Tissue-Specific Expression of the Porcine DHRS3 Gene and Its Impact on the Proliferation and Differentiation of Myogenic Cells
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Sample Collection
2.2. RNA Extraction and cDNA Synthesis from Tissue Samples
2.3. Primer Design, PCR Amplification, and Sequencing
2.4. RT-qPCR
2.5. Isolation, Culture, and Induced Differentiation of Porcine Myoblasts
2.6. Overexpression Plasmid, shRNA, and Transfection
2.7. EdU Assay
2.8. Flow Cytometry
2.9. Statistical Analysis
3. Results
3.1. Tissue-Specific Expression Analysis of DHRS3 in Pigs
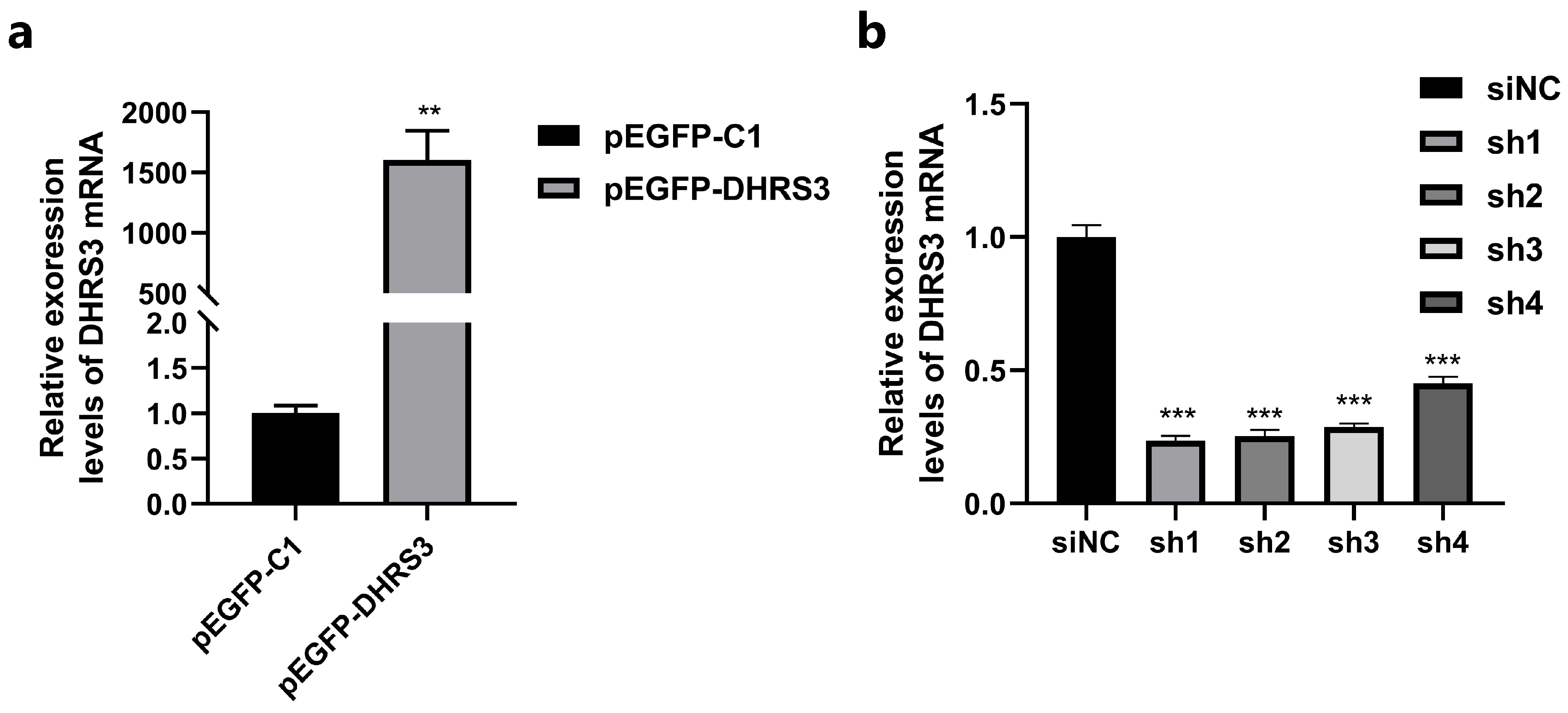
3.2. Transfection Efficiency Assessment
3.3. DHRS3 Gene Inhibits Proliferation of Porcine Myoblasts
3.4. Effects of the DHRS3 Gene on Apoptosis-Related Genes
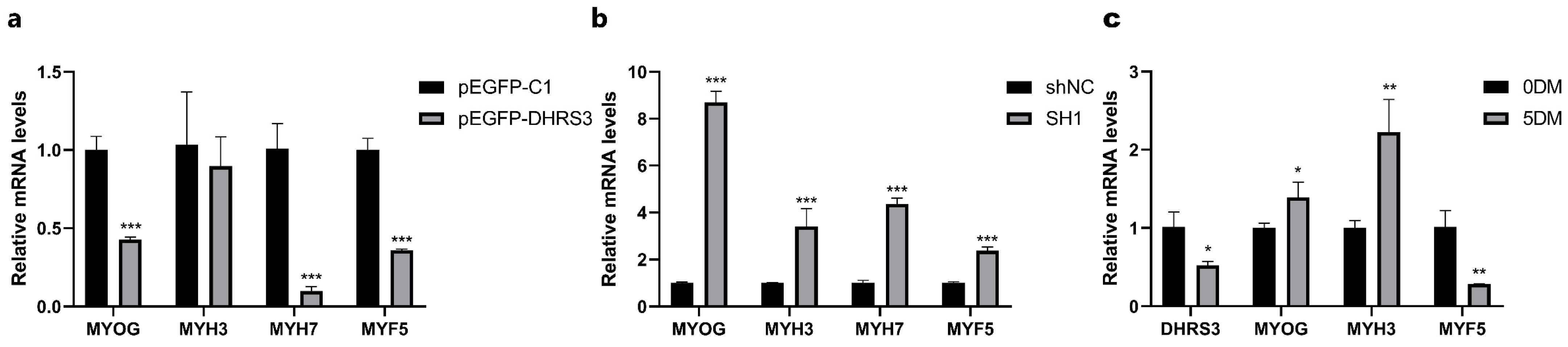
3.5. The DHRS3 Gene Affects Myoblast Differentiation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shahriyari, M.; Islam, M.R.; Sakib, S.M.; Rin, M.; Rika, A.; Krüger, D.; Kaurani, L.; Gisa, V.; Winterhoff, M.; Anandakumar, H.; et al. Engineered skeletal muscle recapitulates human muscle development, regeneration and dystrophy. J. Cachexia Sarcopenia Muscle 2022, 13, 3106–3121. [Google Scholar] [CrossRef]
- Frontera, W.R.; Ochala, J. Skeletal muscle: A brief review of structure and function. Calcif. Tissue Int. 2014, 96, 183–195. [Google Scholar] [CrossRef]
- Kim, C.J.; Hadjiargyrou, M. Mustn1 in Skeletal Muscle: A Novel Regulator? Genes 2024, 15, 829. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Ege, D.; Salehi, S.; Boccaccini, A.R. Ionic medicine: Exploiting metallic ions to stimulate skeletal muscle tissue regeneration. Acta Biomater. 2024, 190, 1–23. [Google Scholar] [CrossRef]
