Assessment of Fish Biomass and Distribution in a Nuclear Power Plant’s Water Intake Zone Using Acoustic and Trawl Methods
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Survey Area and Station Design
2.2. Biological Sample Data Analysis
2.3. Acoustic Survey Data Collection
2.4. Acoustic Survey Data Analysis
3. Results
3.1. Fish Resource Trawl Survey
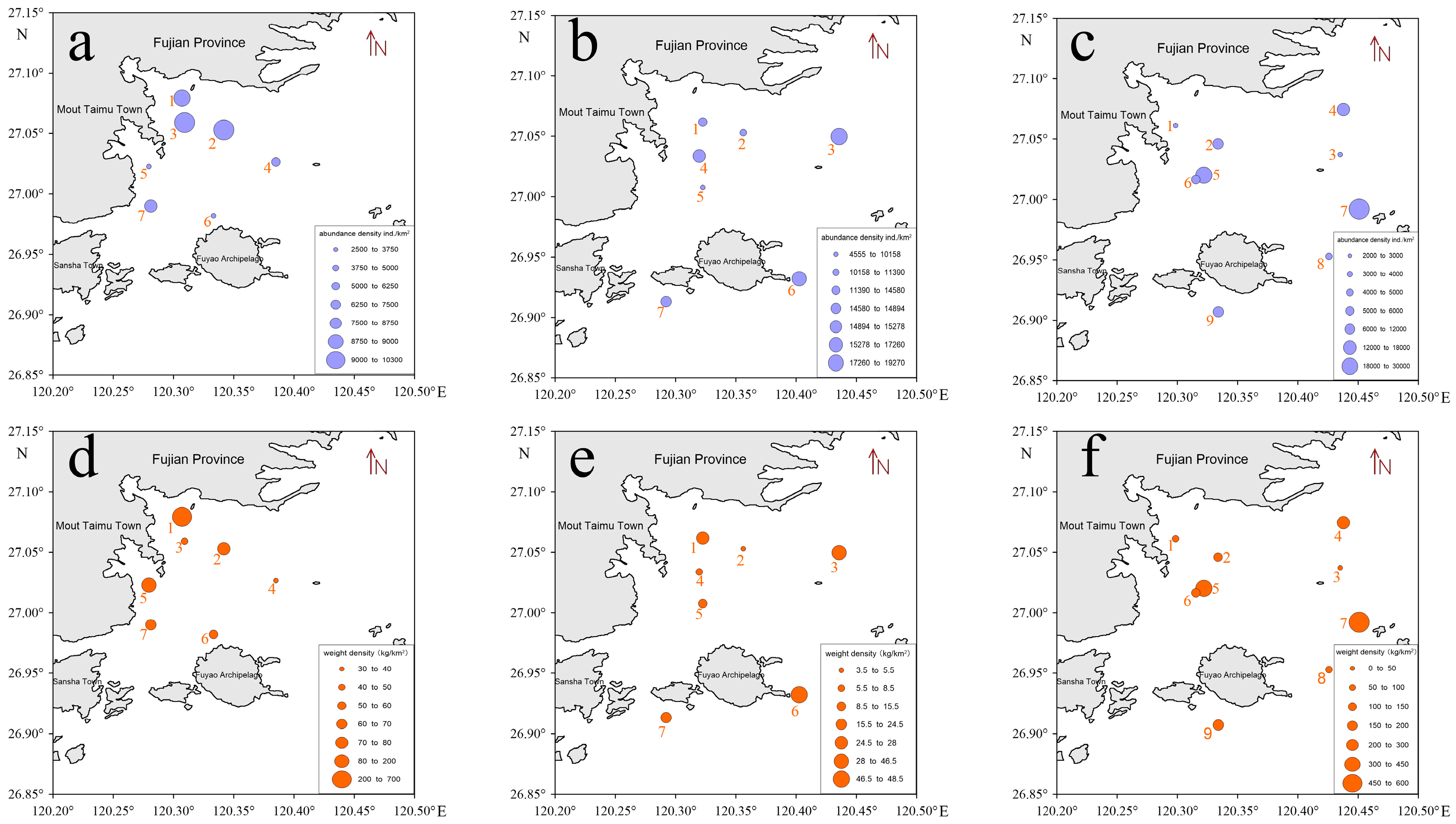
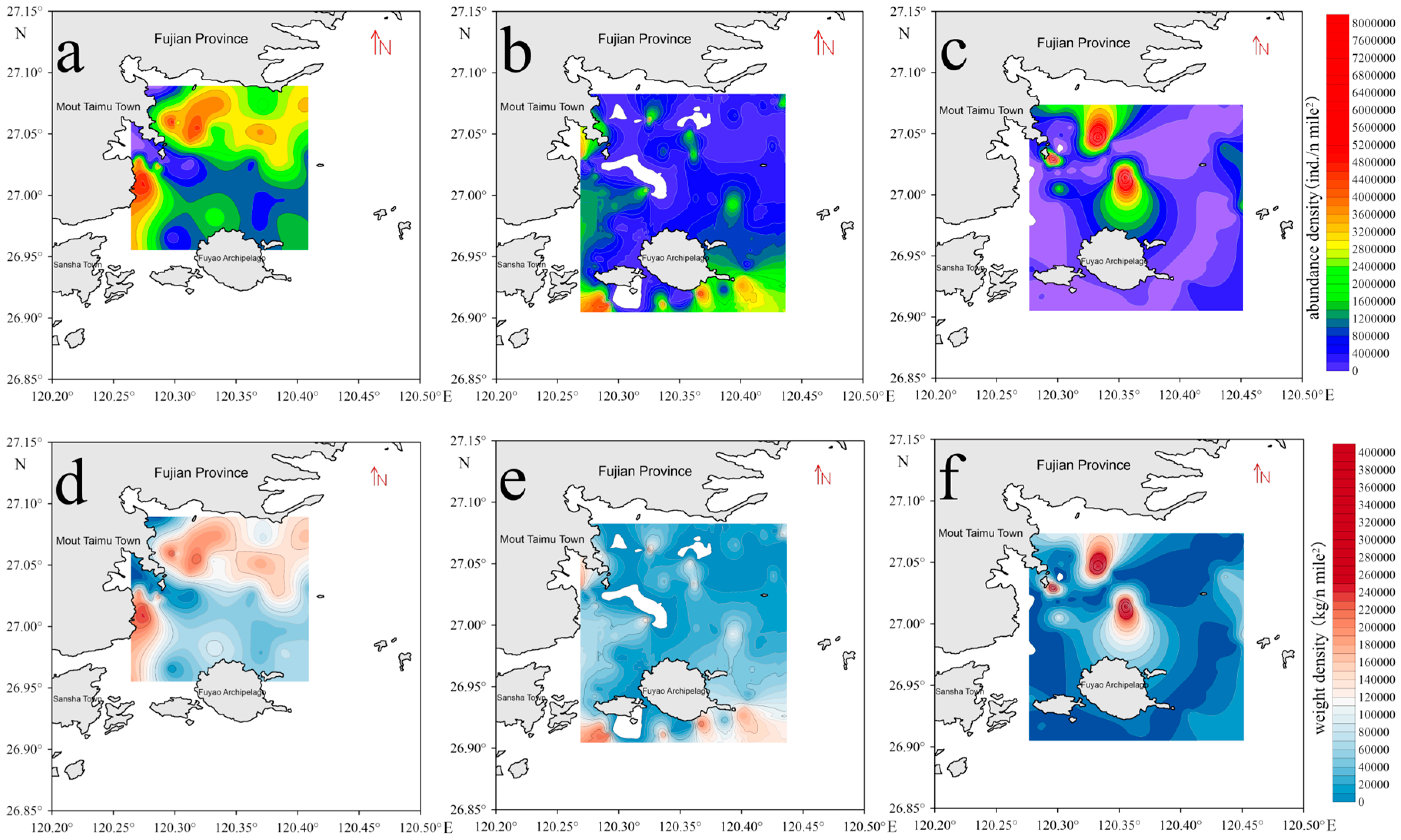
3.1.1. Spatiotemporal Distribution of Fish Resource Abundance Density
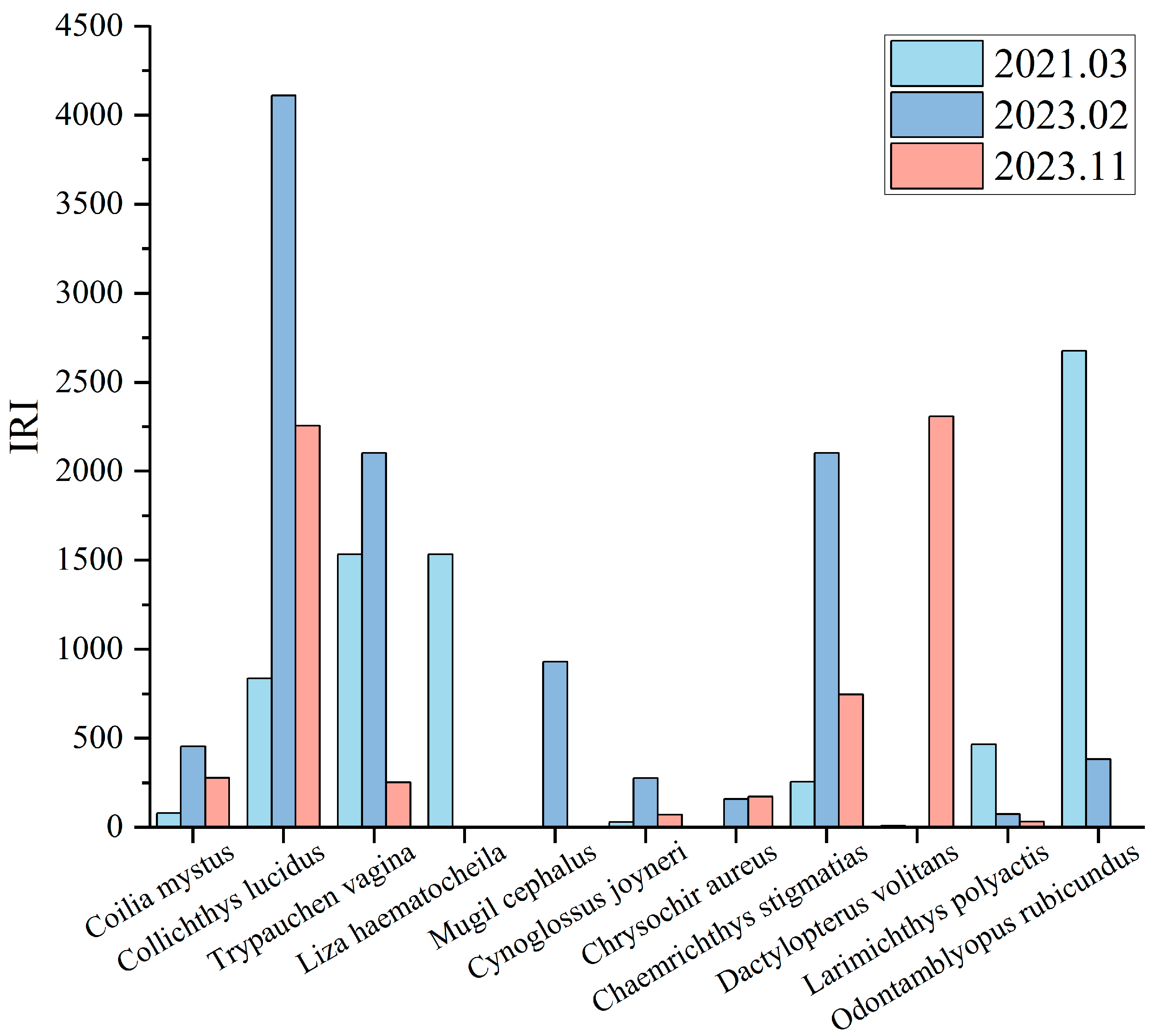
3.1.2. Dominant Species Composition
3.2. Acoustic Survey Investigation
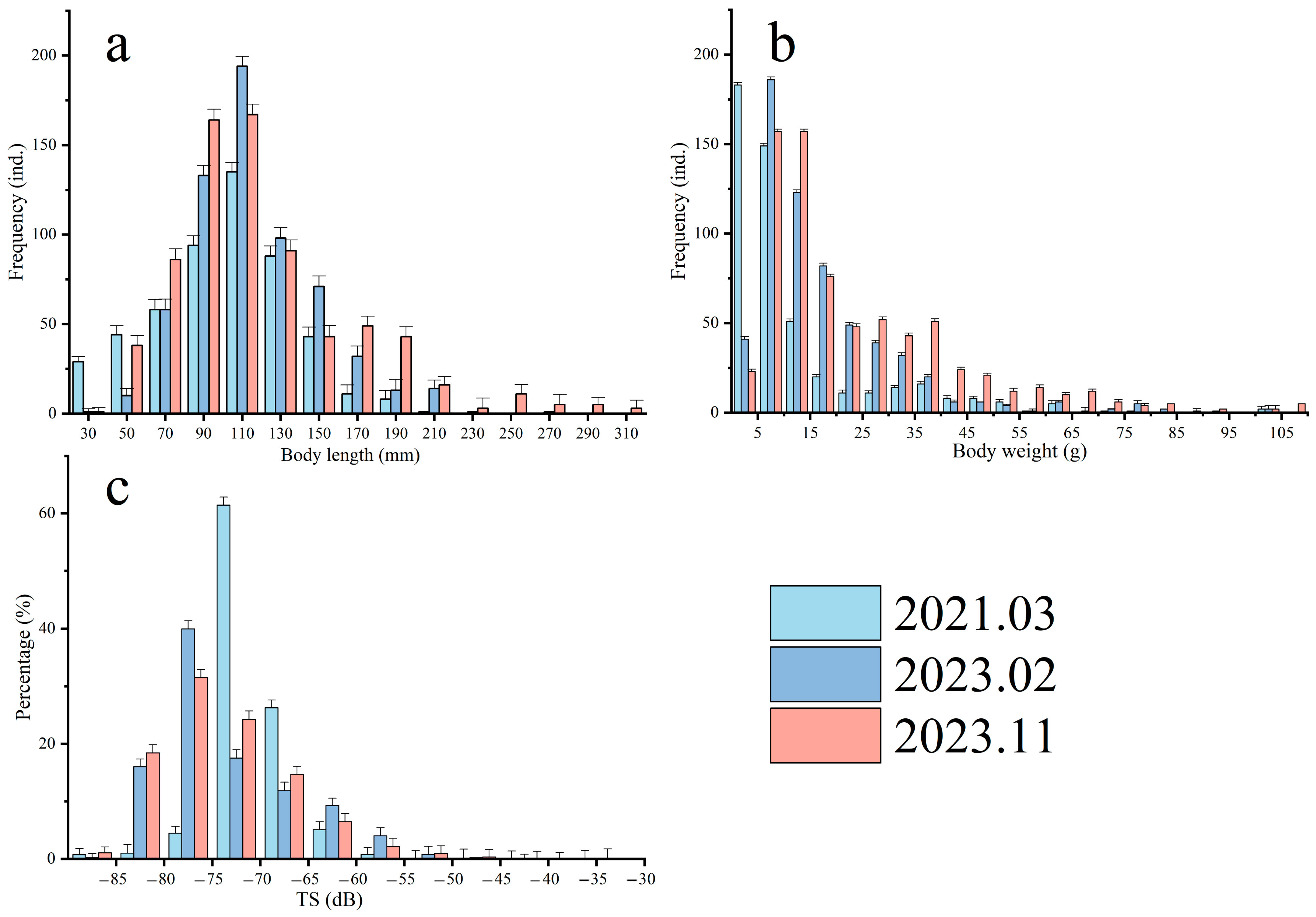
3.2.1. Distribution of Fish Target Strength and Body Length
3.2.2. Acoustic Assessment of Fish Resources
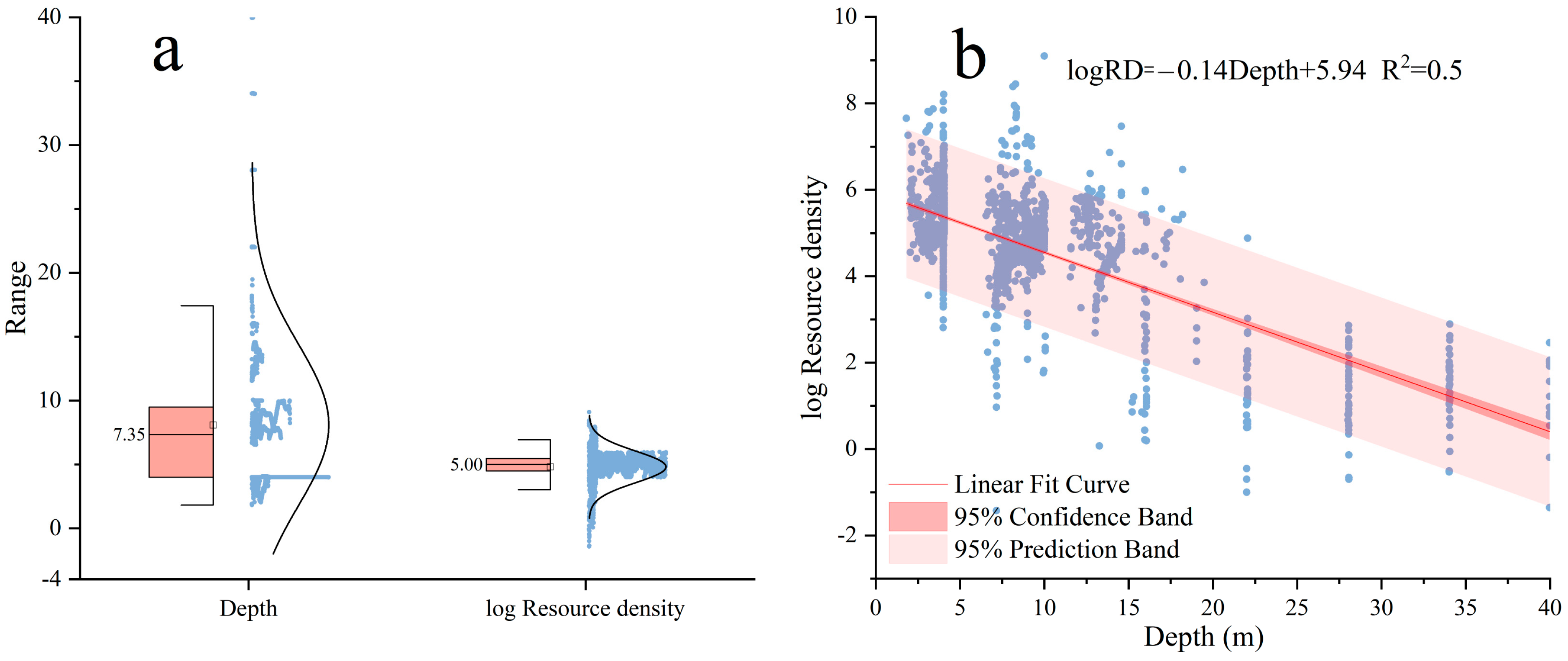
3.2.3. Influence of Water Depth on Fish Resource Density
4. Discussion
4.1. Spatiotemporal Distribution of Species
4.2. Diversity and Variation Analysis of Dominant Species
4.3. Acoustic Evaluation of Fish Target Strength and Resources
4.4. Error Sources of Trawl and Underwater Acoustic Techniques
4.5. Impact of the Research Results on the Safety of the Cooling Water Intake System of Nuclear Power Plants
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wenqi, S.; Shuai, Y.; Lina, S.; Yujin, Z.; Ning, X.; Xueqin, L.; Yuxian, M. A preliminary study on numerical simulation warning of the risk of ice blocking in water intake of the Hongyanhe Nuclear Power Plant. Mar. Sci. Bull. 2020, 39, 439–446. [Google Scholar]
- Lv, Z.; Zhang, H.; Liang, J.; Zhao, T.; Xu, Y.; Lei, Y. Microalgae removal technology for the cold source of nuclear power plant: A review. Mar. Pollut. Bull. 2022, 183, 114087. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Xing, X.; Lin, C.; Xiong, M.; Zhang, X.; Zhang, Z.; Wu, S.; Zhang, S.; Shi, Y.; Wu, Z.; et al. Advances in the investigation and risk assessment of cold source blockages in nuclear power plants in China. Nucl. Eng. Des. 2024, 420, 112998. [Google Scholar] [CrossRef]
