Maternal Social Hierarchy, Morphometric Traits, Live Weight, and Metabolic Status as Related to the Offspring Pre-Weaning Growth in Crossbred Dairy Goats
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Note
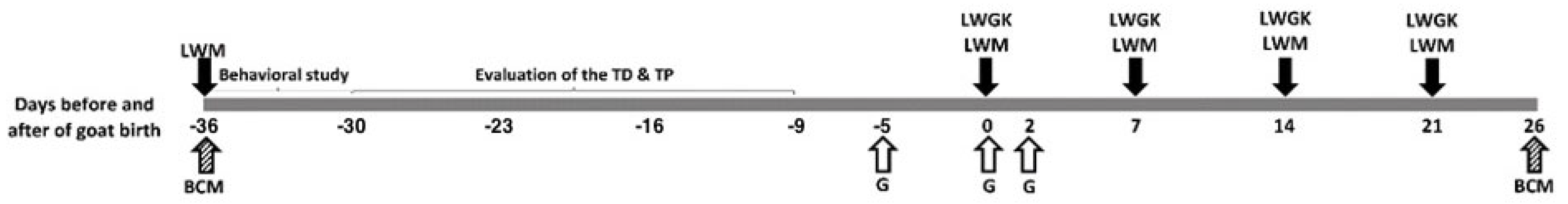
2.2. Location of the Study Area, Animals, and Management
2.3. Behavioral Study to Define the Maternal Social Rank
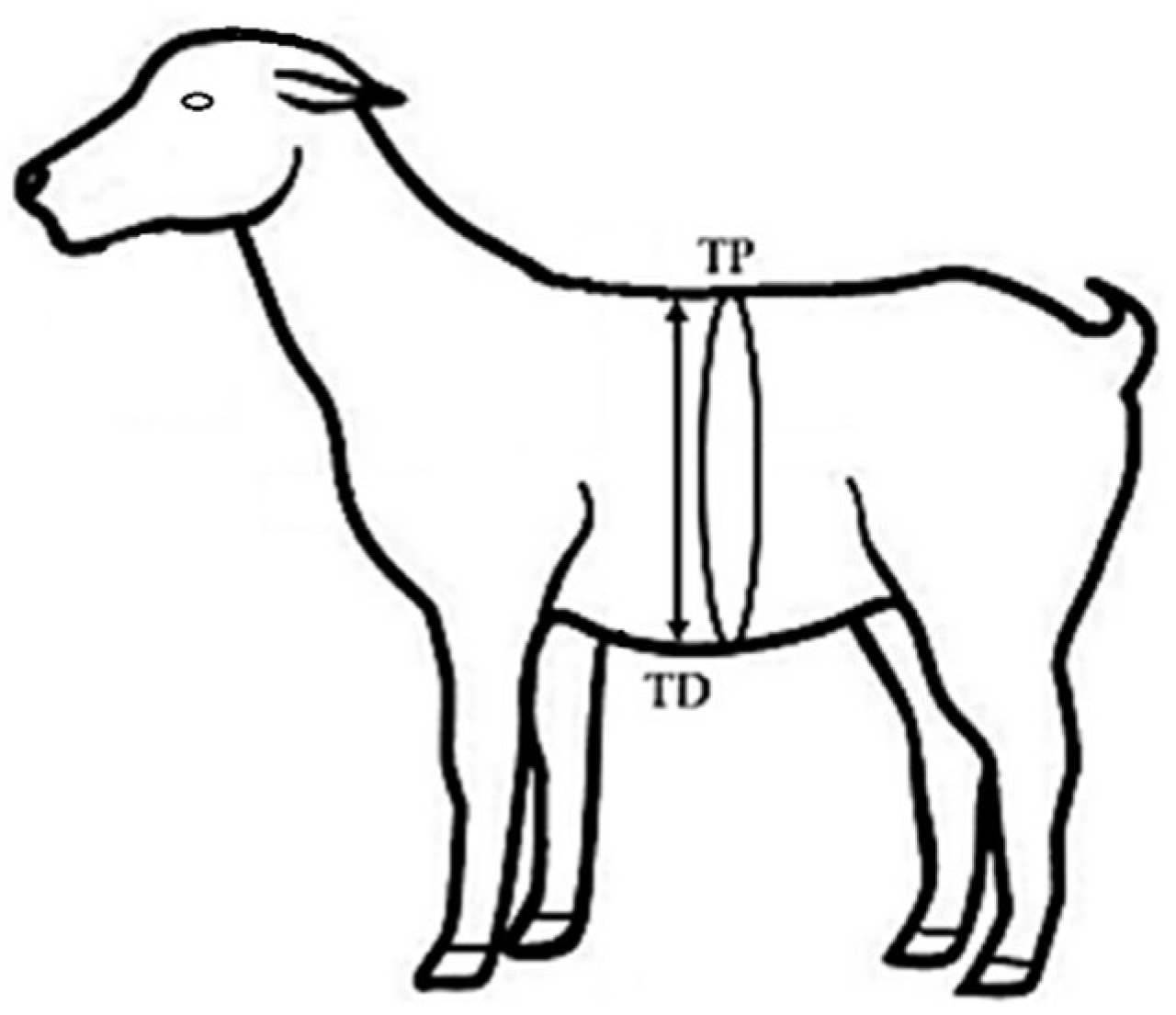
2.4. Quantification of the Response Variables According to Maternal Social Rank
2.5. Maternal Serum Glucose Levels and Recording of the Pre-Weaning Growth of the Offspring
2.6. Statistical Analyses
3. Results
3.1. Social Rank, Time, and Doe’s Live Weight: Simple Effects
3.2. Social Rank, Time, and Doe’s Live Weight Across Time: Interaction Effects
3.3. Social Rank, Time, and Doe’s Body Condition Score Across Time: Interaction Effects
3.4. Social Rank, Time, and Doe’s Morphometry: Simple Effects
3.5. Social Rank, Time, and Doe’s Morphometry Across Time: Interaction Effects
3.6. Social Rank, Time, and Doe’s Serum Glucose Concentration Across Time: Interaction Effects
3.7. Maternal Social Rank, Time, and Kid’s Pre-Weaning Live Weight Across Time: Interaction Effects
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LW | Live weight |
| BCS | Body condition score |
| TD | Thoracic diameter |
| TP | Thoracic perimeter |
| GLUC | Serum glucose content |
| LWGK | Kid’s live weight |
| SR | Social rank |
| HSR | High social rank |
| LSR | Low social rank |
| LWM | Live weight of the mother |
References
- Pitcher, B.J.; Briefer, E.F.; Baciadonna, L.; McElligott, A.G. Cross-modal recognition of familiar conspecifics in goats. R. Soc. Open Sci. 2017, 4, 160346. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, F.; Serrapica, M.; Braghieri, A.; Claps, S.; Serrapica, F.; De Rosa, G. Can we monitor adaptation of juvenile goats to a new social environment through continuous qualitative behaviour assessment? PLoS ONE 2018, 13, e0200165. [Google Scholar] [CrossRef]