- Morozzi, G.; Beccafico, S.; Bianchi, R.; Riuzzi, F.; Bellezza, I.; Giambanco, I.; Arcuri, C.; Minelli, A.; Donato, R. Oxidative stress-induced S100B accumulation converts myoblasts into brown adipocytes via an NF-κB/YY1/miR-133 axis and NF-κB/YY1/BMP-7 axis. Cell Death Differ. 2017, 24, 2077–2088. [Google Scholar] [CrossRef]
- Brown, L.D. Endocrine regulation of fetal skeletal muscle growth: Impact on future metabolic health. J. Endocrinol. 2014, 221, R13–R29. [Google Scholar] [CrossRef]
- Kallberg, Y.; Oppermann, U.; Jörnvall, H.; Persson, B. Short-chain dehydrogenases/reductases (SDRs). Eur. J. Biochem. 2002, 269, 4409–4417. [Google Scholar] [CrossRef]
- Kramm, A.; Kisiela, M.; Schulz, R.; Maser, E. Short-chain dehydrogenases/reductases in cyanobacteria. FEBS J. 2012, 279, 1030–1043. [Google Scholar] [CrossRef]
- Franco, G.; Marco, A.; Sergio, T. Gene Structure Evolution of the Short-Chain Dehydrogenase/Reductase (SDR) Family. Genes 2023, 14, 110. [Google Scholar] [CrossRef]
- Da Costa, M.; Gevaert, O.; Van Overtveldt, S.; Lange, J.; Joosten, H.; Desmet, T.; Beerens, K. Structure-function relationships in NDP-sugar active SDR enzymes: Fingerprints for functional annotation and enzyme engineering. Biotechnol. Adv. 2021, 48, 107705. [Google Scholar] [CrossRef]
- Li, Z.; Tan, Y.; Li, X.; Quan, J.; Bode, A.M.; Cao, Y.; Luo, X. DHRS2 inhibits cell growth and metastasis in ovarian cancer by downregulation of CHKα to disrupt choline metabolism. Cell Death Dis. 2022, 13, 845. [Google Scholar] [CrossRef] [PubMed]
- Oppermann, U.; Filling, C.; Jörnvall, H. Forms and functions of human SDR enzymes. Chem. Biol. Interact. 2001, 130–132, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.K.; Lee, S.; Belyaeva, O.V.; Wu, L.; Kedishvili, N.Y. Characterization of human short chain dehydrogenase/reductase SDR16C family members related to retinol dehydrogenase 10. Chem. Biol. Interact. 2017, 276, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, Y.; Zheng, Z.; Zeng, X.; Liu, D.; Wang, C.; Ting, K. MicroRNA-223 Suppresses Osteoblast Differentiation by Inhibiting DHRS3. Cell Physiol. Biochem. 2018, 47, 667–679. [Google Scholar] [CrossRef]
