The Prevalence and Genetic Diversity of Avian Malaria in Wild Birds in the Republic of Korea
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. DNA Extraction
2.3. Real-Time Polymerase Chain Reaction
2.4. Conventional Polymerase Chain Reaction
2.5. Climate Data: Temperature and Precipitation
2.6. Lineage Analysis
2.7. Phylogenetic Analysis
2.8. Statistical Analysis
3. Results
3.1. Prevalence of Plasmodium spp.
3.2. Monthly Average Temperatures and Precipitation for Each Year
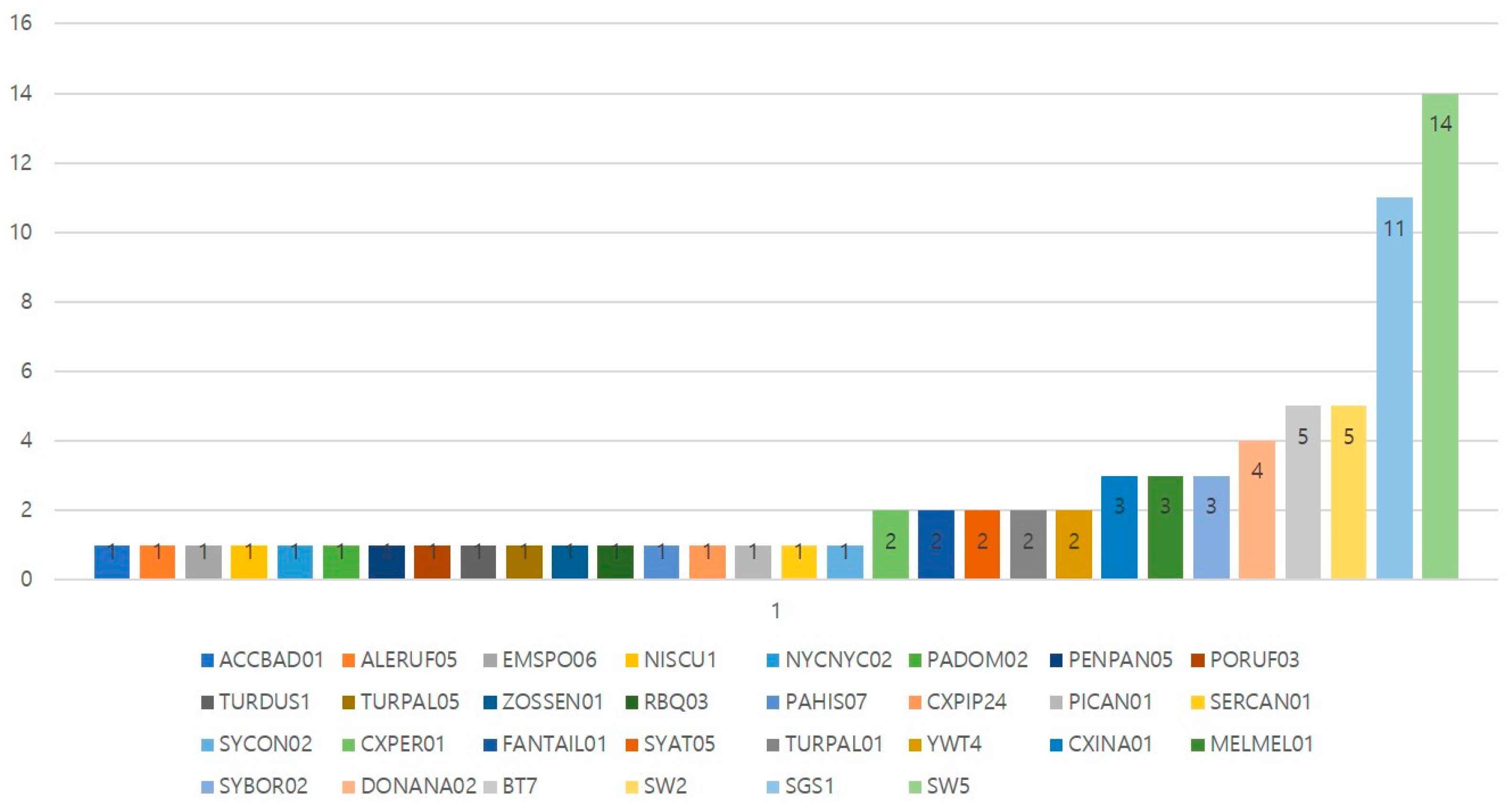
3.3. Lineage
3.4. Median-Joining Network
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Daszak, P.; Cunningham, A.A.; Hyatt, A.D. Emerging infectious diseases of wildlife-threats to biodiversity and human health. Science 2000, 287, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Ostfeld, R.S.; Brunner, J.L. Climate change and Ixodes tick-borne diseases of humans. Philos. Trans. R Soc. Lond. B Biol. Sci. 2015, 370, 20140051. [Google Scholar] [CrossRef] [PubMed]
- Loiseau, C.; Harrigan, R.J.; Cornel, A.J.; Guers, S.L.; Dodge, M.; Marzec, T.; Carlson, J.S.; Seppi, B.; Sehgal, R.N. First evidence and predictions of Plasmodium transmission in Alaskan bird populations. PLoS ONE 2012, 7, e44729. [Google Scholar] [CrossRef]
- Atkinson, C.T.; Dusek, R.J.; Woods, K.L.; Iko, W.M. Pathogenicity of avian malaria in experimentally-infected Hawaii Amakihi. J. Wildl. Dis. 2000, 36, 197–204. [Google Scholar] [CrossRef]
- Ilgūnas, M.; Bukauskaitė, D.; Palinauskas, V.; Iezhova, T.A.; Dinhopl, N.; Nedorost, N.; Weissenbacher-Lang, C.; Weissenböck, H.; Valkiūnas, G. Mortality and pathology in birds due to Plasmodium (Giovannolaia) homocircumflexum infection, with emphasis on the exoerythrocytic development of avian malaria parasites. Malar. J. 2016, 15, 256–266. [Google Scholar] [CrossRef]
- Asghar, M.; Hasselquist, D.; Hansson, B.; Zehtindjiev, P.; Westerdahl, H.; Bensch, S. Chronic infection. Hidden costs of infection: Chronic malaria accelerates telomere degradation and senescence in wild birds. Science 2015, 347, 436–438. [Google Scholar] [CrossRef]
