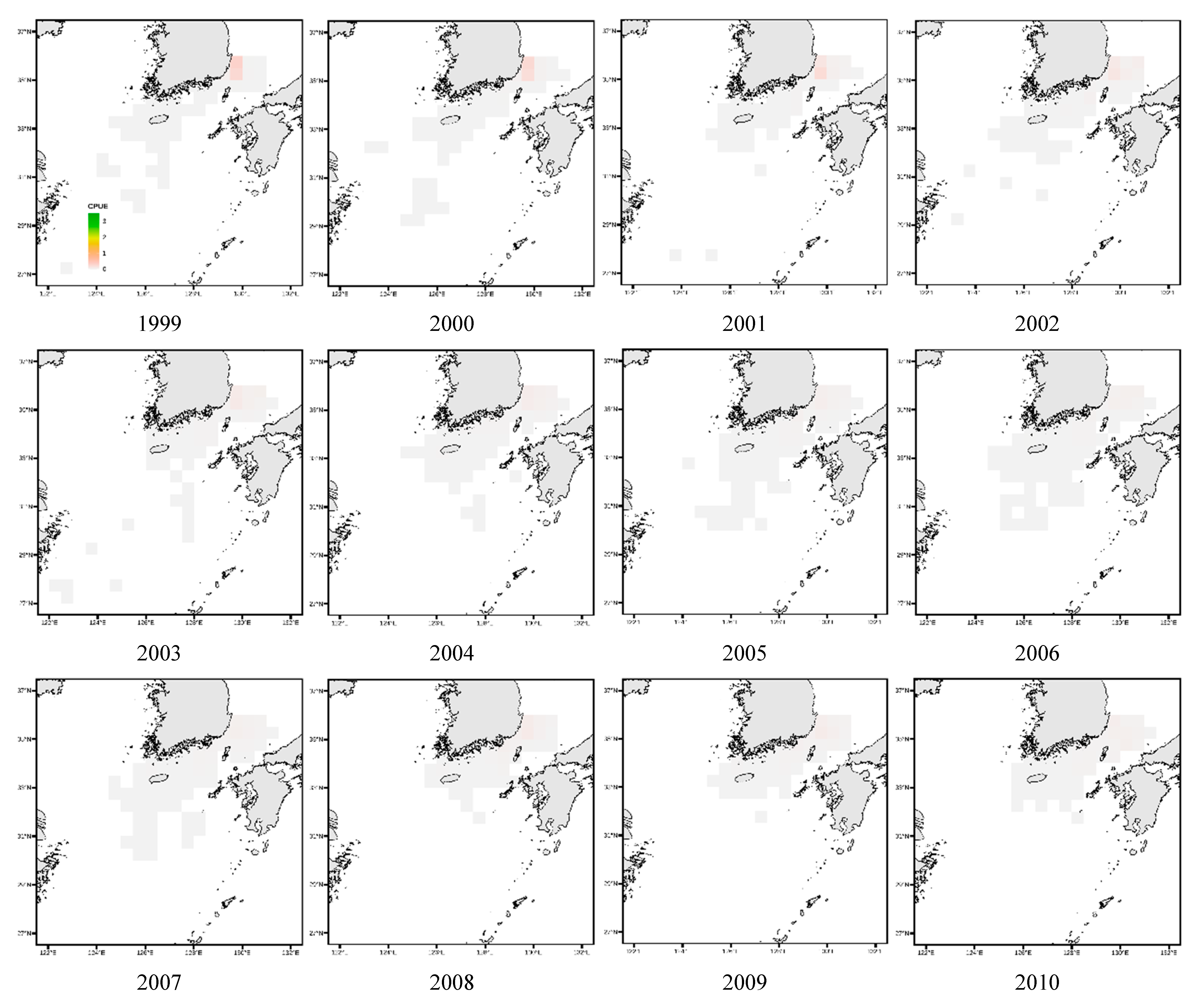
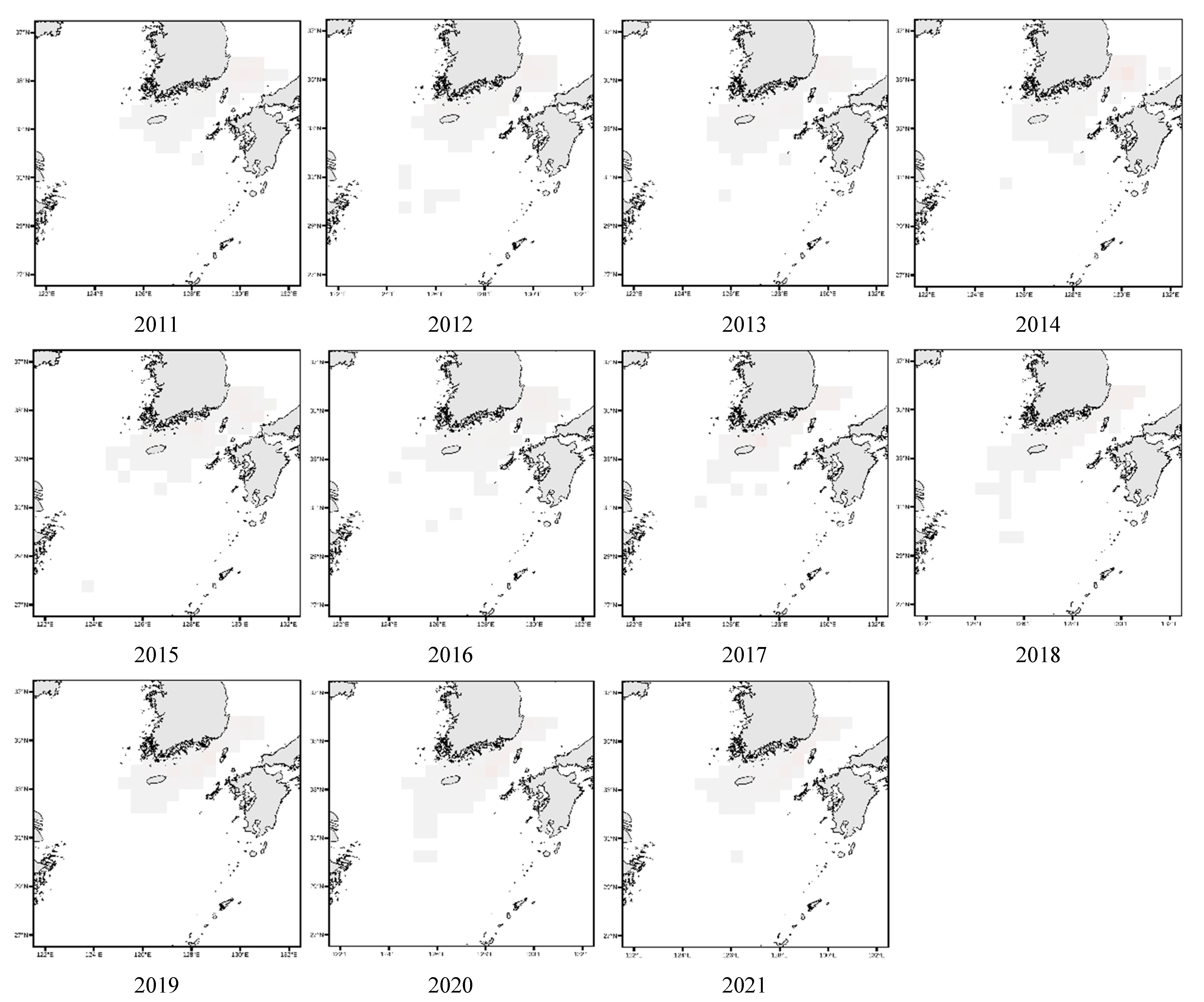
3.4. Spatial Distribution of Key Species
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CAR | Catching Activity Record |
| KODC | Korea Oceanographic Data Center |
| IQR | InterQuartile Range |
| CPUE | Catch Per Unit Effort |
| MPAs | Marine Protected Areas |
| PDO | Pacific Decadal Oscillation |
| GAMs | Generalized Additive Models |
| GLMs | Generalized Linear Models |
References
- Costanza, R.; Andrade, F.; Antunes, P.; den Belt, M.V.; Boersma, D.; Boesch, D.F.; Young, M. Principles for sustainable governance of the oceans. Science 1998, 281, 198–199. [Google Scholar] [CrossRef] [PubMed]
- Duarte, C.M. Marine biodiversity and ecosystem services: An elusive link. J. Exp. Mar. Bio. Ecol. 2000, 250, 117–131. [Google Scholar] [CrossRef] [PubMed]
- Heap, C.; Warwick, R.M.; d’Ozouville, L. A European science plan on marine biodiversity. J. Exp. Mar. Bio. Ecol. 1998, 250, 133–149. [Google Scholar]
- Chapin, F.S., III; Walker, B.H.; Hobbs, R.J.; Hooper, D.U.; Lawton, J.H.; Sala, O.E.; Tilman, D. Biotic control over the functioning of ecosystems. Science 1997, 277, 500–504. [Google Scholar] [CrossRef]
- Sala, E.; Knowlton, N. Global marine biodiversity trends. Annu. Rev. Environ. Resourc. 2006, 31, 93–122. [Google Scholar] [CrossRef]
- Free, C.M.; Thorson, J.T.; Pinsky, M.L.; Oken, K.L.; Wiedenmann, J.; Jensen, O.P. Impacts of historical warming on marine fisheries production. Science 2019, 363, 979–983. [Google Scholar] [CrossRef]
- Lee, J.H.; Seo, Y.I.; Yoon, S.C.; Kang, H.; Choi, J.H.; Choi, M.J.; Gim, J. A study on the variation of the Korean marine ecosystem through biodiversity attributes. J. Korean Soc. Fish. Ocean Technol. 2023, 59, 315–327. [Google Scholar] [CrossRef]
- Kirby, R.R.; Beaugrand, G.; Lindley, J.A. Synergistic effects of climate and fishing in a marine ecosystem. Ecosystems 2009, 12, 548–561. [Google Scholar] [CrossRef]