- Ni, J.; Chen, H.; Dai, L.; Ji, J.; Lin, J.; Yu, T. Early warning and monitoring of the safety risk of coastal nuclear power plant cold source under the stress from Phaeocystis globosa blooms. Mar. Freshw. Res. 2024, 75, MF23179. [Google Scholar] [CrossRef]
- Lu, C.; Xiong, M.; Xing, X.; Zhang, Z.; Zhang, X.; Song, Y.; Wu, Z.; Wu, Y. Acoustic assessment of Collichthys Lucidus resources in the winter of 2023 in the cooling water area of Ningde Nuclear Power Plant. Fish. Inf. Strategy 2024, 39, 39–48. [Google Scholar] [CrossRef]
- Cai, L.; Rao, Y.; Zhao, X.; Yang, D.; Lin, J.; Fu, S.; Zhou, X. Two risk indices for benthic macrofauna entrapment evaluation on the water intake systems in coastal nuclear power plants. J. Appl. Oceanogr. 2022, 41, 655–662. [Google Scholar] [CrossRef]
- Nie, H.; Tian, X.; Yang, L.; Kang, J.; Wang, X.; Lu, H.; Guan, C.; Zhou, C. Risk biological monitoring of cold source water intake area in eastern Liaodong Bay nuclear power plant of 2021. Mar. Environ. Sci. 2022, 41, 430–436. [Google Scholar]
- Xing, X.F.; Zhang, Z.L.; Tang, J.M.; Guo, T.X.; Wang, S.; Tao, Z. Study on the Issue about Water Intake Blockage by Marine Organism in Nuclear Power Plant. Nucl. Saf. 2021, 20, 103–109. [Google Scholar] [CrossRef]
- Tang, Z.; Cheng, F.; Jin, X.; Sun, L.; Bao, R.; Liu, Y. An automatic marine-organism monitoring system for the intake water of the nuclear power plant. Ann. Nucl. Energy 2017, 109, 208–211. [Google Scholar] [CrossRef]