- Hernández-Ospina, M.C.; Chitan-Guerrero, D.; Alvarez-Londoño, J.; Bohada-Murillo, M.; Martínez-Sánchez, E.T.; Rivera-Páez, F.A.; Castaño-Villa, G.J. Avian haemosporidians of the genera Plasmodium and Haemoproteus from resident and Neotropical migrant birds in Colombia. Parasitol. Res. 2024, 123, 252–264. [Google Scholar] [CrossRef]
- Marzal, A.; Magallanes, S.; Salas-Rengifo, T.; Muriel, J.; Navarro, C.; Vecco, D.; Guerra-Saldaña, C.; Mendo, L.; Paredes, V.; González-Blázquez, M.; et al. Prevalence and diversity of avian malaria parasites in illegally traded white-winged parakeets in Peruvian Amazonas. Anim. Conserv. 2024, 27, 364–373. [Google Scholar] [CrossRef]
- Ko, K.N.; Kang, S.C.; Jung, J.Y.; Bae, J.H.; Kim, J.H. Avian malaria associated with Plasmodium spp. Infection in a penguin in Jeju Island. Kor. J. Vet. Res. 2008, 48, 197–201. [Google Scholar]
- Kim, M.; Bae, J.; Oh, B.; Rhim, H.; Yang, M.S.; Yang, S.; Kim, B.; Han, J.I. Surveillance of wild animals carrying infectious agents based on high-throughput screening platform in the Republic of Korea. BMC Vet. Res. 2023, 19, 158–166. [Google Scholar] [CrossRef]
- Rhim, H.; Bae, J.; Kim, H.; Han, J.I. Prevalence and phylogenetic analysis of avian haemosporidia in wild birds in the Republic of Korea. J. Wildl. Dis. 2018, 54, 772–781. [Google Scholar] [CrossRef]
- National Institute of Korean Language. Available online: https://stdict.korean.go.kr/main/main.do (accessed on 26 February 2025).
- Cellier-Holzem, E.; Esparza-Salas, R.; Garnier, S.; Sorci, G. Effect of repeated exposure to Plasmodium relictum (lineage SGS1) on infection dynamics in domestic canaries. Int. J. Parasitol. 2010, 40, 1447–1453. [Google Scholar] [CrossRef] [PubMed]
- Bensch, S.; Hellgren, O.; Perez-Tris, J. MalAvi: A public database of malaria parasites and related haemosporidians in avian hosts based on mitochondrial cytochrome b lineages. Mol. Ecol. Resour. 2009, 9, 1353–1358. [Google Scholar] [CrossRef] [PubMed]
- Beadell, J.S.; Gering, E.; Austin, J.; Dumbacher, J.P.; Peirce, M.A.; Pratt, T.K.; Atkinson, C.T.; Fleischer, R.C. Prevalence and differential host-specificity of two avian blood parasite genera in the Australo-Papuan region. Mol. Ecol. 2004, 13, 3829–3844. [Google Scholar] [CrossRef] [PubMed]
- Korea Meteorological Administration. Available online: http://www.climate.go.kr/home/ (accessed on 12 March 2025).