- Pikitch, E.K.; Santora, C.; Babcock, E.A.; Bakun, A.; Bonfil, R.; Conover, D.O.; Sainsbury, K.J. Ecosystem-based fishery management. Science 2004, 305, 346–347. [Google Scholar] [CrossRef]
- Long, R.D.; Charles, A.; Stephenson, R.L. Key principles of marine ecosystem-based management. Mar. Policy 2015, 57, 53–60. [Google Scholar] [CrossRef]
- Crain, C.M.; Halpern, B.S.; Beck, M.W.; Kappel, C.V. Understanding and managing human threats to the coastal marine environment. Ann. N. Y. Acad. Sci. 2009, 1162, 39–62. [Google Scholar] [CrossRef] [PubMed]
- Costello, C.; Gaines, S.D.; Lynham, J. Can catch shares prevent fisheries collapse? Science 2008, 321, 1678–1681. [Google Scholar] [CrossRef] [PubMed]
- Pauly, D.; Watson, R.; Alder, J. Global trends in world fisheries: Impacts on marine ecosystems and food security. Philos. Trans. R Soc. Lond. B Biol. Sci. 2005, 360, 5–12. [Google Scholar] [CrossRef]
- Zhang, C.I. A study on the ecosystem-based management system for fisheries resource in Korea. J. Korean Soc. Fish. Ocean. Technol. 2006, 42, 240–258. [Google Scholar] [CrossRef]
- Strong, J.A.; Andonegi, E.; Bizsel, K.C.; Danovaro, R.; Elliott, M.; Franco, A.; Graces, E.; Little, S.; Mazik, K.; Moncheva, S.; et al. Marine biodiversity and ecosystem function relationships: The potential for practical monitoring applications. Estuar. Coast. Shelf Sci. 2015, 161, 46–64. [Google Scholar] [CrossRef]
- Guisande, C.; Patti, B.; Vaamonde, A.; Manjarrés-Hernández, A.; Pelayo-Villamil, P.; García-Roselló, E.; Granado-Lorencio, C. Factors affecting species richness of marine elasmobranchs. Biol. Conserv. 2013, 22, 1703–1714. [Google Scholar] [CrossRef]
- Wernberg, T.; Russell, B.D.; Moore, P.J.; Ling, S.D.; Smale, D.A.; Campbell, A.; Connell, S.D. Impacts of climate change in a global hotspot for temperate marine biodiversity and ocean warming. J. Exp. Mar. Bio. Ecol. 2011, 400, 7–16. [Google Scholar] [CrossRef]