- Lundová, T.; Zemanová, L.; Malčeková, B.; Skarka, A.; Štambergová, H.; Havránková, J.; Šafr, M.; Wsól, V. Molecular and biochemical characterisation of human short-chain dehydrogenase/reductase member 3 (DHRS3). Chem. Biol. Interact. 2015, 234, 178–187. [Google Scholar] [CrossRef]
- Kirschner, R.D.; Rother, K.; Müller, G.A.; Engeland, K. The retinal dehydrogenase/reductase retSDR1/DHRS3 gene is activated by p53 and p63 but not by mutants derived from tumors or EEC/ADULT malformation syndromes. Cell Cycle 2010, 9, 2177–2188. [Google Scholar] [CrossRef]
- Adams, M.K.; Belyaeva, O.V.; Wu, L.; Chaple, I.F.; Dunigan-Russell, K.; Popov, K.M.; Kedishvili, N.Y. Characterization of subunit interactions in the hetero-oligomeric retinoid oxidoreductase complex. Biochem. J. 2021, 478, 3597–3611. [Google Scholar] [CrossRef]
- Johns, E.; Ma, Y.; Louphrasitthiphol, P.; Peralta, C.; Hunter, M.V.; Raymond, J.H.; Molina, H.; Goding, C.R.; White, R.M. The Lipid Droplet Protein DHRS3 Is a Regulator of Melanoma Cell State. Pigment Cell Melanoma Res. 2024, 38, e13208. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, J.; Zhang, C.; Tong, H.; Li, S.; Yan, Y. Effects of DHRS3 in C2C12 Myoblast Differentiation and Mouse Skeletal Muscle Injury. J. Northeast. Agric. Univ. (Engl. Ed.) 2021, 28, 10. Available online: https://kns.cnki.net/kcms2/article/abstract?v=_mP9DtK6WVcW4V6VjYPckVfIvpj2sWL6HvctjmIZd8Zk3UaEwhg1aPZj8WK8xB51Zksfq6BRjiKES2BIgbNYdaBxSB0qPDXat_595x9Pd2zCSvko5G9cjsWcg5E6YZHgvlEti9E0Cvjrsne-hFJUH8zdLbJekGwn4SYtMYZmLWv4IJBw1PYalDR4yH-rpKjh&uniplatform=NZKPT&language=CHS (accessed on 19 October 2021).