- Zobel, G.; Neave, H.W.; Webster, J. Understanding natural behavior to improve dairy goat (Capra hircus) management systems. Transl. Anim. Sci. 2019, 3, 212–224. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Zuñiga, M.S.; Meza-Herrera, C.A.; Calderón-Leyva, G.; López-Villalobos, N.; Navarrete-Molina, C.; Bustamante-Andrade, J.A.; Sifuentes-Lamónt, P.I.; Flores-Salas, J.M.; Véliz-Deras, F.G. Interactions between Social Hierarchy and Some Udder Morphometric Traits upon Colostrum and Milk Physicochemical Characteristics in Crossbred Dairy Goats. Agriculture 2022, 12, 734. [Google Scholar] [CrossRef]
- Miranda-de la Lama, G.C.; Mattiello, S. The importance of social behaviour for goat welfare in livestock farming. Small Rumin. Res. 2010, 90, 1–10. [Google Scholar] [CrossRef]
- Rault, J.L. Be kind to others: Prosocial behaviours and their implications for animal welfare. Appl. Anim. Behav. Sci. 2019, 210, 113–123. [Google Scholar] [CrossRef]
- Di Virgilio, A.; Morales, J.M. Towards evenly distributed grazing patterns: Including social context in sheep management strategies. PeerJ 2016, 4, e2152. [Google Scholar] [CrossRef]
- Hussein, A.N.; Al-Marashdeh, O.; Bryant, R.H.; Edwards, G. Relationship between social dominance and milk production of dairy cows grazing pasture. New Zealand Soc. Anim. Prod. 2016, 76, 69–72. [Google Scholar]
- Bica, G.S.; Pinheiro Machado Filho, L.C.; Teixeira, D.L.; De Sousa, K.T.; Hötzel, M.J. Time of grain supplementation and social dominance modify feeding behavior of heifers in rotational grazing systems. Front. Vet. Sci. 2020, 7, 61. [Google Scholar] [CrossRef]
- Zuñiga-Garcia, S.; Meza-Herrera, C.A.; Mendoza-Cortina, A.; Otal, J.; Perez-Marín, C.; Lopez-Flores, N.M.; Carrillo, E.; Calderon-Leyva, G.; Gutierrez-Guzman, U.N.; Véliz-Deras, F.G. Effect of social rank upon estrus induction and some reproductive outcomes in anestrus goats treated with progesterone + eCG. Animals 2020, 10, 1125. [Google Scholar] [CrossRef]
