Cold Atmospheric Plasma in Oncology: A Review and Perspectives on Its Application in Veterinary Oncology
Simple Summary
Abstract
1. Introduction
2. Methods
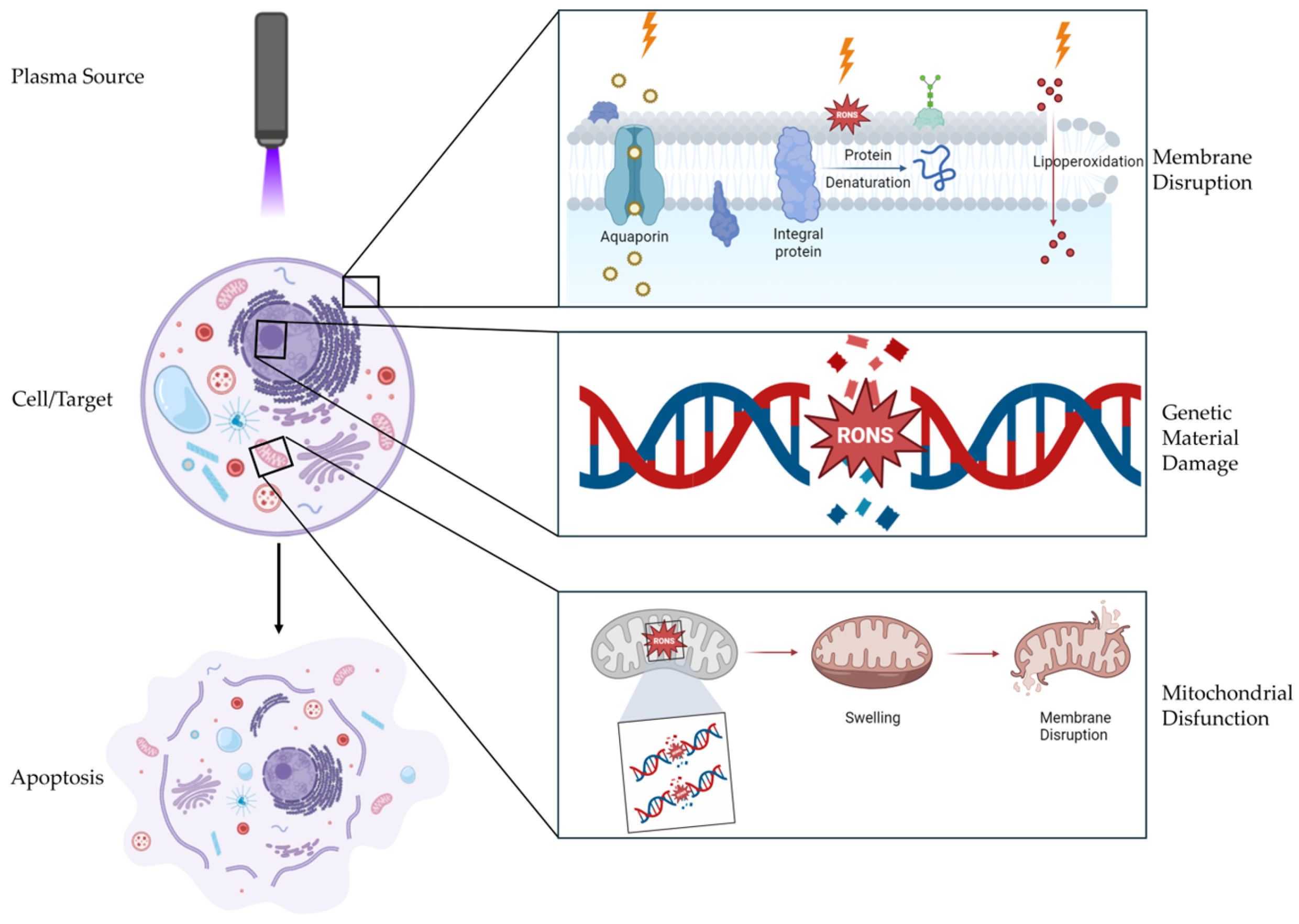
3. Mechanisms of Action of CAP
3.1. Reactive Oxygen Species (ROS)
3.2. Reactive Nitrogen Species (RNS)
3.3. Physical Effects
3.3.1. Electromagnetic Waves (EMs)
3.3.2. UV Radiation
3.3.3. Thermal Radiation
4. CAP Devices for Clinical Use
| Device Type | Generation Mechanism | Key Characteristics | Common Clinical Applications | Limitations |
|---|---|---|---|---|
| Dielectric Barrier Discharge (DBD) | - Generates direct plasma discharge between two electrodes separated by a dielectric barrier [37]. | - Cover larger areas more easily [40]. - Does not require additional gas supply equipment [40]. | - Treatment of superficial acute or chronic wounds, skin disinfection, and tissue regeneration [44,48]. | - Less effective on irregular surfaces [39]. |
| Plasma Jets | - Generates indirect plasma discharge by expelling ionized gas in a directed jet [41]. | - Produces plasma at a distance from the generating electrode [38]. - Greater adaptability treating for irregular surfaces [32,42]. | - Treatment of superficial acute or chronic wounds, skin disinfection, and tissue regeneration [49,50]. - Suitable for therapies for delicate tissues, such as mucous membranes [51]. | - Loss of a significant portion of plasma through the nozzle other openings [42]. |
| Hybrid Devices | - Combines microdischarges on a grounded mesh electrode [36]. | - Integrates advantages of both DBD and plasma jets [36]. - Produces a uniform discharge [39]. - Device is relatively easy to control [39] | - Currently applied only at the experimental level [39]. | - Increased susceptibility to component wear and subsequent deterioration [39]. |
5. Applicability of the CAP
6. Preclinical Trials
6.1. In Vitro Trials
6.2. In Vivo Trials
7. Clinical Trials
8. Limitations and Perspectives
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data availability statement
Conflicts of Interest
References
- Rai, V.; Gupta, Y.; Srivastava, S.P.; Shukla, A.; Bano, N.; Khan, S. Targeted Therapies in Cancer Treatment: Unveiling the Latest Breakthroughs and Promising Approaches. J. Res. Appl. Sci. Biotechnol. 2024, 2, 175–183. [Google Scholar] [CrossRef]
- Shifana, A.S.; Adnan, M.; Gupta, A.; Ajazuddin; Jain, P. A Comprehensive Review on Novel Pathways in Cancer Treatment: Clinical Applications and Future Prospects. Curr. Cancer Drug Targets 2024, 24. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Rawat, A.; Sahu, V.; Chaurasia, Y.; Kumar, A.; Rathore, S.; Tripathi, R. Richa Tripathi Current Advances in Cancer Treatment: A Comprehensive Review of Therapeutic Strategies and Emerging Innovations. World J. Biol. Pharm. Health Sci. 2024, 18, 274–282. [Google Scholar] [CrossRef]
- Kugler, P.; Becker, S.; Welz, C.; Wiesmann, N.; Sax, J.; Buhr, C.R.; Thoma, M.H.; Brieger, J.; Eckrich, J. Cold Atmospheric Plasma Reduces Vessel Density and Increases Vascular Permeability and Apoptotic Cell Death in Solid Tumors. Cancers 2022, 14, 2432. [Google Scholar] [CrossRef]
- Keidar, M.; Yan, D.; Beilis, I.I.; Trink, B.; Sherman, J.H. Plasmas for Treating Cancer: Opportunities for Adaptive and Self-Adaptive Approaches. Trends Biotechnol. 2018, 36, 586–593. [Google Scholar] [CrossRef]
- Domonkos, M.; Tichá, P.; Trejbal, J.; Demo, P. Applications of Cold Atmospheric Pressure Plasma Technology in Medicine, Agriculture and Food Industry. Appl. Sci. 2021, 11, 4809. [Google Scholar] [CrossRef]
- Kaushik, N.; Mitra, S.; Baek, E.J.; Nguyen, L.N.; Bhartiya, P.; Kim, J.H.; Choi, E.H.; Kaushik, N.K. The Inactivation and Destruction of Viruses by Reactive Oxygen Species Generated through Physical and Cold Atmospheric Plasma Techniques: Current Status and Perspectives. J. Adv. Res. 2023, 43, 59–71. [Google Scholar] [CrossRef]
- Keidar, M.; Beilis, I.I. Plasma Medicine. In Plasma Engineering, 2nd ed.; Keidar, M., Beilis, I.I., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 455–539. [Google Scholar] [CrossRef]
- Sklias, K.; Sousa, J.S.; Girard, P.M. Role of Short- and Long-Lived Reactive Species on the Selectivity and Anti-Cancer Action of Plasma Treatment in Vitro. Cancers 2021, 13, 615. [Google Scholar] [CrossRef]
- Privat-Maldonado, A.; Schmidt, A.; Lin, A.; Weltmann, K.-D.; Wende, K.; Bogaerts, A.; Bekeschus, S. ROS from Physical Plasmas: Redox Chemistry for Biomedical Therapy. Oxidative Med. Cell. Longev. 2019, 2019, 9062098. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, Y.; Xu, M.; Chen, H.; Lu, X.; Ostrikov, K. Cold Atmospheric Pressure Plasmas in Dermatology: Sources, Reactive Agents, and Therapeutic Effects. Plasma Process. Polym. 2020, 17, 1900218. [Google Scholar]