- Wu, Q.; He, G.; Wen, H.; Lin, X.; He, S.; Chang, X. Study on Cooling Water Source Seawater Filtration System of Nuclear Power Plant. IOP Conf. Ser. Earth Environ. Sci. 2020, 514, 042014. [Google Scholar] [CrossRef]
- Andreychev, A.V. A New Methodology for Studying the Activity of Underground Mammals. Biol. Bull. 2018, 45, 937–943. [Google Scholar] [CrossRef]
- Prosekov, A.; Kuznetsov, A.; Rada, A.; Ivanova, S. Methods for Monitoring Large Terrestrial Animals in the Wild. Forests 2020, 11, 808. [Google Scholar] [CrossRef]
- Brehmer, P.; Lafont, T.; Georgakarakos, S.; Josse, E.; Gerlotto, F.; Collet, C. Omnidirectional multibeam sonar monitoring: Applications in fisheries science. Fish Fish. 2006, 7, 165–179. [Google Scholar] [CrossRef]
- Gerlotto, F.M.; Brehmer, P.A.; Fernandes, P.G.; Reid, D.G.; Copland, P.; Georgakarakos, S.; Paramo, J. Application of multibeam sonar in marine ecology and fisheries research: New fields and limitations. J. Acoust. Soc. Am. 2003, 114, 2299. [Google Scholar] [CrossRef]
- Peña, H.; Macaulay, G.J.; Ona, E.; Vatnehol, S.; Holmin, A.J. Estimating individual fish school biomass using digital omnidirectional sonars, applied to mackerel and herring. ICES J. Mar. Sci. 2021, 78, 940–951. [Google Scholar] [CrossRef]
- Van Hoeck, R.V.; Paxton, A.B.; Bohnenstiehl, D.R.; Taylor, J.C.; Fodrie, F.J.; Peterson, C.H. Passive acoustic monitoring complements traditional methods for assessing marine habitat enhancement outcomes. Ecosphere 2021, 12, e03840. [Google Scholar] [CrossRef]
- Mizuno, K.; Asada, A.; Ban, S.; Uehara, Y.; Ishida, T.; Okuda, N. Validation of a high-resolution acoustic imaging sonar method by estimating the biomass of submerged plants in shallow water. Ecol. Inform. 2018, 46, 179–184. [Google Scholar] [CrossRef]
- Faulkner, A.V.; Maxwell, S.L. Adult Sockeye Salmon Assessment in a Tidal, Turbid River: A Comparison of Sonar and Test Fishing Methods. N. Am. J. Fish. Manag. 2020, 40, 852–864. [Google Scholar] [CrossRef]