- Zuñiga-Garcia, S.; Meza-Herrera, C.A.; Mendoza-Cortina, A.; Perez-Marin, C.; Lopez-Flores, N.M.; Guillén-Muñoz, J.M.; Arellano-Rodriguez, G.; Gutierrez-Guzman, U.N.; Bustamante-Andrade, J.A.; Luna-Orozco, J.R.; et al. Does Size Matters? Relationships among Social Dominance and Some Morphometric Traits upon Out-of-Season Reproductive Outcomes in Anestrus Dairy Goats Treated with P4+ eCG. Biology 2020, 9, 354. [Google Scholar] [CrossRef] [PubMed]
- Clark, S.; Mora-García, M.B.M. A 100-year review: Advances in goat milk research. J. Dairy Sci. 2017, 100, 10026–10044. [Google Scholar] [CrossRef] [PubMed]
- Isidro-Requejo, L.M.; Meza-Herrera, C.A.; Pastor-López, F.J.; Maldonado, J.A.; Salinas-González, H. Physicochemical characterization of goat milk produced in the Comarca Lagunera, Mexico. Anim. Sci. J. 2019, 90, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Koluman, N.; Silanikove, N. The advantages of goats for future adaptation to climate change: A conceptual overview. Small Rumin. Res. 2018, 163, 34–38. [Google Scholar] [CrossRef]
- Meza-Herrera, C.A.; Vergara-Hernández, H.P.; Paleta-Ochoa, A.; Álvarez-Ruíz, A.R.; Veliz-Deras, F.G.; Arellano-Rodriguez, G.; Rosales-Nieto, C.A.; Macias-Cruz, U.; Rodriguez-Martinez, R.; Carrillo, E. Glutamate supply reactivates ovarian function while increases serum insulin and triiodothyronine concentrations in Criollo × Saanen-alpine yearlings’ goats during the anestrous season. Animals 2020, 10, 234. [Google Scholar] [CrossRef]
- Secretaría de Agricultura, G. Desarrollo Rural, Pesca y Alimentación, NORMA Oficial Mexicana NOM-062-ZOO-1999, Especificaciones Técnicas Para la Producción, Cuidado y Uso de los Animales de Laboratorio. Available online: https://www.gob.mx/senasica/documentos/nom-062-zoo-1999 (accessed on 13 January 2024).