- Yang, Y.; Wang, Y.; Wei, S.; Wang, X.; Zhang, J. Effects and Mechanisms of Non-Thermal Plasma-Mediated ROS and Its Applications in Animal Husbandry and Biomedicine. Int. J. Mol. Sci. 2023, 24, 15889. [Google Scholar] [CrossRef] [PubMed]
- Moszczyńska, J.; Roszek, K.; Wiśniewski, M. Non-Thermal Plasma Application in Medicine—Focus on Reactive Species Involvement. Int. J. Mol. Sci. 2023, 24, 12667. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Wang, Q.; Adhikari, M.; Malyavko, A.; Lin, L.; Zolotukhin, D.B.; Yao, X.; Kirschner, M.; Sherman, J.H.; Keidar, M. A Physically Triggered Cell Death via Transbarrier Cold Atmospheric Plasma Cancer Treatment. ACS Appl. Mater. Interfaces 2020, 12, 34548–34563. [Google Scholar] [CrossRef] [PubMed]
- Jha, N.; Ryu, J.J.; Choi, E.H.; Kaushik, N.K. Generation and Role of Reactive Oxygen and Nitrogen Species Induced by Plasma, Lasers, Chemical Agents, and Other Systems in Dentistry. Oxidative Med. Cell. Longev. 2017, 2017, 7542540. [Google Scholar] [CrossRef]
- Cernei, N.; Sandru, S.; Cobilețchi, S.; Grabovschi, I.; Civirjic, I.; Baltaga, R. Current Affairs in the Use of Medical Ozone. Biological Effects. Mechanisms of Action. Mold. J. Health Sci. 2023, 10, 65–72. [Google Scholar] [CrossRef]
- Tian, M.; Xu, D.; Li, B.; Wang, S.; Qi, M.; Zhang, H.; Liu, Z.; Liu, D.; Chen, H.; Kong, M.G. Metabolome Analysis of Selective Inactivation of Human Melanoma and Normal Cells by Cold Atmospheric Plasma. Plasma Chem. Plasma Process. 2021, 41, 591–605. [Google Scholar] [CrossRef]
- Drummond, G.R.; Selemidis, S.; Griendling, K.K.; Sobey, C.G. Combating Oxidative Stress in Vascular Disease: NADPH Oxidases as Therapeutic Targets. Nat. Rev. Drug Discov. 2011, 10, 453–471. [Google Scholar] [CrossRef]
- Attri, P.; Kaushik, N.K.; Kaushik, N.; Hammerschmid, D.; Privat-Maldonado, A.; De Backer, J.; Shiratani, M.; Choi, E.H.; Bogaerts, A. Plasma Treatment Causes Structural Modifications in Lysozyme, and Increases Cytotoxicity towards Cancer Cells. Int. J. Biol. Macromol. 2021, 182, 1724–1736. [Google Scholar] [CrossRef]
- Graves, D.B. Reactive Species from Cold Atmospheric Plasma: Implications for Cancer Therapy. Plasma Process. Polym. 2014, 11, 1120–1127. [Google Scholar] [CrossRef]
- Kaushik, N.K.; Kaushik, N.; Min, B.; Choi, K.H.; Hong, Y.J.; Miller, V.; Fridman, A.; Choi, E.H. Cytotoxic Macrophage-Released Tumour Necrosis Factor-Alpha (TNF- α) as a Killing Mechanism for Cancer Cell Death after Cold Plasma Activation. J. Phys. D Appl. Phys. 2016, 49, 084001. [Google Scholar] [CrossRef]
- Kaushik, N.K.; Kaushik, N.; Adhikari, M.; Ghimire, B.; Linh, N.N.; Mishra, Y.K.; Lee, S.-J.; Choi, E.H. Preventing the Solid Cancer Progression via Release of Anticancer-Cytokines in Co-Culture with Cold Plasma-Stimulated Macrophages. Cancers 2019, 11, 842. [Google Scholar] [CrossRef] [PubMed]
- Miller, V.; Lin, A.; Fridman, A. Why Target Immune Cells for Plasma Treatment of Cancer. Plasma Chem. Plasma Process. 2016, 36, 259–268. [Google Scholar] [CrossRef]
- Adamovich, I.; Baalrud, S.D.; Bogaerts, A.; Bruggeman, P.J.; Cappelli, M.; Colombo, V.; Czarnetzki, U.; Ebert, U.; Eden, J.G.; Favia, P.; et al. The 2017 Plasma Roadmap: Low Temperature Plasma Science and Technology. J. Phys. D Appl. Phys. 2017, 50, 323001. [Google Scholar] [CrossRef]
- Metelmann, H.-R.; Von Woedtke, T.; Weltmann, K.-D. Comprehensive Clinical Plasma Medicine, Metelmann, H.-R., von Woedtke, T., Weltmann, K.-D., Eds.; 1st ed.; Springer International Publishing: Cham, Switzerland, 2019; ISBN 978-3-319-67626-5. [Google Scholar]
- Gay-Mimbrera, J.; García, M.C.; Isla-Tejera, B.; Rodero-Serrano, A.; García-Nieto, A.V.; Ruano, J. Clinical and Biological Principles of Cold Atmospheric Plasma Application in Skin Cancer. Adv. Ther. 2016, 33, 894–909. [Google Scholar] [CrossRef]
- Kletschkus, K.; Gelbrich, N.; Burchardt, M.; Kramer, A.; Bekeschus, S.; Stope, M.B. Emission of Ultraviolet Radiation from 220 to 280 NM by a Cold Physical Plasma Generating Device. Health Phys. 2020, 119, 153–159. [Google Scholar] [CrossRef]
- Kos, S.; Blagus, T.; Cemazar, M.; Filipic, G.; Sersa, G.; Cvelbar, U. Safety Aspects of Atmospheric Pressure Helium Plasma Jet Operation on Skin: In Vivo Study on Mouse Skin. PLoS ONE 2017, 12, e0174966. [Google Scholar] [CrossRef]
- Bouceiro Mendes, R.; Alpalhão, M.; Filipe, P. UVB Phototherapy in the Treatment of Vitiligo: State of the Art and Clinical Perspectives. Photodermatol. Photoimmunol. Photomed. 2022, 38, 215–223. [Google Scholar] [CrossRef]