- Auber, A.; Gohin, F.; Goascoz, N.; Schlaich, I. Decline of cold-water fish species in the Bay of Somme (English Channel, France) in response to ocean warming. Estuar. Coast. Shelf Sci. 2017, 189, 189–202. [Google Scholar] [CrossRef]
- Shannon, C.E.; Wiener, W. The Mathematical Theory of Communication; University of Illinois Press: Urbana, IL, USA, 1963; Volume 177. [Google Scholar]
- Perry, A.L.; Low, P.J.; Ellis, J.R.; Reynolds, J.D. Climate change and distribution shifts in marine fishes. Science 2005, 308, 1912–1915. [Google Scholar] [CrossRef]
- Taylor, S.J.; Letham, B. Forecasting at scale. Am. Stat. 2018, 72, 37–45. [Google Scholar] [CrossRef]
- Hahsler, M.; Grün, B.; Hornik, K. arules-A computational environment for mining association rules and frequent item sets. J. Stat. Softw. 2005, 14, 1–25. [Google Scholar] [CrossRef]
- Lim, S.; Kim, D.H.; Hong, J.B. A Study on Stock Assessment of Japanese Flying Squid (Todarodes pacificus) in Korea· China· Japan Waters. Environ. Resour. Econ. Rev. 2022, 31, 451–480. [Google Scholar] [CrossRef]
- McConnaughey, R.A.; Hiddink, J.G.; Jennings, S.; Pitcher, C.R.; Kaiser, M.J.; Suuronen, P.; Hilborn, R. Choosing best practices for managing impacts of trawl fishing on seabed habitats and biota. Fish Fish. 2020, 21, 319–337. [Google Scholar] [CrossRef]
- Zhou, S.; Smith, A.D. Effect of fishing intensity and selectivity on trophic structure and fishery production. Mar. Ecol. Prog. Ser. 2017, 585, 185–198. [Google Scholar] [CrossRef][Green Version]
- Maestro, M.; Pérez-Cayeiro, M.L.; Chica-Ruiz, J.A.; Reyes, H. Marine protected areas in the 21st century: Current situation and trends. Ocean Coast. Manag. 2019, 171, 28–36. [Google Scholar] [CrossRef]
- Poloczanska, E.S.; Brown, C.J.; Sydeman, W.J.; Kiessling, W.; Schoeman, D.S.; Moore, P.J.; Brander, K.; Bruno, J.F.; Buckley, L.B.; Burrows, M.T.; et al. Global imprint of climate change on marine life. Nat. Clim. Change 2013, 3, 919–925. [Google Scholar] [CrossRef]