- American Dairy Science Association. Guide for the Care and Use of Agricultural Animals in Agricultural Research and Teaching, 4th ed.; American Dairy Science Association: Champaign, IL, USA, 2020. [Google Scholar]
- National Academy of Medicine. Guide for the Care and Use of Laboratory Animals, 1 ed.; National Academy of Medicine: México City, Mexico, 2010. [Google Scholar]
- Instituto Nacional de Estadística y Geografía México en Cifras. Available online: https://www.inegi.org.mx/app/areasgeograficas/ (accessed on 9 February 2024).
- National Research Council. Nutrient Requirements of Small Ruminants: Sheep, Goats, Cervids, and New World Camelids; The National Academies Press: Washington, DC, USA, 2007. [Google Scholar]
- Walkden-Brown, S.W.; Restall, B.J.; Scaramuzzi, R.J.; Martin, G.B.; Blackberry, M.A. Seasonality in male Australian cashmere goats: Long term effects of castration and testosterone or oestradiol treatment on changes in LH, FSH and prolactin concentrations, and body growth. Small Rumin. Res. 1997, 26, 239–252. [Google Scholar] [CrossRef]
- Miranda-de la Lama, G.C.; Sepúlveda, W.S.; Montaldo, H.H.; María, G.A.; Galindo, F. Social strategies associated with identity profiles in dairy goats. Appl. Snimal Behav. Sci. 2011, 134, 48–55. [Google Scholar] [CrossRef]
- Alvarez, L.; Martin, G.B.; Galindo, F.; Zarco, L.A. Social dominance of female goats affects their response to the male effect. Appl. Anim. Behav. Sci. 2003, 84, 119–126. [Google Scholar] [CrossRef]
- Alvarez, L.; Zarco, L.; Galindo, F.; Blache, D.; Martin, G.B. Social rank and response to the “male effect” in the Australian Cashmere goat. Anim. Reprod. Sci. 2007, 102, 258–266. [Google Scholar] [CrossRef]
- Alvarez, L.; Ramos, A.L.; Zarco, L. The ovulatory and LH responses to the male effect in dominant and subordinate goats. Small Rumin. Res. 2009, 83, 29–33. [Google Scholar] [CrossRef]
- Andersen, I.L.; Bøe, K.E. Resting pattern and social interactions in goats—The impact of size and organisation of lying space. Appl. Anim. Behav. Sci. 2007, 108, 89–103. [Google Scholar] [CrossRef]
- Barroso, F.G.; Alados, C.L.; Boza, J. Social hierarchy in the domestic goat: Effect on food habits and production. Appl. Anim. Behav. Sci. 2000, 7, 35–53. [Google Scholar] [CrossRef] [PubMed]
- Côté, S. Dominance hierarchies in female mountain goats: Stability, aggressiveness and determinants of rank. Behaviour 2000, 137, 1541–1566. [Google Scholar] [CrossRef]
- Côté, S.D.; Festa-Bianchet, M. Reproductive success in female mountain goats: The influence of age and social rank. Anim. Behav. 2001, 62, 173–181. [Google Scholar] [CrossRef]
- Fournier, F.; Festa-Bianchet, M. Social dominance in adult female mountain goats. Anim. Behav. 1995, 49, 1449–1459. [Google Scholar] [CrossRef]
- Santiago-Moreno, J.; Gómez-Brunet, A.; Toledano-Díaz, A.; Pulido-Pastor, A.; López-Sebastián, A. Social dominance and breeding activity in Spanish ibex (Capra pyrenaica) maintained in captivity. Reprod. Fertil. Dev. 2007, 19, 436–442. [Google Scholar] [CrossRef]