- Li, Y.; Cao, Z.; Guo, J.; Li, Q.; Zhu, W.; Kuang, Y.; Chen, X. Assessment of Efficacy and Safety of UV-Based Therapy for Psoriasis: A Network Meta-Analysis of Randomized Controlled Trials. Ann. Med. 2022, 54, 159–169. [Google Scholar] [CrossRef]
- Lucena, S.; Salazar, N.; Gracia-Cazaña, T.; Zamarrón, A.; González, S.; Juarranz, Á.; Gilaberte, Y. Combined Treatments with Photodynamic Therapy for Non-Melanoma Skin Cancer. Int. J. Mol. Sci. 2015, 16, 25912–25933. [Google Scholar] [CrossRef]
- Holanda, A.G.A.; Cesário, B.C.; Silva, V.M.; Francelino, L.E.C.; Nascimento, B.H.M.; Damasceno, K.F.A.; Ishikawa, U.; Farias, N.B.S.; Junior, R.F.A.; Barboza, C.A.G.; et al. Use of Cold Atmospheric Plasma in the Treatment of Squamous Cell Carcinoma: In Vitro Effects and Clinical Application in Feline Tumors: A Pilot Study. Top. Companion Anim. Med. 2023, 53–54, 100773. [Google Scholar] [CrossRef]
- Saadati, F.; Mahdikia, H.; Abbaszadeh, H.-A.; Abdollahifar, M.-A.; Khoramgah, M.S.; Shokri, B. Comparison of Direct and Indirect Cold Atmospheric-Pressure Plasma Methods in the B16F10 Melanoma Cancer Cells Treatment. Sci. Rep. 2018, 8, 7689. [Google Scholar] [CrossRef]
- Laroussi, M. Cold Plasma in Medicine and Healthcare: The New Frontier in Low Temperature Plasma Applications. Front. Phys. 2020, 8, 74. [Google Scholar] [CrossRef]
- Qin, J.; Zhang, J.; Fan, G.; Wang, X.; Zhang, Y.; Wang, L.; Zhang, Y.; Guo, Q.; Zhou, J.; Zhang, W.; et al. Cold Atmospheric Plasma Activates Selective Photothermal Therapy of Cancer. Molecules 2022, 27, 5941. [Google Scholar] [CrossRef] [PubMed]
- Isbary, G.; Shimizu, T.; Li, Y.-F.; Stolz, W.; Thomas, H.M.; Morfill, G.E.; Zimmermann, J.L. Cold Atmospheric Plasma Devices for Medical Issues. Expert. Rev. Med. Devices 2013, 10, 367–377. [Google Scholar] [CrossRef]
- Almeida-Ferreira, C.; Rodrigues, F.; Marto, C.M.; Botelho, M.F.; Laranjo, M. Cold Atmospheric Plasma for Breast Cancer Treatment: What Next? Med. Gas. Res. 2025, 15, 110–111. [Google Scholar] [CrossRef]
- Yan, D.; Sherman, J.H.; Keidar, M. Cold Atmospheric Plasma, a Novel Promising Anti-Cancer Treatment Modality. Oncotarget 2017, 8, 15977–15995. [Google Scholar] [CrossRef]
- Braný, D.; Dvorská, D.; Halašová, E.; Škovierová, H. Cold Atmospheric Plasma: A Powerful Tool for Modern Medicine. Int. J. Mol. Sci. 2020, 21, 2932. [Google Scholar] [CrossRef]
- Weltmann, K.-D.; von Woedtke, T. Plasma Medicine—Current State of Research and Medical Application. Plasma Phys. Control. Fusion 2017, 59, 014031. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, G.; Obenchain, R.; Zhang, R.; Bai, F.; Fang, T.; Wang, H.; Lu, Y.; Wirz, R.E.; Gu, Z. Cold Atmospheric Plasma Delivery for Biomedical Applications. Mater. Today 2022, 54, 153–188. [Google Scholar] [CrossRef]
- Murillo, D.; Huergo, C.; Gallego, B.; Rodríguez, R.; Tornín, J. Exploring the Use of Cold Atmospheric Plasma to Overcome Drug Resistance in Cancer. Biomedicines 2023, 11, 208. [Google Scholar] [CrossRef]
- Reuter, S.; von Woedtke, T.; Weltmann, K.-D. The KINPen—A Review on Physics and Chemistry of the Atmospheric Pressure Plasma Jet and Its Applications. J. Phys. D Appl. Phys. 2018, 51, 233001. [Google Scholar] [CrossRef]
- Brehmer, F.; Haenssle, H.A.; Daeschlein, G.; Ahmed, R.; Pfeiffer, S.; Görlitz, A.; Simon, D.; Schön, M.P.; Wandke, D.; Emmert, S. Alleviation of Chronic Venous Leg Ulcers with a Hand-held Dielectric Barrier Discharge Plasma Generator (PlasmaDerm® VU-2010): Results of a Monocentric, Two-armed, Open, Prospective, Randomized and Controlled Trial (NCT 01415622). J. Eur. Acad. Dermatol. Venereol. 2015, 29, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Bennett, A.; Urayama, T.; Papangelis, K.; Yuen, P.; Yu, N. Characterisation of a Cold Atmospheric Pressure Plasma Torch for Medical Applications: Demonstration of Device Safety. Appl. Sci. 2021, 11, 11864. [Google Scholar] [CrossRef]
- Izadjoo, M.; Zack, S.; Kim, H.; Skiba, J. Medical Applications of Cold Atmospheric Plasma: State of the Science. J. Wound Care 2018, 27, S4–S10. [Google Scholar] [CrossRef]
- Reza Lotfi, M.; Khani, M.; Moradi, A.; Razaghiha, E.; Shokri, B. Development and Characterization of a Spark Plasma Device Designed for Medical and Aesthetic Applications. Heliyon 2024, 10, e33042. [Google Scholar] [CrossRef]
- Abu Rached, N.; Kley, S.; Storck, M.; Meyer, T.; Stücker, M. Cold Plasma Therapy in Chronic Wounds—A Multicenter, Randomized Controlled Clinical Trial (Plasma on Chronic Wounds for Epidermal Regeneration Study): Preliminary Results. J. Clin. Med. 2023, 12, 5121. [Google Scholar] [CrossRef]