- Kemper, J.H.; Raat, A.J.P. Hydroacoustic assessment of the fish stock in Theodorushaven, a small Dutch harbour. Fish. Manag. Ecol. 1997, 4, 63–71. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, G.; Chen, Z.; Yu, J.; Fan, J.; Qiu, Y. Acoustic estimation of fishery resources in southern continental shelf of Nansha area. S. China Fish. Sci. 2015, 11, 1–10. [Google Scholar]
- Linke, S.; Gifford, T.; Desjonquères, C. Six steps towards operationalising freshwater ecoacoustic monitoring. Freshw. Biol. 2020, 65, 1–6. [Google Scholar] [CrossRef]
- Wang, X.; Li, Q.; Yin, J.; Han, X.; Hao, W. An Adaptive Denoising and Detection Approach for Underwater Sonar Image. Remote Sens. 2019, 11, 396. [Google Scholar] [CrossRef]
- Wu, J.; Guo, H. A Method for Sonar Image Segmentation Based on Combination of MRF and Region Growing. In Proceedings of the 2015 Fifth International Conference on Communication Systems and Network Technologies, Gwalior, India, 4–6 April 2015; pp. 457–460. [Google Scholar]
- Yang, H.; Li, L.; Li, G. A New Denoising Method for Underwater Acoustic Signal. IEEE Access 2020, 8, 201874–201888. [Google Scholar] [CrossRef]
- Mello, L.G.S.; Rose, G.A. The acoustic dead zone: Theoretical vs. empirical estimates, and its effect on density measurements of semi-demersal fish. ICES J. Mar. Sci. 2009, 66, 1364–1369. [Google Scholar] [CrossRef]
- Scalabrin, C.; Marfia, C.; Boucher, J. How much fish is hidden in the surface and bottom acoustic blind zones? ICES J. Mar. Sci. 2009, 66, 1355–1363. [Google Scholar] [CrossRef]
- GB/T 12763.6-2007; Specifications for Oceanographic Survey—Part 6: Marine Biological Survey. National Standardization Management Committee of China: Beijing, China, 2007.