- So, J.; Kang, H.; Kim, S.; Jang, C.; Koo, B.; Kwon, Y.; Kim, Y. The Future Projection of Marine Ecosystem for the Seas around Korea I (Development of a Pilot Atlas for the Past Change and Future Projection of Marine Ecosystem in the Seas around Korea)—Final Report; Korea Institute of Ocean Science and Technology: Busan, Republic of Korea, 2018. [Google Scholar]
- Schippers, P.; Abarca, E.L.; Verboom, J.; Wamelink, G.W.W.; Vos, C.C.; de Boer, W.F.; Harvey, J.A.; Essens, T.; Grashof-Bokdam, C.J.; WallisDeVries, M.F.; et al. Biodiversity conservation in climate change driven transient communities. Biodivers. Conserv. 2021, 30, 2885–2906. [Google Scholar] [CrossRef]
- Thomas, C.D.; Franco, A.M.; Hill, J.K. Range retractions and extinction in the face of climate warming. Trends Ecol. Evol. 2006, 21, 415–416. [Google Scholar] [CrossRef]
- Ju, S.J.; Kim, S.J. Assessment of the impact of climate change on marine ecosystem in the south sea of Korea. Ocean Polar Res. 2012, 34, 197–199. [Google Scholar] [CrossRef]
- Ju, S.J.; Kim, S.J. Assessment of the Impact of Climate Change on Marine Ecosystem in the South Sea of Korea II. Ocean Polar Res. 2013, 35, 123–125. [Google Scholar] [CrossRef]
- Dulvy, N.K.; Rogers, S.I.; Jennings, S.; Stelzenmüller, V.; Dye, S.R.; Skjoldal, H.R. Climate change and deepening of the North Sea fish assemblage: A biotic indicator of warming seas. J. Appl. Ecol. 2008, 45, 1029–1039. [Google Scholar] [CrossRef]
- Guerra, Á.; Allcock, L.; Pereira, J. Cephalopod life history, ecology and fisheries: An introduction. Fish. Res. 2010, 106, 117–124. [Google Scholar] [CrossRef]
- Somero, G.N. The physiology of climate change: How potentials for acclimatization and genetic adaptation will determine ‘winners’ and ‘losers’. J. Exp. Biol. 2010, 213, 912–920. [Google Scholar] [CrossRef] [PubMed]
- Rubin, S.P. Fish: Section 4.8 in Climate Change and the Olympic Coast National Marine Sanctuary: Interpreting Potential Futures; Report ONMS-13-01; U.S. Geological Survey, Western Fisheries Research Center: Seattle, WA, USA, 2013. [Google Scholar]