- Mirpour, S.; Fathollah, S.; Mansouri, P.; Larijani, B.; Ghoranneviss, M.; Mohajeri Tehrani, M.; Amini, M.R. Cold Atmospheric Plasma as an Effective Method to Treat Diabetic Foot Ulcers: A Randomized Clinical Trial. Sci. Rep. 2020, 10, 10440. [Google Scholar] [CrossRef]
- Daeschlein, G.; Napp, M.; Lutze, S.; Arnold, A.; von Podewils, S.; Guembel, D.; Jünger, M. Skin and Wound Decontamination of Multidrug-resistant Bacteria by Cold Atmospheric Plasma Coagulation. JDDG J. Der Dtsch. Dermatol. Ges. 2015, 13, 143–149. [Google Scholar] [CrossRef]
- Kusakci-Seker, B.; Demirayak-Akdemir, M. The Effect of Non-thermal Atmospheric Pressure Plasma Application on Wound Healing after Gingivectomy. Int. Wound J. 2020, 17, 1376–1383. [Google Scholar] [CrossRef]
- Min, T.; Xie, X.; Ren, K.; Sun, T.; Wang, H.; Dang, C.; Zhang, H. Therapeutic Effects of Cold Atmospheric Plasma on Solid Tumor. Front. Med. 2022, 9, 884887. [Google Scholar] [CrossRef]
- Fofana, M.; Buñay, J.; Judée, F.; Baron, S.; Menecier, S.; Nivoix, M.; Perisse, F.; Vacavant, A.; Balandraud, X. Selective Treatments of Prostate Tumor Cells with a Cold Atmospheric Plasma Jet. Clin. Plasma Med. 2020, 17–18, 100098. [Google Scholar] [CrossRef]
- Poramapijitwat, P.; Thana, P.; Sukum, P.; Liangdeng, Y.; Kuensaen, C.; Boonyawan, D. Selective Cytotoxicity of Lung Cancer Cells—A549 and H1299—Induced by Ringer’s Lactate Solution Activated by a Non-Thermal Air Plasma Jet Device, Nightingale®. Plasma Chem. Plasma Process. 2023, 43, 805–830. [Google Scholar] [CrossRef]
- Jung, J.-M.; Yoon, H.-K.; Kim, S.-Y.; Yun, M.-R.; Kim, G.-H.; Lee, W.-J.; Lee, M.-W.; Chang, S.-E.; Won, C.-H. Anticancer Effect of Cold Atmospheric Plasma in Syngeneic Mouse Models of Melanoma and Colon Cancer. Molecules 2023, 28, 4171. [Google Scholar] [CrossRef] [PubMed]
- Daeschlein, G.; Scholz, S.; Lutze, S.; Arnold, A.; von Podewils, S.; Kiefer, T.; Tueting, T.; Hardt, O.; Haase, H.; Grisk, O.; et al. Comparison between Cold Plasma, Electrochemotherapy and Combined Therapy in a Melanoma Mouse Model. Exp. Dermatol. 2013, 22, 582–586. [Google Scholar] [CrossRef]
- Kenari, A.J.; Siadati, S.N.; Abedian, Z.; Sohbatzadeh, F.; Amiri, M.; Gorji, K.E.; Babapour, H.; Zabihi, E.; Ghoreishi, S.M.; Mehraeen, R.; et al. Therapeutic Effect of Cold Atmospheric Plasma and Its Combination with Radiation as a Novel Approach on Inhibiting Cervical Cancer Cell Growth (HeLa Cells). Bioorg Chem. 2021, 111, 104892. [Google Scholar] [CrossRef]
- Canady, J.; Murthy, S.R.K.; Zhuang, T.; Gitelis, S.; Nissan, A.; Ly, L.; Jones, O.Z.; Cheng, X.; Adileh, M.; Blank, A.T.; et al. The First Cold Atmospheric Plasma Phase I Clinical Trial for the Treatment of Advanced Solid Tumors: A Novel Treatment Arm for Cancer. Cancers 2023, 15, 3688. [Google Scholar] [CrossRef]
- Troitskaya, O.; Golubitskaya, E.; Biryukov, M.; Varlamov, M.; Gugin, P.; Milakhina, E.; Richter, V.; Schweigert, I.; Zakrevsky, D.; Koval, O. Non-Thermal Plasma Application in Tumor-Bearing Mice Induces Increase of Serum HMGB1. Int. J. Mol. Sci. 2020, 21, 5128. [Google Scholar] [CrossRef]
- Fang, T.; Cao, X.; Shen, B.; Chen, Z.; Chen, G. Injectable Cold Atmospheric Plasma-Activated Immunotherapeutic Hydrogel for Enhanced Cancer Treatment. Biomaterials 2023, 300, 122189. [Google Scholar] [CrossRef]
- Harley, J.C.; Suchowerska, N.; McKenzie, D.R. Cancer Treatment with Gas Plasma and with Gas Plasma–Activated Liquid: Positives, Potentials and Problems of Clinical Translation. Biophys. Rev. 2020, 12, 989–1006. [Google Scholar] [CrossRef]
- Dubuc, A.; Monsarrat, P.; Virard, F.; Merbahi, N.; Sarrette, J.-P.; Laurencin-Dalicieux, S.; Cousty, S. Use of Cold-Atmospheric Plasma in Oncology: A Concise Systematic Review. Ther. Adv. Med. Oncol. 2018, 10, 1758835918786475. [Google Scholar] [CrossRef]
- Yoshikawa, N.; Nakamura, K.; Kajiyama, H. Current Understanding of Plasma-Activated Solutions for Potential Cancer Therapy. Free Radic. Res. 2023, 57, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Takeda, S.; Yamada, S.; Hattori, N.; Nakamura, K.; Tanaka, H.; Kajiyama, H.; Kanda, M.; Kobayashi, D.; Tanaka, C.; Fujii, T.; et al. Intraperitoneal Administration of Plasma-Activated Medium: Proposal of a Novel Treatment Option for Peritoneal Metastasis From Gastric Cancer. Ann. Surg. Oncol. 2017, 24, 1188–1194. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Horkowitz, A.; Wang, Q.; Keidar, M. On the Selective Killing of Cold Atmospheric Plasma Cancer Treatment: Status and Beyond. Plasma Process. Polym. 2021, 18, e2100020. [Google Scholar] [CrossRef]
- Solé-Martí, X.; Espona-Noguera, A.; Ginebra, M.-P.; Canal, C. Plasma-Conditioned Liquids as Anticancer Therapies In Vivo: Current State and Future Directions. Cancers 2021, 13, 452. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Kim, S.-O.; Wei, Y.; Li, J. A Flexible Cold Microplasma Jet Using Biocompatible Dielectric Tubes for Cancer Therapy. Appl. Phys. Lett. 2010, 96, 203701. [Google Scholar] [CrossRef]