- Ungerfeld, R.; Correa, O. Social dominance of female dairy goats influences the dynamics of gastrointestinal parasite eggs. Appl. Anim. Behav. Sci. 2007, 105, 249–253. [Google Scholar] [CrossRef]
- Alvarez, L.; Arvizu, R.R.; Luna, J.A.; Zarco, L.A. Social ranking and plasma progesterone levels in goats. Small Rumin. Res. 2010, 90, 161–164. [Google Scholar] [CrossRef]
- Ungerfeld, R. Sexual behavior of medium-ranked rams toward non-estrual ewes is stimulated by the presence of low-ranked rams. J. Vet. Behav. 2012, 7, 84–87. [Google Scholar] [CrossRef]
- Ungerfeld, R.; Lacuesta, L. Social rank during pre-pubertal development and reproductive performance of adult rams. Anim. Reprod. Sci. 2010, 121, 101–105. [Google Scholar] [CrossRef]
- Boissy, A.; Manteuffel, G.; Jensen, M.B.; Moe, R.O.; Spruijt, B.; Keeling, L.J.; Winckler, C.; Forkman, B.; Dimitrov, I.; Langbein, J.; et al. Assessment of positive emotions in animals to improve their welfare. Physiol. Behav. 2007, 92, 375–397. [Google Scholar] [CrossRef]
- Keeling, L.J.; Gonyou, H.W. Social Behaviour in Farm Animals. Available online: http://sherekashmir.informaticspublishing.com/356/1/9780851993973.pdf (accessed on 9 February 2024).
- Rault, J.L. Friends with benefits: Social support and its relevance for farm animal welfare. Appl. Anim. Behav. Sci. 2012, 210, 113–123. [Google Scholar] [CrossRef]
- Manousidis, T.; Kyriazopoulos, A.P.; Parissi, Z.M.; Abraham, E.M.; Korakis, G.; Abas, Z. Grazing behavior, forage selection and diet composition of goats in a Mediterranean woody rangeland. Small Rumin. Res. 2016, 145, 142–153. [Google Scholar] [CrossRef]
- Neave, H.W.; Von Keyserlingk, M.A.; Weary, D.M.; Zobel, G. Feed intake and behavior of dairy goats when offered an elevated feed bunk. J. Dairy Sci. 2018, 101, 3303–3310. [Google Scholar] [CrossRef] [PubMed]
- Hartley, A.; Shrader, A.M.; Chamaillé-Jammes, S. Can intrinsic foraging efficiency explain dominance status? A test with functional response experiments. Oecologia 2019, 189, 105–110. [Google Scholar] [CrossRef]
- Jena, S.; Malik, D.S.; Kaswan, S.; Sharma, A.; Kashyap, N.; Singh, U. Relationship of udder morphometry with milk yield and body condition traits in Beetal goats. Indian J. Anim. Sci. 2019, 89, 204–208. [Google Scholar] [CrossRef]
- Keskin, S.; Kor, A.; Karaca, S. Use of factor analysis scores in multiple linear regression model for determining relationships between milk yield and some udder traits in Goats. J. Appl. Anim. Res. 2007, 31, 185–188. [Google Scholar] [CrossRef]
- Susilorini, T.E.; Maylinda, S.; Surjowardojo, P. Importance of body condition score for milk production traits in Peranakan Etawah goats. J. Biol. Agric. Healthc. 2014, 4, 151–157. [Google Scholar]
- Pascual-Alonso, M.; María, G.A.; Sepúlveda, W.S.; Villarroel, M.; Aguayo-Ulloa, L.; Galindo, F.; Miranda-De La Lama, G.C. Identity profiles based on social strategies, morphology, physiology, and cognitive abilities in goats. J. Vet. Behav. 2013, 8, 458–465. [Google Scholar] [CrossRef]