- Perpetua, M.; Gorospe, J.G.; Torres, M.A.; Demayo, C.G. Diet composition based on stomach content of the Streaked spinefoot (Siganus javus) from three coastal bays in Mindanao, Philippines. AES Bioflux 2013, 5, 49–61. [Google Scholar]
- Huang, L.-M.; Li, J.; Zhang, Y.-Z.; Xie, Y.-J.; Liu, Q.-D.; Jin, X.-S. Current fishery resource assessment in the Minjiang River Estuary and its neighboring waters. J. Trop. Oceanogr. 2010, 29, 142–148. [Google Scholar]
- Chen, G.; Li, N.; Chen, P.; Li, Y.; Yu, J.; Li, X. Target strength measurements of pearl-spotted spinefoot (Siganus oramin) in the South China Sea. J. Fish. Sci. China 2010, 17, 1293–1299. [Google Scholar]
- Zhang, J.; Jiang, Y.; Chen, Z.; Gong, Y.; Chen, G. Preliminary study on the nautical area scattering coefficient and distribution of mesopelagic fish species in the central-southern part of the South China Sea. J. Fish. Sci. China 2017, 24, 120–135. [Google Scholar] [CrossRef]
- Zhu, Z.; Tong, J.; Xue, M.; Qiu, C.; Lyu, S.; Liu, B. Investigations on Target Strength Estimation Methods: A Case Study of Chub Mackerel (Scomber japonicus) in the Northwest Pacific Ocean. Fishes 2024, 9, 307. [Google Scholar] [CrossRef]
- Li, B.; Tang, Y.; Sun, J.; Li, P.; Hu, Y.; Zhang, J. Spatial distribution of fisheries resources in Sanmenxia Reservoir in the Yellow River based on an acoustic method. J. Dalian Ocean Univ. 2016, 31, 563–571. [Google Scholar] [CrossRef]
- Shang, X.; Kong, L.; Wu, C. On target strength of several important economic fishes. J. Oceanol. Limnol. 2015, 46, 636–641. [Google Scholar] [CrossRef]
- Dunning, J.; Jansen, T.; Fenwick, A.J.; Fernandes, P.G. A new in-situ method to estimate fish target strength reveals high variability in broadband measurements. Fish. Res. 2023, 261, 106611. [Google Scholar] [CrossRef]
- Kindong, R.; Chen, J.; Dai, L.; Gao, C.; Han, D.; Tian, S.; Wu, J.; Ma, Q.; Tang, J. The effect of environmental conditions on seasonal and inter-annual abundance of two species in the Yangtze River estuary. Mar. Freshw. Res. 2021, 72, 493–506. [Google Scholar] [CrossRef]
- Brazo, A.; Marques, R.; Zimmermann, M.; Aspillaga, E.; Hereu, B.; Saragoni, G.; Mercière, A.; Crec’Hriou, R.; Mercader, M.; Verdoit-Jarraya, M.; et al. Seasonal influence on the bathymetric distribution of an endangered fish within a marine protected area. Sci. Rep. 2021, 11, 13342. [Google Scholar] [CrossRef]
- Berry, T.E.; Saunders, B.J.; Coghlan, M.L.; Stat, M.; Jarman, S.; Richardson, A.J.; Davies, C.H.; Berry, O.; Harvey, E.S.; Bunce, M. Marine environmental DNA biomonitoring reveals seasonal patterns in biodiversity and identifies ecosystem responses to anomalous climatic events. PLoS Genet. 2019, 15, e1007943. [Google Scholar] [CrossRef]
- Fang, W.; Fan, T.; Wang, S.; Yu, X.; Lu, A.; Wang, X.; Zhou, W.; Yuan, H.; Zhang, L. Seasonal changes driving shifts in microbial community assembly and species coexistence in an urban river. Sci. Total Environ. 2023, 905, 167027. [Google Scholar] [CrossRef]
- Park, J.-Y.; Stock, C.A.; Dunne, J.P.; Yang, X.; Rosati, A. Seasonal to multiannual marine ecosystem prediction with a global Earth system model. Science 2019, 365, 284–288. [Google Scholar] [CrossRef]
- Souza, A.T.; Ilarri, M.I.; Timóteo, S.; Marques, J.C.; Martins, I. Assessing the effects of temperature and salinity oscillations on a key mesopredator fish from European coastal systems. Sci. Total Environ. 2018, 640–641, 1332–1345. [Google Scholar] [CrossRef]
- Kindong, R.; Wu, J.; Gao, C.; Dai, L.; Tian, S.; Dai, X.; Chen, J. Seasonal changes in fish diversity, density, biomass, and assemblage alongside environmental variables in the Yangtze River Estuary. Environ. Sci. Pollut. Res. 2020, 27, 25461–25474. [Google Scholar] [CrossRef]
- Ghosh, S.; Matthews, B.; Petchey, O.L. Temperature and biodiversity influence community stability differently in birds and fishes. Nat. Ecol. Evol. 2024, 8, 1835–1846. [Google Scholar] [CrossRef]
- Wang, Z.; Zeng, C.; Cao, L. Mapping the biodiversity conservation gaps in the East China sea. J. Environ. Manag. 2023, 336, 117667. [Google Scholar] [CrossRef] [PubMed]
- DuFour, M.R.; Mayer, C.M.; Qian, S.S.; Vandergoot, C.S.; Kraus, R.T.; Kocovsky, P.M.; Warner, D.M. Inferred fish behavior its implications for hydroacoustic surveys in nearshore habitats. Fish. Res. 2018, 199, 63–75. [Google Scholar] [CrossRef]
- Yao, W.; Liu, H.; Chen, Y.; Zhang, W.; Zhong, Y.; Fan, H.; Li, L.; Bamal, S. Simulating Spawning and Juvenile Rainbow Trout (Oncorhynchus mykiss) Habitat in Colorado River Based on High-Flow Effects. Water 2017, 9, 150. [Google Scholar] [CrossRef]
- Littlefair, J.E.; Hrenchuk, L.E.; Blanchfield, P.J.; Rennie, M.D.; Cristescu, M.E. Thermal stratification and fish thermal preference explain vertical eDNA distributions in lakes. Mol. Ecol. 2021, 30, 3083–3096. [Google Scholar] [CrossRef] [PubMed]
- Do, A.N.T.; Tran, H.D.; Ashley, M. Employing a novel hybrid of GA-ANFIS model to predict distribution of whiting fish larvae and juveniles from tropical estuaries in the context of climate change. Ecol. Inform. 2022, 71, 101780. [Google Scholar] [CrossRef]