- Zirnheld, J.L.; Zucker, S.N.; DiSanto, T.M.; Berezney, R.; Etemadi, K. Nonthermal Plasma Needle: Development and Targeting of Melanoma Cells. IEEE Trans. Plasma Sci. 2010, 38, 948–952. [Google Scholar] [CrossRef]
- Georgescu, N.; Lupu, A.R. Tumoral and Normal Cells Treatment With High-Voltage Pulsed Cold Atmospheric Plasma Jets. IEEE Trans. Plasma Sci. 2010, 38, 1949–1955. [Google Scholar] [CrossRef]
- Keidar, M.; Walk, R.; Shashurin, A.; Srinivasan, P.; Sandler, A.; Dasgupta, S.; Ravi, R.; Guerrero-Preston, R.; Trink, B. Cold Plasma Selectivity and the Possibility of a Paradigm Shift in Cancer Therapy. Br. J. Cancer 2011, 105, 1295–1301. [Google Scholar] [CrossRef]
- Bakhtiyari-Ramezani, M.; Nohekhan, M.; Akbari, M.E.; Abbasvandi, F.; Bayat, M.; Akbari, A.; Nasiri, M. Comparative Assessment of Direct and Indirect Cold Atmospheric Plasma Effects, Based on Helium and Argon, on Human Glioblastoma: An in Vitro and in Vivo Study. Sci. Rep. 2024, 14, 3578. [Google Scholar] [CrossRef]
- Welz, C.; Emmert, S.; Canis, M.; Becker, S.; Baumeister, P.; Shimizu, T.; Morfill, G.E.; Harréus, U.; Zimmermann, J.L. Cold Atmospheric Plasma: A Promising Complementary Therapy for Squamous Head and Neck Cancer. PLoS ONE 2015, 10, e0141827. [Google Scholar] [CrossRef]
- Ermakov, A.M.; Ermakova, O.N.; Afanasyeva, V.A.; Popov, A.L. Dose-Dependent Effects of Cold Atmospheric Argon Plasma on the Mesenchymal Stem and Osteosarcoma Cells In Vitro. Int. J. Mol. Sci. 2021, 22, 6797. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Chung, T.H. Cold Atmospheric Plasma Jet-Generated RONS and Their Selective Effects on Normal and Carcinoma Cells. Sci. Rep. 2016, 6, 20332. [Google Scholar] [CrossRef]
- Ma, Y.; Ha, C.S.; Hwang, S.W.; Lee, H.J.; Kim, G.C.; Lee, K.-W.; Song, K. Non-Thermal Atmospheric Pressure Plasma Preferentially Induces Apoptosis in P53-Mutated Cancer Cells by Activating ROS Stress-Response Pathways. PLoS ONE 2014, 9, e91947. [Google Scholar] [CrossRef]
- Naciri, M.; Dowling, D.; Al-Rubeai, M. Differential Sensitivity of Mammalian Cell Lines to Non-Thermal Atmospheric Plasma. Plasma Process. Polym. 2014, 11, 391–400. [Google Scholar] [CrossRef]
- Yan, D.; Talbot, A.; Nourmohammadi, N.; Sherman, J.H.; Cheng, X.; Keidar, M. Toward Understanding the Selective Anticancer Capacity of Cold Atmospheric Plasma—A Model Based on Aquaporins (Review). Biointerphases 2015, 10, 040801. [Google Scholar] [CrossRef]
- Pasqual-Melo, G.; Nascimento, T.; Sanches, L.J.; Blegniski, F.P.; Bianchi, J.K.; Sagwal, S.K.; Berner, J.; Schmidt, A.; Emmert, S.; Weltmann, K.-D.; et al. Plasma Treatment Limits Cutaneous Squamous Cell Carcinoma Development In Vitro and In Vivo. Cancers 2020, 12, 1993. [Google Scholar] [CrossRef]
- Karki, S.B.; Gupta, T.T.; Yildirim-Ayan, E.; Eisenmann, K.M.; Ayan, H. Miniature Non-Thermal Plasma Induced Cell Cycle Arrest and Apoptosis in Lung Carcinoma Cells. Plasma Chem. Plasma Process. 2020, 40, 99–117. [Google Scholar] [CrossRef]
- Tornin, J.; Mateu-Sanz, M.; Rodríguez, A.; Labay, C.; Rodríguez, R.; Canal, C. Pyruvate Plays a Main Role in the Antitumoral Selectivity of Cold Atmospheric Plasma in Osteosarcoma. Sci. Rep. 2019, 9, 10681. [Google Scholar] [CrossRef]
- Vaquero, J.; Judée, F.; Vallette, M.; Decauchy, H.; Arbelaiz, A.; Aoudjehane, L.; Scatton, O.; Gonzalez-Sanchez, E.; Merabtene, F.; Augustin, J.; et al. Cold-Atmospheric Plasma Induces Tumor Cell Death in Preclinical In Vivo and In Vitro Models of Human Cholangiocarcinoma. Cancers 2020, 12, 1280. [Google Scholar] [CrossRef]
- Van Loenhout, J.; Flieswasser, T.; Freire Boullosa, L.; De Waele, J.; Van Audenaerde, J.; Marcq, E.; Jacobs, J.; Lin, A.; Lion, E.; Dewitte, H.; et al. Cold Atmospheric Plasma-Treated PBS Eliminates Immunosuppressive Pancreatic Stellate Cells and Induces Immunogenic Cell Death of Pancreatic Cancer Cells. Cancers 2019, 11, 1597. [Google Scholar] [CrossRef]
- Cheng, F.; Yan, D.; Chen, J.; Keidar, M.; Sotomayor, E. Cold Plasma with Immunomodulatory Properties Has Significant Anti-Lymphoma Activities in Vitro and In Vivo. Blood 2019, 134, 5307. [Google Scholar] [CrossRef]
- Bisag, A.; Bucci, C.; Coluccelli, S.; Girolimetti, G.; Laurita, R.; De Iaco, P.; Perrone, A.M.; Gherardi, M.; Marchio, L.; Porcelli, A.M.; et al. Plasma-Activated Ringer’s Lactate Solution Displays a Selective Cytotoxic Effect on Ovarian Cancer Cells. Cancers 2020, 12, 476. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Moon, H.; Ku, B.; Lee, K.; Hwang, C.Y.; Baek, S.J. Anticancer Effects of Cold Atmospheric Plasma in Canine Osteosarcoma Cells. Int. J. Mol. Sci. 2020, 21, 4556. [Google Scholar] [CrossRef] [PubMed]