- Tian, J.; Du, Y.; Wang, B.; Ji, M.; Li, H.; Xia, Y.; Zhang, K.; Li, Z.; Xie, W.; Gong, W.; et al. Hif1α/DHRS3a Pathway Participates in Lipid Droplet Accumulation via Retinol and Ppar-γ in Fish Hepatocytes. Int. J. Mol. Sci. 2023, 24, 10236. [Google Scholar] [CrossRef]
- Deisenroth, C.; Itahana, Y.; Tollini, L.; Jin, A.; Zhang, Y. p53-inducible DHRS3 Is an Endoplasmic Reticulum Protein Associated with Lipid Droplet Accumulation. J. Biol. Chem. 2011, 286, 28343–28356. [Google Scholar] [CrossRef] [PubMed]
- Forcina, L.; Miano, C.; Pelosi, L.; Musarò, A. An Overview About the Biology of Skeletal Muscle Satellite Cells. Curr. Genomics 2019, 20, 24–37. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, W.; Li, P.; Wang, H.; Zhang, Y.; Zan, L. Myocyte enhancer factor 2A promotes proliferation and its inhibition attenuates myogenic differentiation via myozenin 2 in bovine skeletal muscle myoblast. PLoS ONE 2018, 13, e196255. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Chen, X.; Huang, Z.; Chen, D.; Yu, B.; Chen, H.; Luo, J.; He, J.; Zheng, P.; Yu, J. Leucine promotes differentiation of porcine myoblasts through the protein kinase B (Akt)/Forkhead box O1 signalling pathway. Br. J. Nutr. 2018, 119, 727–733. [Google Scholar] [CrossRef]
- Reynhout, S.; Janssens, V. Physiologic functions of PP2A: Lessons from genetically modified mice. Biochim. Biophys. Acta Mol. Cell Res. 2019, 1866, 31–50. [Google Scholar] [CrossRef]
- Reza, Z.; Qiuyan, C.; A Catharine, R. DHRS3, a retinal reductase, is differentially regulated by retinoic acid and lipopolysaccharide-induced inflammation in THP-1 cells and rat liver. Am. J. Physiol.-Gastroint. Liver Physiol. 2012, 303, G578–G588. [Google Scholar] [CrossRef]
- Hardebeck, S.; Schreiber, S.; Adick, A.; Langer, K.; Jose, J. A FRET-Based Assay for the Identification of PCNA Inhibitors. Int. J. Mol. Sci. 2023, 24, 11858. [Google Scholar] [CrossRef]
- Røst, L.M.; Ræder, S.B.; Olaisen, C.; Søgaard, C.K.; Sharma, A.; Bruheim, P.; Otterlei, M. PCNA regulates primary metabolism by scaffolding metabolic enzymes. Oncogene 2023, 42, 613–624. [Google Scholar] [CrossRef]
- Herrera, M.C.; Chymkowitch, P.; Robertson, J.M.; Eriksson, J.; Bøe, S.O.; Alseth, I.; Enserink, J.M. Cdk1 gates cell cycle-dependent tRNA synthesis by regulating RNA polymerase III activity. Nucleic Acids Res. 2018, 46, 11698–11711. [Google Scholar] [CrossRef]
- Tyagi, N.; Deshmukh, S.K.; Srivastava, S.K.; Azim, S.; Ahmad, A.; AL-Ghadhban, A.; Singh, A.P.; Carter, J.E.; Wang, B.; Singh, S. ETV4 Facilitates Cell-Cycle Progression in Pancreatic Cells through Transcriptional Regulation of Cyclin D1. Mol. Cancer Res. 2018, 16, 187–196. [Google Scholar] [CrossRef]
- Vermeulen, K.; Berneman, Z.N.; Van Bockstaele, D.R. Cell cycle and apoptosis. Cell Prolif. 2003, 36, 113–175. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Gao, S.; Han, Y.; Ning, H.; Zhou, Y.; Guan, H.; Liu, X.; Yan, S.; Zhou, P. BECN1 promotes radiation-induced G2/M arrest through regulation CDK1 activity: A potential role for autophagy in G2/M checkpoint. Cell Death Discov. 2020, 6, 70. [Google Scholar] [CrossRef] [PubMed]