- Li, D.; Dorber, M.; Barbarossa, V.; Verones, F. Global characterization factors for quantifying the impacts of increasing water temperature on freshwater fish. Ecol. Indic. 2022, 142, 109201. [Google Scholar] [CrossRef]
- Zhang, C.; Guo, H.; Chen, W. Comparative early growth patterns across four dominant fish species in a marine protected area in the East China Sea. Reg. Stud. Mar. Sci. 2023, 61, 102862. [Google Scholar] [CrossRef]
- Yi, D.; Zhang, X.; Zhang, S.; Gao, S. Population structure of Collichthys lucidus in three fishing grounds in the East sea and the Yellow Sea of China. Ocean Coast. Manag. 2024, 249, 106941. [Google Scholar] [CrossRef]
- Lin, X.P.; Zhu, Z.J.; Li, P.F. Feeding habits of Harpadon nehereus in the East China Sea region. Mar. Fish. 2010, 32, 290–296. [Google Scholar]
- Pan, X.; Cheng, J. Feeding ecology of Harpadon nehereus in areas adjacent to Changjiang River estuary. J. Fish. Sci. China 2011, 18, 1132–1140. [Google Scholar] [CrossRef]
- Hobbs, D.; Bigot, M.; Smith, R.E.W. Rio Doce Acoustic Surveys of Fish Biomass and Aquatic Habitat. Integr. Environ. Assess. Manag. 2020, 16, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, E.K.; Forney, K.A.; Barlow, J. Using paired visual and passive acoustic surveys to estimate passive acoustic detection parameters for harbor porpoise abundance estimates. J. Acoust. Soc. Am. 2017, 141, 219–230. [Google Scholar] [CrossRef]
- Wood, C.M.; Kahl, S.; Chaon, P.; Peery, M.Z.; Klinck, H. Survey coverage, recording duration and community composition affect observed species richness in passive acoustic surveys. Methods Ecol. Evol. 2021, 12, 885–896. [Google Scholar] [CrossRef]
- Chu, D.; Michael Jech, J.; Lavery, A. Inference of geometrical and behavioural parameters of individual fish from echo-trace-analysis. Deep. Sea Res. Part I Oceanogr. Res. Pap. 2003, 50, 515–527. [Google Scholar] [CrossRef]
- Letessier, T.B.; Proud, R.; Meeuwig, J.J.; Cox, M.J.; Hosegood, P.J.; Brierley, A.S. Estimating Pelagic Fish Biomass in a Tropical Seascape Using Echosounding and Baited Stereo-Videography. Ecosystems 2022, 25, 1400–1417. [Google Scholar] [CrossRef]
- Minami, K.; Kita, C.; Shirakawa, H.; Kawauchi, Y.; Shao, H.; Tomiyasu, M.; Iwahara, Y.; Takahara, H.; Kitagawa, T.; Miyashita, K. Acoustic characteristics of a potentially important macroalgae, Sargassum horneri, for coastal fisheries. Fish. Res. 2021, 240, 105955. [Google Scholar] [CrossRef]
- Yan, N.; Mukai, T.; Yamamoto, J.U.N.; Hasegawa, K.; Kudo, N. Acoustic characteristics of three bladderless fishes. J. Acoust. Soc. Am. 2019, 146, 2965. [Google Scholar] [CrossRef]
- Stevens, J.R.; Jech, J.M.; Zydlewski, G.B.; Brady, D.C. Estimating target strength of estuarine pelagic fish assemblages using fisheries survey data. J. Acoust. Soc. Am. 2021, 150, 2553–2565. [Google Scholar] [CrossRef]
- Jůza, T.; Muška, M.; Blabolil, P.; Kočvara, L.; Sajdlová, Z.; Dumpis, J.; Medne, R. Spatial fish distribution in autumn in a shallow mesotrophic lake, assessed by hydroacoustic surveys, trawling, and beach seining. J. Fish Biol. 2024, 104, 1525–1536. [Google Scholar] [CrossRef]
- Mitchell, P.J.; Bolam, S.G.; Close, H.L.; Garcia, C.; Monk, J.; Alliji, K. Distribution of demersal fish assemblages along the west coast of St Lucia: Implications for planning no-take marine reserves. Aquat. Conserv. Mar. Freshw. Ecosyst. 2021, 31, 1354–1366. [Google Scholar] [CrossRef]
- Olsen, E.; Axelsen, B.E.; Moland, E.; Utne-Palm, A.C.; Elamin, E.M.; Mukhtar, M.A.; Saleh, A.M.; Elamin, S.M.; Iragi, M.A.; Gumaa, S.G.F. Distribution and diversity of fish species along the Sudanese Red Sea coast based on three combined trap and gillnet surveys. Fish. Res. 2021, 242, 106032. [Google Scholar] [CrossRef]
- Fall, J.; Gjøsæter, H.; Tvete, I.F.; Aldrin, M. Classification of acoustic survey data: A comparison between seven teams of experts. Fish. Res. 2024, 274, 107005. [Google Scholar] [CrossRef]
- De Robertis, A.; Taylor, K.; Williams, K.; Wilson, C.D. Species and size selectivity of two midwater trawls used in an acoustic survey of the Alaska Arctic. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2017, 135, 40–50. [Google Scholar] [CrossRef]
- Danby, R.E.; Clarke, E.D.; Kynoch, R.J.; Reid, D.G.; Fernandes, P.G. Estimating and accounting for fish losses under the footrope of a survey trawl: The case of northern shelf anglerfish. Fish. Res. 2022, 255, 106431. [Google Scholar] [CrossRef]
- Annasawmy, P.; Ternon, J.-F.; Cotel, P.; Cherel, Y.; Romanov, E.V.; Roudaut, G.; Lebourges-Dhaussy, A.; Ménard, F.; Marsac, F. Micronekton distributions and assemblages at two shallow seamounts of the south-western Indian Ocean: Insights from acoustics and mesopelagic trawl data. Prog. Oceanogr. 2019, 178, 102161. [Google Scholar] [CrossRef]
- Ingvaldsen, R.B.; Eriksen, E.; Gjøsæter, H.; Engås, A.; Schuppe, B.K.; Assmann, K.M.; Cannaby, H.; Dalpadado, P.; Bluhm, B.A. Under-ice observations by trawls and multi-frequency acoustics in the Central Arctic Ocean reveals abundance and composition of pelagic fauna. Sci. Rep. 2023, 13, 1000. [Google Scholar] [CrossRef]