- Limanowski, R.; Yan, D.; Li, L.; Keidar, M. Preclinical Cold Atmospheric Plasma Cancer Treatment. Cancers 2022, 14, 3461. [Google Scholar] [CrossRef]
- Alimohammadi, M.; Golpour, M.; Sohbatzadeh, F.; Hadavi, S.; Bekeschus, S.; Niaki, H.A.; Valadan, R.; Rafiei, A. Cold Atmospheric Plasma Is a Potent Tool to Improve Chemotherapy in Melanoma In Vitro and In Vivo. Biomolecules 2020, 10, 1011. [Google Scholar] [CrossRef]
- Soni, V.; Adhikari, M.; Simonyan, H.; Lin, L.; Sherman, J.H.; Young, C.N.; Keidar, M. In Vitro and In Vivo Enhancement of Temozolomide Effect in Human Glioblastoma by Non-Invasive Application of Cold Atmospheric Plasma. Cancers 2021, 13, 4485. [Google Scholar] [CrossRef]
- Mahdikia, H.; Saadati, F.; Freund, E.; Gaipl, U.S.; Majidzadeh-a, K.; Shokri, B.; Bekeschus, S. Gas Plasma Irradiation of Breast Cancers Promotes Immunogenicity, Tumor Reduction, and an Abscopal Effect in Vivo. Oncoimmunology 2021, 10, 1859731. [Google Scholar] [CrossRef]
- Dezhpour, A.; Ghafouri, H.; Jafari, S.; Nilkar, M. Effects of Cold Atmospheric-Pressure Plasma in Combination with Doxorubicin Drug against Breast Cancer Cells in Vitro and in Vivo. Free Radic. Biol. Med. 2023, 209, 202–210. [Google Scholar] [CrossRef]
- Partecke, L.I.; Evert, K.; Haugk, J.; Doering, F.; Normann, L.; Diedrich, S.; Weiss, F.-U.; Evert, M.; Huebner, N.O.; Guenther, C.; et al. Tissue Tolerable Plasma (TTP) Induces Apoptosis in Pancreatic Cancer Cells in Vitro and in Vivo. BMC Cancer 2012, 12, 473. [Google Scholar] [CrossRef]
- Liedtke, K.R.; Bekeschus, S.; Kaeding, A.; Hackbarth, C.; Kuehn, J.-P.; Heidecke, C.-D.; von Bernstorff, W.; von Woedtke, T.; Partecke, L.I. Non-Thermal Plasma-Treated Solution Demonstrates Antitumor Activity against Pancreatic Cancer Cells in Vitro and in Vivo. Sci. Rep. 2017, 7, 8319. [Google Scholar] [CrossRef]
- Utsumi, F.; Kajiyama, H.; Nakamura, K.; Tanaka, H.; Mizuno, M.; Ishikawa, K.; Kondo, H.; Kano, H.; Hori, M.; Kikkawa, F. Effect of Indirect Nonequilibrium Atmospheric Pressure Plasma on Anti-Proliferative Activity against Chronic Chemo-Resistant Ovarian Cancer Cells In Vitro and In Vivo. PLoS ONE 2013, 8, e81576. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Yoshikawa, N.; Mizuno, Y.; Ito, M.; Tanaka, H.; Mizuno, M.; Toyokuni, S.; Hori, M.; Kikkawa, F.; Kajiyama, H. Preclinical Verification of the Efficacy and Safety of Aqueous Plasma for Ovarian Cancer Therapy. Cancers 2021, 13, 1141. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.G.; Xiang, B.; Merlino, D.J.; Baybutt, T.R.; Sahu, J.; Fridman, A.; Snook, A.E.; Miller, V. Non-Thermal Plasma Induces Immunogenic Cell Death in Vivo in Murine CT26 Colorectal Tumors. Oncoimmunology 2018, 7, e1484978. [Google Scholar] [CrossRef] [PubMed]
- Freund, E.; Liedtke, K.R.; van der Linde, J.; Metelmann, H.-R.; Heidecke, C.-D.; Partecke, L.-I.; Bekeschus, S. Physical Plasma-Treated Saline Promotes an Immunogenic Phenotype in CT26 Colon Cancer Cells in Vitro and in Vivo. Sci. Rep. 2019, 9, 634. [Google Scholar] [CrossRef]
- Jinno, R.; Komuro, A.; Yanai, H.; Ono, R. Antitumor Abscopal Effects in Mice Induced by Normal Tissue Irradiation Using Pulsed Streamer Discharge Plasma. J. Phys. D Appl. Phys. 2022, 55, 17LT01. [Google Scholar] [CrossRef]
- Qi, M.; Xu, D.; Wang, S.; Li, B.; Peng, S.; Li, Q.; Zhang, H.; Fan, R.; Chen, H.; Kong, M.G. In Vivo Metabolic Analysis of the Anticancer Effects of Plasma-Activated Saline in Three Tumor Animal Models. Biomedicines 2022, 10, 528. [Google Scholar] [CrossRef]
- Wang, Y.; Mang, X.; Li, D.; Wang, Z.; Chen, Y.; Cai, Z.; Tan, F. Cold Atmospheric Plasma Sensitizes Head and Neck Cancer to Chemotherapy and Immune Checkpoint Blockade Therapy. Redox Biol. 2024, 69, 102991. [Google Scholar] [CrossRef]
- Song, C.-H.; Attri, P.; Ku, S.-K.; Han, I.; Bogaerts, A.; Choi, E.H. Cocktail of Reactive Species Generated by Cold Atmospheric Plasma: Oral Administration Induces Non-Small Cell Lung Cancer Cell Death. J. Phys. D Appl. Phys. 2021, 54, 185202. [Google Scholar] [CrossRef]
- Chen, G.; Chen, Z.; Wang, Z.; Obenchain, R.; Wen, D.; Li, H.; Wirz, R.E.; Gu, Z. Portable Air-Fed Cold Atmospheric Plasma Device for Postsurgical Cancer Treatment. Sci. Adv. 2021, 7, eabg5686. [Google Scholar] [CrossRef]
- Lin, L.; Wang, L.; Liu, Y.; Xu, C.; Tu, Y.; Zhou, J. Non-thermal Plasma Inhibits Tumor Growth and Proliferation and Enhances the Sensitivity to Radiation In vitro and In vivo. Oncol. Rep. 2018, 40, 3405–3415. [Google Scholar] [CrossRef]