- Kim, Y.H.; Jung, H.K.; Lee, C.I. Changes in the spawning ground environment of the common squid, Todarodes pacificus due to climate change. Ocean Polar Res. 2018, 40, 127–143. [Google Scholar] [CrossRef]
- Kim, Y.H. The population ecology of the common squid (Todarodes pacificus) in the northwest Pacific Marginal Seas. Ph.D. Thesis, Pukyung National University, Busan, Republic of Korea, 2015; 127p. [Google Scholar]




| Variable | Explanation |
|---|---|
| Date | The total number of days for which operational data were reported to the Safety Operation Headquarters was 8248. |
| Fishing method | There are 88 types of coastal industries in Korea. |
| Sea | The fishing areas include the East Sea, South Sea, West Sea, and other sea areas including the Pacific Ocean below Japan. The division of sea areas in the data is based on Figure 1 for consistency. |
| Zone | This refers to the ocean basin; meanwhile, a small zone is a small area divided into nine ocean basins. In this study, the ocean basin was analyzed. |
| Fish name | The name of the species of fish caught. |
| Quantity | The catch volume, reported by fishermans, is based on specific spatial and temporal data, and the unit is kilograms (kg). |
| Year | Number of Fishing Days | Fishing Frequency | Number of Trenches Where Fishing Activities Took Place (Sea) | |||
|---|---|---|---|---|---|---|
| East | South | West | ETC | |||
| 1999 | 214 | 80,259 | 69 | 108 | 68 | 4 |
| 2000 | 365 | 142,957 | 73 | 139 | 78 | 5 |
| 2001 | 365 | 192,517 | 86 | 156 | 79 | 2 |
| 2002 | 364 | 212,506 | 95 | 170 | 74 | 6 |
| 2003 | 365 | 282,699 | 119 | 167 | 74 | 1 |
| 2004 | 366 | 345,548 | 129 | 158 | 77 | 0 |
| 2005 | 365 | 345,291 | 153 | 156 | 76 | 0 |
| 2006 | 365 | 403,085 | 138 | 147 | 80 | 0 |
| 2007 | 365 | 420,316 | 138 | 143 | 71 | 0 |
| 2008 | 366 | 364,140 | 125 | 146 | 70 | 0 |
| 2009 | 365 | 320,661 | 140 | 142 | 62 | 0 |
| 2010 | 365 | 305,918 | 139 | 150 | 68 | 3 |
| 2011 | 365 | 314,866 | 144 | 149 | 66 | 1 |