- Castro, N.; Gómez-González, L.A.; Earley, B.; Argüello, A. SUse of clinic refractometer at farm as a tool to estimate the IgG content in goat colostrum. J. Appl. Anim. Res. 2018, 46, 1505–1508. [Google Scholar] [CrossRef]
- Erdil, A.; Erbıyık, H. Selection strategy via analytic hierarchy process: An application for a small enterprise in milk sector. Procedia-Soc. Behav. Sci. 2015, 195, 2618–2628. [Google Scholar] [CrossRef][Green Version]
- Erduran, H.; Dag, B. Comparison of phenotypic and heterotic effects affecting milk yield, composition and udder morphometry of Hair and F1, F2 and G1 generation cross-breeds of Alpine× Hair and Saanen× Hair dairy goats in a semi-intensive system. Trop. Anim. Health Prod. 2022, 54, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, C.P.; Datta, S.; Mandal, D.; Das, A.K.; Roy, D.C.; Roy, A.; Tudu, N.K. Body condition scoring in goat: Impact and significance. J. Entomol. Zool. Stud. 2019, 7, 554–560. [Google Scholar]
- Kessler, E.C.; Bruckmaier, R.M.; Gross, J.J. Immunoglobulin G content and colostrum composition of different goat and sheep breeds in Switzerland and Germany. J. Dairy Sci. 2019, 102, 5542–5549. [Google Scholar] [CrossRef]
- Kessler, E.C.; Bruckmaier, R.M.; Gross, J.J. Comparative estimation of colostrum quality by Brix refractometry in bovine, caprine, and ovine colostrum. J. Dairy Sci. 2021, 104, 2438–2444. [Google Scholar] [CrossRef]
- Ribeiro, S.M.; Brandão, F.G.; do Nascimento, D.M.; Rocha, F.L.; Gomes, d.S.C.M.; Ferreira, D.F.E.; Pereira, d.S.A.; Arrivabene, M.; Vasconcelos, T.C. Use of digital Brix refractometer to estimate total protein levels in Santa Inês ewes’ colostrum and lambs’ blood serum. Small Rumin. Res. 2020, 182, 78–80. [Google Scholar]
| Behavior | Description |
|---|---|
| Hitting | When an individual hits another individual with its head/horns |
| Threatening | When an individual makes a threatening movement with its head/horns or its trunk and tries to hit another individual |
| Pushing | When an individual pushes another with its body but without hitting and displaces it from a certain place |
| Chasing | When an individual chases another around the pen |
| Fleeing | When an individual moves rapidly away from another |
| Evading | When an individual avoids another’s threats or presence |
| Variable | Social Rank | Days Regarding Kidding Date 1 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| LSR | HSR | s.e. | −36 | 0 | +07 | +14 | +21 | s.e. 3 | |
| LWM (kg) | 49.3 b 2 | 55.7 a | 1.8 | 52.6 ab | 58.8 a | 51.5 ab | 50.2 ab | 49.1 b | 2.9 |
| Days Regarding Kidding Date 1 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| −36 | 0 | 07 | 14 | 21 | s.e. 2 | ||||||
| Variable | LSR | HSR | LSR | HSR | LSR | HSR | LSR | HSR | LSR | HSR | |
| LWM (kg) | 49.5 cdef | 55.8 b | 55.7 b | 62.0 a | 48.5 def | 54.6 bc | 47.3 ef | 53.2 bcd | 45.3 f | 52.9 bcde | 2.0 |
| Variable | Initial 1 | Final 2 | ||
|---|---|---|---|---|
| LSR | HSR | LSR | HSR | |
| BCS (units) | 2.2 ± 0.14 a | 2.4 ± 0.13 a | 1.8 ± 0.14 b | 1.8 ± 0.13 b |
| Variable | Social Rank | Days Regarding Kidding Date 1 | ||||||
|---|---|---|---|---|---|---|---|---|