- Friedman, P.C.; Miller, V.; Fridman, G.; Lin, A.; Fridman, A. Successful Treatment of Actinic Keratoses Using Nonthermal Atmospheric Pressure Plasma: A Case Series. J. Am. Acad. Dermatol. 2017, 76, 349–350. [Google Scholar] [CrossRef] [PubMed]
- Wirtz, M.; Stoffels, I.; Dissemond, J.; Schadendorf, D.; Roesch, A. Actinic Keratoses Treated with Cold Atmospheric Plasma. J. Eur. Acad. Dermatol. Venereol. 2018, 32, E37–E39. [Google Scholar] [CrossRef] [PubMed]
- Arisi, M.; Soglia, S.; Guasco Pisani, E.; Venturuzzo, A.; Gelmetti, A.; Tomasi, C.; Zane, C.; Rossi, M.; Lorenzi, L.; Calzavara-Pinton, P. Cold Atmospheric Plasma (CAP) for the Treatment of Actinic Keratosis and Skin Field Cancerization: Clinical and High-Frequency Ultrasound Evaluation. Dermatol. Ther. 2021, 11, 855–866. [Google Scholar] [CrossRef]
- Schuster, M.; Seebauer, C.; Rutkowski, R.; Hauschild, A.; Podmelle, F.; Metelmann, C.; Metelmann, B.; von Woedtke, T.; Hasse, S.; Weltmann, K.-D.; et al. Visible Tumor Surface Response to Physical Plasma and Apoptotic Cell Kill in Head and Neck Cancer. J. Cranio-Maxillofac. Surg. 2016, 44, 1445–1452. [Google Scholar] [CrossRef]
- Metelmann, H.; Seebauer, C.; Rutkowski, R.; Schuster, M.; Bekeschus, S.; Metelmann, P. Treating Cancer with Cold Physical Plasma: On the Way to Evidence-based Medicine. Contrib. Plasma Phys. 2018, 58, 415–419. [Google Scholar] [CrossRef]
- Metelmann, H.-R.; Nedrelow, D.S.; Seebauer, C.; Schuster, M.; von Woedtke, T.; Weltmann, K.-D.; Kindler, S.; Metelmann, P.H.; Finkelstein, S.E.; Von Hoff, D.D.; et al. Head and Neck Cancer Treatment and Physical Plasma. Clin. Plasma Med. 2015, 3, 17–23. [Google Scholar] [CrossRef]
- Pefani-Antimisiari, K.; Athanasopoulos, D.K.; Marazioti, A.; Sklias, K.; Rodi, M.; de Lastic, A.-L.; Mouzaki, A.; Svarnas, P.; Antimisiaris, S.G. Synergistic Effect of Cold Atmospheric Pressure Plasma and Free or Liposomal Doxorubicin on Melanoma Cells. Sci. Rep. 2021, 11, 14788. [Google Scholar] [CrossRef]
- Mateu-Sanz, M.; Ginebra, M.-P.; Tornín, J.; Canal, C. Cold Atmospheric Plasma Enhances Doxorubicin Selectivity in Metastasic Bone Cancer. Free Radic. Biol. Med. 2022, 189, 32–41. [Google Scholar] [CrossRef]
- Momeni, S.; Shanei, A.; Sazgarnia, A.; Azmoonfar, R.; Ghorbani, F. Increased Radiosensitivity of Melanoma Cells through Cold Plasma Pretreatment Mediated by ICG. J. Radiat. Res. 2023, 64, 751–760. [Google Scholar] [CrossRef]
- Wu, E.; Nie, L.; Liu, D.; Lu, X.; Ostrikov, K. Plasma Poration: Transdermal Electric Fields, Conduction Currents, and Reactive Species Transport. Free Radic. Biol. Med. 2023, 198, 109–117. [Google Scholar] [CrossRef]
- Vijayarangan, V.; Delalande, A.; Dozias, S.; Pouvesle, J.-M.; Robert, E.; Pichon, C. New Insights on Molecular Internalization and Drug Delivery Following Plasma Jet Exposures. Int. J. Pharm. 2020, 589, 119874. [Google Scholar] [CrossRef] [PubMed]
- Biscop, E.; Lin, A.; Van Boxem, W.; Van Loenhout, J.; De Backer, J.; Deben, C.; Dewilde, S.; Smits, E.; Bogaerts, A. Influence of Cell Type and Culture Medium on Determining Cancer Selectivity of Cold Atmospheric Plasma Treatment. Cancers 2019, 11, 1287. [Google Scholar] [CrossRef] [PubMed]
- Boeckmann, L.; Schäfer, M.; Bernhardt, T.; Semmler, M.L.; Jung, O.; Ojak, G.; Fischer, T.; Peters, K.; Nebe, B.; Müller-Hilke, B.; et al. Cold Atmospheric Pressure Plasma in Wound Healing and Cancer Treatment. Appl. Sci. 2020, 10, 6898. [Google Scholar] [CrossRef]
| Tumor Model | Plasma Device | Exposure (Mode/Time) | Results |
|---|---|---|---|
| Melanoma (B16F10 cells) [56] | kINPen 09® plasma jet (argon gas, flow rate ~ 8 L/min) and DBD plasma, voltage (14 kV), pulse repetition rate (100-400 Hz), electric powers dissipated in the gas discharge (167–237 mW) | Direct exposure/exposure for 2 × 3 min with DBD (30 s pause after each 3 min treatment) and 5 min with plasma jet | Synergism with electrochemotherapy, leading to prolonged survival |
| Pancreatic cancer (6606PDA cells) [92] | kINPen MED® plasma jet/argon gas | Indirect exposure/intraperitoneal injection/1 mL of plasma-activated medium (PAM) for 10 min | Tumor growth reduction; increase in median survival and apoptosis; decrease in tumor proliferation; treatment considered safe |
| Gastric cancer (GCIY-EGFP cells) [64] | Plasma jet/argon gas/10 kV, powered by a 60 Hz commercial power supply | Indirect exposure—intraperitoneal injection/6 mL of plasma-activated medium (PAM) for 5 min | Tumor growth reduction; increase in median survival and apoptosis; decrease in tumor proliferation; treatment considered safe |