- Copeland, A.M.; Au, W.W.; Polovina, J. Comparison between trawl volume and composition and acoustic backscatter. J. Acoust. Soc. Am. 2016, 140, 3361. [Google Scholar] [CrossRef]
- Thomas, R.E.; Gauthier, S.; Grandin, C.; Hicks, A.; Parker-Stetter, S. To trawl or not to trawl: Questioning core assumptions of trawl placement choice in fisheries acoustics surveys. Fish. Res. 2024, 270, 106897. [Google Scholar] [CrossRef]
- Masmitja, I.; Navarro, J.; Gomariz, S.; Aguzzi, J.; Kieft, B.; O’Reilly, T.; Katija, K.; Bouvet, P.J.; Fannjiang, C.; Vigo, M.; et al. Mobile robotic platforms for the acoustic tracking of deep-sea demersal fishery resources. Sci. Robot. 2020, 5, eabc3701. [Google Scholar] [CrossRef]
- Hirose, M.; Mukai, T.; Iida, K.; Hwang, D. Target strength estimation of the large jellyfish Nemopilema nomurai. J. Acoust. Soc. Am. 2006, 120, 3106. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, Z.; An, C. Research on the detection and early warning technology of harmful marine organisms in the water intake of nuclear power plant by 3D image sonar. E3S Web Conf. 2021, 290, 03013. [Google Scholar] [CrossRef]
- Lee, Y.-W.; Oh, W.-S.; Kim, D.-S.; Hwang, D.-J.; Lee, K.-H. A Study on the Characteristics of Fishery Resources Distribution in Coastal Waters of Yeongil Bay Using Acoustic Survey. Appl. Sci. 2021, 11, 6627. [Google Scholar] [CrossRef]
- Song, M.; Gong, D.; Cui, X.; Yu, X.; Xu, P.; Xing, B.; Yin, L. Study on feeding behavior and biological sound of Sebastes schlegelii. Front. Mar. Sci. 2023, 10, 1131522. [Google Scholar] [CrossRef]
- Hailong, Z.; Wei, L.; Chenghong, W.; Min, C.; Yuxiang, Z. Codend Mesh Selectivity of Trawl in the Eastern Waters at Hainan Island. Fish. Sci. 2025, 44, 91–99. [Google Scholar] [CrossRef]
- Ji, X. An approach to the mesh size of trawlnet and its effect on trawling speed. J. Fish. China 1986, 10, 19–27. [Google Scholar]
- Zwolinski, J.; Fernandes, P.G.; Marques, V.; Stratoudakis, Y. Estimating fish abundance from acoustic surveys: Calculating variance due to acoustic backscatter and length distribution error. Can. J. Fish. Aquat. Sci. 2009, 66, 2081–2095. [Google Scholar] [CrossRef]
- Alieva, A.K.; Nasibulina, B.M.; Bakhshalizadeh, S.; Kurochkina, T.F.; Popov, N.N.; Barbol, B.I.; Bănăduc, D.; Jussupbekova, N.M.; Kuanysheva, G.A.; Ali, A.M. The Low Ontogenetic Diet Diversity and Flexibility of the Pike-Perch, Sander lucioperca (Linnaeus, 1758) (Osteichthyes, Percidae): A Case Study. Fishes 2023, 8, 395. [Google Scholar] [CrossRef]
- Brownscombe, J.W.; Cooke, S.J.; Algera, D.A.; Hanson, K.C.; Eliason, E.J.; Burnett, N.J.; Danylchuk, A.J.; Hinch, S.G.; Farrell, A.P. Ecology of Exercise in Wild Fish: Integrating Concepts of Individual Physiological Capacity, Behavior, and Fitness Through Diverse Case Studies. Integr. Comp. Biol. 2017, 57, 281–292. [Google Scholar] [CrossRef]
- Yasuda, T.; Kinoshita, J.; Niino, Y.; Okuyama, J. Vertical migration patterns linked to body and environmental temperatures in chub mackerel. Prog. Oceanogr. 2023, 213, 103017. [Google Scholar] [CrossRef]
- Yong, T. Research advance in fisheries resources assessment by using the acoustic technology in China:a review. J. Dalian Ocean Univ. 2023, 38, 185–195. [Google Scholar] [CrossRef]
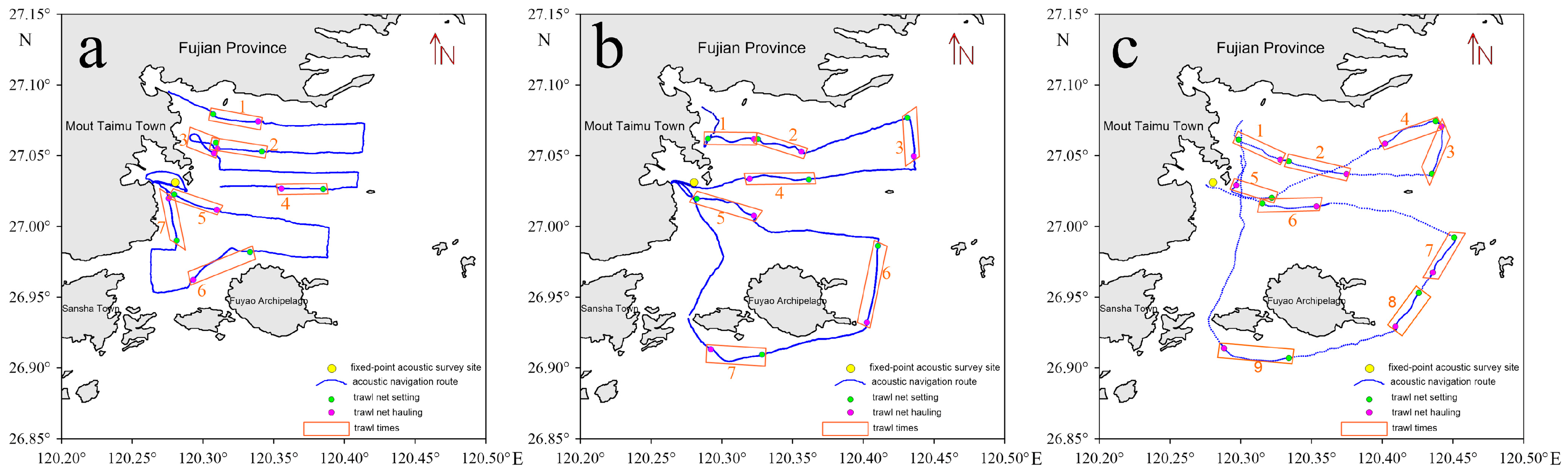
| Time | Net Set | Longitude of Net Setting | Latitude of Net Setting | Longitude of Net Hauling | Latitude of Net Hauling | Average Speed (n mile/h) | Towing Duration (h) |
|---|---|---|---|---|---|---|---|
| March 2021 | 1 | 120°18.430′ | 27°04.760′ | 120°20.334′ | 27°04.455′ | 3.7 | 0.50 |
| 2 | 120°20.496′ | 27°03.182′ | 120°18.612′ | 27°03.293′ | 3.3 | 0.50 | |
| 3 | 120°18.549′ | 27°03.549′ | 120°18.465′ | 27°03.101′ | 4.0 | 0.50 | |
| 4 | 120°23.100′ | 27°01.588′ | 120°21.326′ | 27°01.605′ | 3.2 | 0.50 | |
| 5 | 120°16.779′ | 27°01.362′ | 120°18.589′ | 27°00.704′ | 3.4 | 0.50 | |
| 6 | 120°19.995′ | 26°58.917′ | 120°17.589′ | 26°57.737′ | 4.0 | 0.50 | |
| 7 | 120°16.877′ | 26°59.400′ | 120°16.548′ | 27°01.190′ | 3.7 | 0.50 | |
| February 2023 | 1 | 120°17.421′ | 27°03.724′ | 120°19.352′ | 27°03.701′ | 3.5 | 0.55 |
| 2 | 120°19.523′ | 27°03.709′ | 120°21.363′ | 27°03.168′ | 3.6 | 0.58 | |
| 3 | 120°25.866′ | 27°04.610′ | 120°26.144′ | 27°02.987′ | 3.5 | 0.52 | |
| 4 | 120°21.695′ | 27°01.999′ | 120°19.179′ | 27°02.027′ | 3.0 | 0.78 | |
| 5 | 120°16.926′ | 27°01.182′ | 120°19.352′ | 27°00.462′ | 3.4 | 0.83 | |
| 6 | 120°24.612′ | 26°59.174′ | 120°24.160′ | 26°55.924′ | 3.8 | 0.85 | |
| 7 | 120°19.708′ | 26°54.562′ | 120°17.521′ | 26°54.793′ | 3.1 | 0.70 | |
| November 2023 | 1 | 120°17.912′ | 27°03.676′ | 120°19.679′ | 27°02.831′ | 3.3 | 0.50 |
| 2 | 120°20.027′ | 27°02.766′ | 120°22.483′ | 27°02.223′ | 3.6 | 0.50 | |
| 3 | 120°26.268′ | 27°04.467′ | 120°24.116′ | 27°03.511′ | 4.5 | 0.50 | |
| 4 | 120°26.110′ | 27°02.234′ | 120°26.513′ | 27°04.242′ | 4.7 | 0.50 | |