| LSR | HSR | s.e. | −30 | −23 | −16 | −09 | s.e. 3 | |
| TD (cm) | 33.9 b 2 | 35.7 a | 0.6 | 31.6 b | 34.9 | 36.0 a | 36.8 a | 0.8 |
| TP (cm) | 108.4 a | 112.6 a | 1.4 | 107.2 b | 109.0 ab | 112.1 ab | 114.4 a | 2.3 |
| Variable | Days Regarding Kidding Date 1 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| −30 | −23 | −16 | −09 | s.e 3 | |||||
| LSR | HSR | LSR | HSR | LSR | HSR | LSR | HSR | ||
| TD (cm) | 30.8 e 2 | 32.3 de | 33.8 cd | 35.9 ab | 35.1 c | 36.9 ab | 35.1 c | 36.9 ab | 0.6 |
| TP (cm) | 105.1 e | 109.0 cd | 106.7 de | 111.2 bc | 109.5 bc | 114.3 ab | 109.5 cde | 114.3 ab | 1.7 |
| Variable | Days Regarding Kidding Date 1 | ||||||
|---|---|---|---|---|---|---|---|
| −05 | 0 | +02 | s.e 2 | ||||
| LSR | HSR | LSR | HSR | LSR | HSR | ||
| GLU (mg dL−1) | 39.4 b | 37.1 b | 101.5 a | 116.0 a | 43.1 b | 41.5 b | 3.3 |
| Variable | Days Regarding Kidding Date 1 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 07 | 14 | 21 | s.e 2 | |||||
| LSR | HSR | LSR | HSR | LSR | HSR | LSR | HSR | ||
| LWGK (kg) | 3.5 e | 3.3 e | 4.8 d | 4.5 d | 6.1 c | 5.7 c | 7.6 a | 6.8 b | 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flores-Salas, J.M.; Castillo-Zuñiga, M.S.; Meza-Herrera, C.A.; Calderon-Leyva, M.G.; Bustamante-Andrade, J.A.; Santiago-Miramontes, M.d.l.A.d.; Moreno-Avalos, S.; Alvarado-Espino, A.S.; Contreras-Villarreal, V.; Véliz-Deras, F.G. Maternal Social Hierarchy, Morphometric Traits, Live Weight, and Metabolic Status as Related to the Offspring Pre-Weaning Growth in Crossbred Dairy Goats. Animals 2025, 15, 1100. https://doi.org/10.3390/ani15081100
Flores-Salas JM, Castillo-Zuñiga MS, Meza-Herrera CA, Calderon-Leyva MG, Bustamante-Andrade JA, Santiago-Miramontes MdlAd, Moreno-Avalos S, Alvarado-Espino AS, Contreras-Villarreal V, Véliz-Deras FG. Maternal Social Hierarchy, Morphometric Traits, Live Weight, and Metabolic Status as Related to the Offspring Pre-Weaning Growth in Crossbred Dairy Goats. Animals. 2025; 15(8):1100. https://doi.org/10.3390/ani15081100
Chicago/Turabian StyleFlores-Salas, Jessica Maria, Ma Silvia Castillo-Zuñiga, Cesar Alberto Meza-Herrera, Ma Guadalupe Calderon-Leyva, Jorge Arturo Bustamante-Andrade, Ma de los Angeles de Santiago-Miramontes, Silvestre Moreno-Avalos, Alan Sebastian Alvarado-Espino, Viridiana Contreras-Villarreal, and Francisco Gerardo Véliz-Deras. 2025. "Maternal Social Hierarchy, Morphometric Traits, Live Weight, and Metabolic Status as Related to the Offspring Pre-Weaning Growth in Crossbred Dairy Goats" Animals 15, no. 8: 1100. https://doi.org/10.3390/ani15081100
APA StyleFlores-Salas, J. M., Castillo-Zuñiga, M. S., Meza-Herrera, C. A., Calderon-Leyva, M. G., Bustamante-Andrade, J. A., Santiago-Miramontes, M. d. l. A. d., Moreno-Avalos, S., Alvarado-Espino, A. S., Contreras-Villarreal, V., & Véliz-Deras, F. G. (2025). Maternal Social Hierarchy, Morphometric Traits, Live Weight, and Metabolic Status as Related to the Offspring Pre-Weaning Growth in Crossbred Dairy Goats. Animals, 15(8), 1100. https://doi.org/10.3390/ani15081100