| 2012 | 366 | 467,570 | 135 | 147 | 62 | 0 |
| 2013 | 365 | 896,084 | 135 | 151 | 67 | 1 |
| 2014 | 365 | 969,692 | 140 | 138 | 67 | 0 |
| 2015 | 365 | 861,863 | 135 | 153 | 65 | 0 |
| 2016 | 366 | 810,794 | 142 | 156 | 70 | 0 |
| 2017 | 365 | 871,717 | 137 | 111 | 67 | 0 |
| 2018 | 365 | 1,051,979 | 144 | 129 | 68 | 1 |
| 2019 | 365 | 1,347,303 | 148 | 125 | 58 | 1 |
| 2020 | 366 | 1,354,784 | 113 | 128 | 66 | 0 |
| 2021 | 365 | 1,264,167 | 120 | 122 | 60 | 0 |
| Month | Fishing Frequency | Number of Trenches Where Fishing Activities Took Place (Sea) | |||
|---|---|---|---|---|---|
| East | South | West | ETC | ||
| 1 | 866,985 | 86 | 165 | 81 | 1 |
| 2 | 672,618 | 88 | 171 | 83 | 2 |
| 3 | 891,609 | 86 | 191 | 83 | 2 |
| 4 | 977,095 | 84 | 183 | 84 | 1 |
| 5 | 1,211,875 | 88 | 171 | 85 | 1 |
| 6 | 1,279,220 | 87 | 154 | 84 | 3 |
| 7 | 1,169,302 | 157 | 126 | 79 | 2 |
| 8 | 1,232,525 | 179 | 117 | 86 | 4 |
| 9 | 1,429,914 | 175 | 115 | 85 | 2 |
| 10 | 1,483,153 | 152 | 143 | 86 | 5 |
| 11 | 1,302,713 | 95 | 150 | 85 | 1 |
| 12 | 1,109,707 | 92 | 157 | 86 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ceong, H.; Kwon, I. Spatiotemporal Changes in Catch Composition of Marine Species Across Seawater Temperature Shift Points in Korean Water. Animals 2025, 15, 1212. https://doi.org/10.3390/ani15091212
Ceong H, Kwon I. Spatiotemporal Changes in Catch Composition of Marine Species Across Seawater Temperature Shift Points in Korean Water. Animals. 2025; 15(9):1212. https://doi.org/10.3390/ani15091212
Chicago/Turabian StyleCeong, Hyithaek, and Inyeong Kwon. 2025. "Spatiotemporal Changes in Catch Composition of Marine Species Across Seawater Temperature Shift Points in Korean Water" Animals 15, no. 9: 1212. https://doi.org/10.3390/ani15091212
APA StyleCeong, H., & Kwon, I. (2025). Spatiotemporal Changes in Catch Composition of Marine Species Across Seawater Temperature Shift Points in Korean Water. Animals, 15(9), 1212. https://doi.org/10.3390/ani15091212