| 5 | 120°19.315′ | 27°01.212′ | 120°17.805′ | 27°01.748′ | 4.8 | 0.50 | |
| 6 | 120°18.914′ | 27°00.980′ | 120°21.211′ | 27°00.849′ | 5.0 | 0.50 | |
| 7 | 120°27.054′ | 26°59.532′ | 120°26.143′ | 26°58.045′ | 2.5 | 0.50 | |
| 8 | 120°25.548′ | 26°57.180′ | 120°24.559′ | 26°55.752′ | 3.3 | 0.50 | |
| 9 | 120°20.035′ | 26°54.420′ | 120°17.290′ | 26°54.835′ | 3.6 | 0.50 |
| Species | b20 | q | Species | b20 | q |
|---|---|---|---|---|---|
| Arius sinensis | −66.1 | 0.8 | Cynoglossus lighti | −71.9 | 0.8 |
| Thryssa mystax | −72.5 | 0.3 | Liza haematocheila | −72.5 | 0.3 |
| Chaeturichthys hexanema | −71.9 | 0.8 | Nibea albiflora | −68.0 | 0.5 |
| Coilia mystus | −72.5 | 0.3 | Sillago sihama | −72.5 | 0.3 |
| Coilia nasus | −72.5 | 0.3 | Cociella punctata | −68.0 | 0.5 |
| Larimichthys crocea | −68.0 | 0.5 | Tridentiger barbatus | −71.9 | 0.8 |
| Trypauchen vagina | −71.9 | 0.8 | Johnius grypotus | −68.0 | 0.5 |
| Eupleurogrammus muticus | −66.1 | 0.5 | Miichthys miiuy | −68.0 | 0.5 |
| Chrysochir aureus | −68.0 | 0.5 | Larimichthys polyactis | −68.0 | 0.5 |
| Collichthys lucidus | −68.0 | 0.5 | Solea ovata | −71.9 | 0.8 |
| Chaemrichthys stigmatias | −71.9 | 0.8 | Sebastiscus marmoratus | −67.7 | 0.3 |
| Cynoglossus joyneri | −71.9 | 0.8 | Lateolabrax japonicus | −72.5 | 0.5 |
| Odontamblyopus rubicundus | −71.9 | 0.8 | Acanthogobius ommaturus | −71.9 | 0.3 |
| Sebastiscus marmoratus | −67.7 | 0.8 | Dactylopterus volitans | −72.5 | 0.5 |
| Thryssa kammalensis | −72.5 | 0.8 | Hemitrygon akajei | −71.9 | 0.8 |
| Harpadon nehereus | −70.6 | 0.3 | Callionymus beniteguri | −69.5 | 0.5 |
| Survey Cruise | Diversity of Swimming Species | Fish Species Count | Fish Percentage (%) | Average Resource Density (ind./km²) | Average Resource Weight Density (kg/km²) |
|---|---|---|---|---|---|
| March 2021 | 54 | 26 | 48.15 | 6855 | 107.63 |
| February 2023 | 43 | 29 | 67.44 | 17,267 | 19.02 |
| November 2023 | 45 | 27 | 60.00 | 16,233 | 198.66 |
| Survey Cruise | Density | F-Value | p-Value | Minimum Value | Maximum Value |
|---|---|---|---|---|---|
| March 2021 | Resource density | 2.567 | <0.05 | 2638 ind./km2 | 10,256 ind./km2 |
| Weight density | 5.773 | <0.01 | 43.347 kg/km2 | 625.060 kg/km2 | |
| February 2023 | Resource density | 2.661 | <0.05 | 4556 ind./km2 | 17,267 ind./km2 |
| Weight density | 2.070 | <0.05 | 3.671 kg/km2 | 46.576 kg/km2 | |
| November 2023 | Resource density | 9.378 | <0.05 | 2343 ind./km2 | 27,979 ind./km2 |
| Weight density | 2.121 | <0.05 | 44.704 kg/km2 | 519.171 kg/km2 |
| Target Strength (dB) | March 2021 | February 2023 | November 2023 | |||
|---|---|---|---|---|---|---|
| Body Length (cm) | Percent (%) | Body Length (cm) | Percent (%) | Body Length (cm) | Percent (%) | |
| −90~−85 | 0.11~0.19 | 0.73 | 0.10~0.14 | 0.20 | 0.12~0.22 | 1.06 |
| −85~−80 | 0.19~0.34 | 1.00 | 0.14~0.25 | 16.01 | 0.22~0.38 | 18.43 |
| −80~−75 | 0.34~0.60 | 4.47 | 0.25~0.44 | 39.98 | 0.38~0.68 | 31.52 |
| −75~−70 | 0.60~1.06 | 61.42 | 0.44~0.78 | 17.52 | 0.68~1.21 | 24.26 |
| −70~−65 | 1.06~1.89 | 26.26 | 0.78~1.39 | 11.88 | 1.21~2.15 | 14.69 |
| −65~−60 | 1.89~3.37 | 5.08 | 1.39~2.47 | 9.26 | 2.15~3.82 | 6.47 |
| −60~−55 | 3.37~5.98 | 0.75 | 2.47~4.39 | 4.03 | 3.82~6.80 | 2.16 |
| −55~−50 | 5.98~10.64 | 0.11 | 4.39~7.81 | 0.76 | 6.80~12.09 | 0.96 |
| −50~−45 | 10.64~18.92 | 0.05 | 7.81~13.88 | 0.20 | 12.09~21.50 | 0.34 |
| −45~−40 | 18.92~33.65 | 0.08 | 13.88~24.69 | 0.10 | 21.50~38.24 | 0.09 |
| −40~−35 | 33.65~59.84 | 0.05 | 24.69~43.90 | 0.05 | 38.24~68.00 | 0.01 |
| −35~−30 | 59.84~106.41 | 0.01 | 43.90~133.40 | 0.00 | 68.00~120.92 | 0.00 |
| Survey Cruise | Integration Value (dB) | Maximum Target Strength (dB) | Minimum Target Strength (dB) | Average Target Strength (dB) | Average Depth (m) |
|---|---|---|---|---|---|
| March 2021 | 95,744.85 | −42.42 | −57.90 | −47.90 ± 13.59 | 19.10 ± 16.32 |
| February 2023 | 30,722.18 | −40.01 | −54.72 | −46.11 ± 12.59 | 8.05 ± 3.95 |
| November 2023 | 512,361.35 | −21.17 | −80.95 | −69.35 ± 6.11 | 6.70 ± 3.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Z.; Song, Y.; Zhao, G.; Shi, Y.; Wu, Y.; Zhang, S. Assessment of Fish Biomass and Distribution in a Nuclear Power Plant’s Water Intake Zone Using Acoustic and Trawl Methods. Animals 2025, 15, 987. https://doi.org/10.3390/ani15070987
Wu Z, Song Y, Zhao G, Shi Y, Wu Y, Zhang S. Assessment of Fish Biomass and Distribution in a Nuclear Power Plant’s Water Intake Zone Using Acoustic and Trawl Methods. Animals. 2025; 15(7):987. https://doi.org/10.3390/ani15070987
Chicago/Turabian StyleWu, Zuli, Yunpeng Song, Guoqing Zhao, Yongchuang Shi, Yumei Wu, and Shengmao Zhang. 2025. "Assessment of Fish Biomass and Distribution in a Nuclear Power Plant’s Water Intake Zone Using Acoustic and Trawl Methods" Animals 15, no. 7: 987. https://doi.org/10.3390/ani15070987
APA StyleWu, Z., Song, Y., Zhao, G., Shi, Y., Wu, Y., & Zhang, S. (2025). Assessment of Fish Biomass and Distribution in a Nuclear Power Plant’s Water Intake Zone Using Acoustic and Trawl Methods. Animals, 15(7), 987. https://doi.org/10.3390/ani15070987