- Cai, F.; Chen, P.; Chen, L.; Biskup, E.; Liu, Y.; Chen, P.; Chang, J.; Jiang, W.; Jing, Y.; Chen, Y.; et al. Human RAD6 promotes G1-S transition and cell proliferation through upregulation of cyclin D1 expression. PLoS ONE 2014, 11, e113727. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zeng, J.; Sun, F.; Tong, X.; Meng, G.; Wu, C.; Ding, X.; Liu, L.; Han, M.; Lu, C.; et al. p27 inhibits CDK6/CCND1 complex formation resulting in cell cycle arrest and inhibition of cell proliferation. Cell Cycle 2018, 17, 2335–2348. [Google Scholar] [CrossRef]
- Gennaro, V.J.; Stanek, T.J.; Peck, A.R.; Sun, Y.; Wang, F.; Qie, S.; Knudsen, K.E.; Rui, H.; Butt, T.; Diehl, J.A.; et al. Control of CCND1 ubiquitylation by the catalytic SAGA subunit USP22 is essential for cell cycle progression through G1 in cancer cells. Proc. Natl. Acad. Sci. USA 2018, 115, E9298–E9307. [Google Scholar] [CrossRef]
- Sha, S.; Kong, X.; Chen, F.; Sun, X.; Hu, S.; Bai, B.; Shi, X.; Tu, Y.; Wu, K.; Zhao, Q.; et al. Hypermethylation of DHRS3 as a Novel Tumor Suppressor Involved in Tumor Growth and Prognosis in Gastric Cancer. Front. Cell Dev. Biol. 2021, 9, 624871. [Google Scholar] [CrossRef]
- Wang, L.; Su, L.; Cen, Y.; Li, J.; Yang, J.; Li, Y. Overexpressed miRNA-nov-1 promotes manganese-induced apoptosis in N27 cells by regulating DHRS3 to activate mTOR signaling pathway. Toxicology 2023, 489, 153472. [Google Scholar] [CrossRef]
- Jiang, R.; Gao, H.; Cong, F.; Zhang, W.; Song, T.; Yu, Z. Circ_DHRS3 positively regulates GREM1 expression by competitively targeting miR-183-5p to modulate IL-1β-administered chondrocyte proliferation, apoptosis and ECM degradation. Int. Immunopharmacol. 2021, 91, 107293. [Google Scholar] [CrossRef]
- Yi, C.; Roshan, M.K.; Bennett, H.P.; Charlotte, A.B.; Charles, K.; Laurie, A.B.; Richard, A.Y.; Stephen, J.T. Global and gene-specific analyses show distinct roles for Myod and Myog at a common set of promoters. EMBO J. 2006, 25, 502–511. [Google Scholar] [CrossRef]
- Tang, J.; Yang, B.; Song, G.; Zhang, X.; Wang, Z.; Mo, Z.; Zan, L.; Wang, H. Effect of bovine myosin heavy chain 3 on proliferation and differentiation of myoblast. Anim. Biotechnol. 2022, 34, 4337–4346. [Google Scholar] [CrossRef]
- Wang, H.; Dou, M.; Li, J.; Cao, P.; Li, J.; Guo, T.; Zhao, D.; Khan, A.; Li, Y.; Li, B.; et al. Expression patterns and correlation analyses of muscle-specific genes in the process of sheep myoblast differentiation. In Vitro Cell Dev. Biol. 2022, 58, 798–809. [Google Scholar] [CrossRef] [PubMed]
- Francetic, T.; Le May, M.; Hamed, M.; Mach, H.; Meyers, D.; Cole, P.A.; Chen, J.; Li, Q. Regulation of Myf5 Early Enhancer by Histone Acetyltransferase P300 during Stem Cell Differentiation. Mol. Biol. 2012, 1, 1000103. [Google Scholar] [CrossRef]
- Jihong, C.; Qiao, L. Implication of retinoic acid receptor selective signaling in myogenic differentiation. Sci. Rep. 2016, 6, 18856. [Google Scholar] [CrossRef]