| Hepatoblastoma (HepG2 cells) [102] | Plasma jet/argon and oxygen gas/output voltage (3 kV), current (40 mA), average power 12 W | Direct exposure for 20 s | Tumor volume regression and increased apoptosis, particularly in combination with radiotherapy; treatment considered safe |
| Cutaneous squamous cell carcinoma (UV induced skin cancer) [78] | kINPen® plasma jet/argon gas | Direct exposure for 3 min | Reduced progression of UVB-induced SCC-like lesions; decreased cell proliferation |
| Cholangiocarcinoma (EGI-1 cells) [81] | Plasma jet/helium gas (flow rate 1 L/min), amplitude (9 kV), duty cycle (14%), repetition frequency (30 kHz) | Direct exposure for 1 min | Tumor size reduction and decreased growth rate; induction of DNA damage and tumor cell apoptosis |
| Ovarian cancer (ES2 cells) [94] | Plasma jet/argon and oxygen gas (flow rate 2 L/min), 10 kV, powered by a 60 mA 60 Hz commercial power supply | Indirect exposure—intraperitoneal injection/10 mL of/Ringer’s solution activated for 5 min | Inhibited tumor progression; improved overall survival; activated immune response |
| Breast cancer (4T1 cells) [89] | Plasma jet/helium gas/frequency (20 kHz), total input power (1 W) | Direct exposure for 300 s | Tumor growth and weight reduction; abscopal effect; increased survival and apoptosis; induction of immunogenic cell death |
| Melanoma (B16F10 cells) and breast cancer (4T1 cells) [101] | Portable ambient air-fed CAP (aCAP)/voltage (6 V) and flow 16.8 L/min | Direct exposure for 1, 2, 3 and 4 min | Synergism with surgery, leading to tumor growth inhibition, prolonged survival, and induction of cancer immunogenic cell death |
| Melanoma (A375 cells), oral squamous cell carcinoma (Tca-8113) and lung cancer (A549) [98] | DBD plasma/voltage applied to the electrodes was sinusoidal at 15 kHz with a peak-to-peak voltage of 6 kV; the total power was 40 w | Indirect exposure—intratumoral injection/5 mL of saline activated for 10 min | Inhibition of tumor growth and cell proliferation; treatment considered safe |
| Colorectal carcinoma (CT26 cells) [97] | DBD plasma/High voltage pulses (25 kV) with 20, 60, or 90 ns pulse width at a repetition rate of 100 pulses/s. | Direct exposure for 10 min | Tumor growth limitation; abscopal effect |
| Breast cancer (4T1 cells) [90] | Plasma jet/Argon gas (flow 2 L/min) or helium (flow 4 L/min) and oxygen (flow 0.2 L/min) gas, voltage (20 kV), frequency (18 kHz) | Indirect exposure/intratumoral injection/5 mL of medium activated for 3 min | Inhibition of tumor growth, particularly in combination with chemotherapy (doxorubicin); reduced doxorubicin-induced liver and kidney toxicity |
| Melanoma (B16F10 cells) and colon cancer (MC38 cells) [55] | MediPL® plasma torch system/argon gas (flow 2 L/min) | Direct exposure/exposure for 2, 5, or 15 min | Reduction in tumor volume and weight; decreased tumor proliferation; dose-dependent apoptosis. |
| Head and neck cancer (Fadu and SCC7 cells) [99] | Piezobrush® PZ2/piezoelectric direct discharge technology | Direct exposure for 1 min | Reduced tumor growth and weight; prolonged survival; synergistic effect with immune checkpoint blockade (PD-L1) and chemotherapy (cisplatin). |
| Glioblastoma (U-87 MG cells) [71] | Plasma jet/helium or argon gas (flow 2 L/min)/frequency (20 kHz), voltage (4.5 kV) | Direct and indirect exposure/variable exposure time, based on IC50 values obtained from in vitro results | Reduction in tumor size; increased survival rate; improved general motor function. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holanda, A.G.A.; Francelino, L.E.C.; Moura, C.E.B.d.; Alves Junior, C.; Matera, J.M.; Queiroz, G.F.d. Cold Atmospheric Plasma in Oncology: A Review and Perspectives on Its Application in Veterinary Oncology. Animals 2025, 15, 968. https://doi.org/10.3390/ani15070968
Holanda AGA, Francelino LEC, Moura CEBd, Alves Junior C, Matera JM, Queiroz GFd. Cold Atmospheric Plasma in Oncology: A Review and Perspectives on Its Application in Veterinary Oncology. Animals. 2025; 15(7):968. https://doi.org/10.3390/ani15070968
Chicago/Turabian StyleHolanda, André Gustavo Alves, Luiz Emanuel Campos Francelino, Carlos Eduardo Bezerra de Moura, Clodomiro Alves Junior, Julia Maria Matera, and Genilson Fernandes de Queiroz. 2025. "Cold Atmospheric Plasma in Oncology: A Review and Perspectives on Its Application in Veterinary Oncology" Animals 15, no. 7: 968. https://doi.org/10.3390/ani15070968
APA StyleHolanda, A. G. A., Francelino, L. E. C., Moura, C. E. B. d., Alves Junior, C., Matera, J. M., & Queiroz, G. F. d. (2025). Cold Atmospheric Plasma in Oncology: A Review and Perspectives on Its Application in Veterinary Oncology. Animals, 15(7), 968. https://doi.org/10.3390/ani15070968