- Moreno-Oñate, M.; Gallardo-Fuentes, L.; Martínez-García, P.M.; Naranjo, S.; Jiménez-Gancedo, S.; Tena, J.J.; Santos-Pereira, J.M. Rewiring of the epigenome and chromatin architecture by exogenously induced retinoic acid signaling during zebrafish embryonic development. Nucleic Acids Res. 2024, 52, 3682–3701. [Google Scholar] [CrossRef]



| Gene | Primer Sequence (5′-3′) | Product Size (bp) | Annealing Temperature (°C) |
|---|---|---|---|
| DHRS3 | F:AGCACCGAGATGTTTCAGGG | 113 | 60 |
| R:AGGGTCTGATTGAGCTGCAC | |||
| GAPDH | F:TTTGTGATGGGCGTGAACC | 171 | 60 |
| R:AGTCTTCTGGGTGGCAGTGAT | |||
| PCNA | F:AAGTGGAGAACTCGGAAATGGAA | 161 | 60 |
| R:CTGTAGGAGAGAGTGGAGTGGCTTT | |||
| CCNB1 | F:GACTGGCTAGTGCAGGTTCAGATG | 137 | 60 |
| R:ATGGCAGTGACACCAACCAGTTG | |||
| CCND1 | F:TGACCTGCCTTAGACCTTA | 140 | 60 |
| R:GCTGCTGTTACTGACTATATC | |||
| CDK1 | F:GAGCGACGCTGACGTGGTA | 72 | 62 |
| R:TGGATGTGGTAGATCCCAGCTT | |||
| BAX | F:TGACGGCAACTTCAACTGGG | 144 | 55 |
| R:AGCAGCCGATCTCGAAGGAA | |||
| BCL2 | F:CAGAGGGGCTACGAGTGGGATG | 89 | 62 |
| R:CCGGGCTGGGAGGAGAAGATG | |||
| Caspase3 | F:AGAAGACCATAGCAAAAGGAGCAG | 155 | 60 |
| R:GTTTGGGTTTGCCAGTTAGAGTTC | |||
| Caspase8 | F:CTGACCTCTTATTTCACTGGTTCGA | 97 | 62 |
| R:CTTTCTGGTATTTATCCCCTTGACA | |||
| MYOG | F:TGCCCAGTGAATGCAGTTCC | 164 | 60 |
| R:ATCCTCCACTGTGATGCTGTCC | |||
| MYH3 | F:ACAGCGGAAAGAAGAAAGTTGC | 157 | 55 |
| R:CCTGGGGTTTTGGTTTCATT | |||
| MYH7 | F:GATGCGGAGATGGCCGCATT | 171 | 60 |
| R:GACTTTGCCACCCTCTCGAGACA | |||
| MYF5 | F:TGCCAGTTCTCGCCTTCTGAGTA | 221 | 62 |
| R:GTGGATTTCCTCTTGCACGCTTT |
| shRNA Name | Sequences (5′-3′) |
|---|---|
| shRNA1 | GGACCATGCATGTCCTCATTA |
| shRNA2 | TGATCTATCTGGTGGTGAAAG |
| shRNA3 | CCTTCTCAAGTCCCAGCATAT |
| shRNA4 | ACACCAGCACCGAGATGTTTC |
| shNC | GTTCTCCGAACGTGTCACGT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Ruan, Y.; Jiang, C.; Sun, J.; An, D.; Zhou, B.; Liu, H.; Li, Z.; Xu, H. Tissue-Specific Expression of the Porcine DHRS3 Gene and Its Impact on the Proliferation and Differentiation of Myogenic Cells. Animals 2025, 15, 1101. https://doi.org/10.3390/ani15081101
Li J, Ruan Y, Jiang C, Sun J, An D, Zhou B, Liu H, Li Z, Xu H. Tissue-Specific Expression of the Porcine DHRS3 Gene and Its Impact on the Proliferation and Differentiation of Myogenic Cells. Animals. 2025; 15(8):1101. https://doi.org/10.3390/ani15081101
Chicago/Turabian StyleLi, Jifeng, Yong Ruan, Chuanmei Jiang, Jinkui Sun, Dongwei An, Bo Zhou, Huan Liu, Ziyang Li, and Houqiang Xu. 2025. "Tissue-Specific Expression of the Porcine DHRS3 Gene and Its Impact on the Proliferation and Differentiation of Myogenic Cells" Animals 15, no. 8: 1101. https://doi.org/10.3390/ani15081101
APA StyleLi, J., Ruan, Y., Jiang, C., Sun, J., An, D., Zhou, B., Liu, H., Li, Z., & Xu, H. (2025). Tissue-Specific Expression of the Porcine DHRS3 Gene and Its Impact on the Proliferation and Differentiation of Myogenic Cells. Animals, 15(8), 1101. https://doi.org/10.3390/ani15081101