- Thompson, J.D.; Gibson, T.J.; Plewniak, F.; Jeanmougin, F.; Higgins, D.G. The CLUSTAL_X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997, 25, 4876–4882. [Google Scholar] [CrossRef]
- Leigh, J.W.; Bryant, D. PopART: Full-feature software for haplotype network construction. Methods Ecol. Evol. 2015, 6, 1110–1116. [Google Scholar] [CrossRef]
- Valkiūnas, G. Avian Malaria Parasites and Other Hamosporidia, 1st ed.; CRC Press: Boca Raton, FL, USA, 2004; pp. 130–180. [Google Scholar]
- Schmitt, C.J.; Edwards, S.V. Passerine birds. Curr. Biol. 2022, 32, R1149–R1154. [Google Scholar] [CrossRef]
- Ribeiro, S.F.; Sebaio, F.; Branquinho, F.C.; Marini, M.A.; Vago, A.R.; Braga, E.M. Avian malaria in Brazilian passerine birds: Parasitism detected by nested PCR using DNA from stained blood smears. Parasitology 2005, 130, 261–267. [Google Scholar] [CrossRef]
- Wilkinson, L.C.; Handel, C.M.; Van Hemert, C.; Loiseau, C.; Sehgal, R.N. Avian malaria in a boreal resident species: Long-term temporal variability, and increased prevalence in birds with avian keratin disorder. Int. J. Parasitol. 2016, 46, 281–290. [Google Scholar] [CrossRef]
- National Institute of Biological Resources. Available online: https://species.nibr.go.kr/index.do (accessed on 26 February 2025).
- Altizer, S.; Bartel, R.; Han, B.A. Animal migration and infectious disease risk. Science 2011, 331, 296–302. [Google Scholar] [CrossRef]
- Han, Y.; Hellgren, O.; Wu, Q.; Liu, J.; Jin, T.; Bensch, S.; Ding, P. Seasonal variations of intensity of avian malaria infection in the Thousand Island Lake System, China. Parasit. Vectors 2023, 16, 218–229. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.B.; Lee, W.G.; Kang, H.J.; Yang, S.C.; Song, B.G.; Shin, E.H.; Kwon, O. Seasonal prevalence and species composition of mosquitoes and chigger mites collected from Daegu, Gunwi and Sangju in South Korea, 2014. J. Ecol. Environ. 2017, 41, 15. [Google Scholar] [CrossRef]
- Im, J.H.; Kim, T.S.; Chung, M.H.; Baek, J.H.; Kwon, H.Y.; Lee, J.S. Current Status and a Perspective of Mosquito-Borne Diseases in the Republic of Korea. Vector Borne Zoonotic Dis. 2021, 21, 69–77. [Google Scholar] [CrossRef]
- Valkiūnas, G.; Duc, M.; Iezhova, T.A. Increase of avian Plasmodium circumflexum prevalence, but not of other malaria parasites and related haemosporidians in northern Europe during the past 40 years. Malar. J. 2022, 21, 105–115. [Google Scholar] [CrossRef]
- Inumaru, M.; Murata, K.; Sato, Y. Prevalence of avian haemosporidia among injured wild birds in Tokyo and environs, Japan. Int. J. Parasitol. Parasites Wildl. 2017, 6, 299–309. [Google Scholar] [CrossRef]
- Meyer, C.L.; Bennett, G.F.; Herman, C.M. Mosquito transmission of Plasmodium (Giovannolaia) circumflexum Kikuth, 1931, to waterfowl in the Tantramar marshes New Brunswick. J. Parasitol. 1974, 60, 905–906. [Google Scholar] [CrossRef]
- Valkiūnas, G.; Ilgūnas, M.; Bukauskaitė, D.; Fragner, K.; Weissenböck, H.; Atkinson, C.T.; Iezhova, T.A. Characterization of Plasmodium relictum, a cosmopolitan agent of avian malaria. Malar. J. 2018, 17, 184–204. [Google Scholar] [CrossRef] [PubMed]
- Beadell, J.S.; Ishtiaq, F.; Covas, R.; Melo, M.; Warren, B.H.; Atkinson, C.T.; Bensch, S.; Graves, G.R.; Jhala, Y.V.; Peirce, M.A.; et al. Global phylogeographic limits of Hawaii’s avian malaria. Proc. Biol. Sci. 2006, 273, 2935–2944. [Google Scholar] [CrossRef]
- Atkinson, C.; Samuel, M.D. Avian malaria Plasmodium relictum in native Hawaiian forest birds: Epizootiology and demographic impacts on ‘apapane Himatione sanguinea. J. Avian Biol. 2010, 41, 357–366. [Google Scholar] [CrossRef]
- Ilgūnas, M.; Palinauskas, V.; Iezhova, T.A.; Valkiūnas, G. Molecular and morphological characterization of two avian malaria parasites (Haemosporida: Plasmodiidae), with description of Plasmodium homonucleophilum n. sp. Zootaxa 2013, 3666, 49–61. [Google Scholar] [CrossRef]
- Valkiūnas, G.; Ilgūnas, M.; Hernández-Lara, C.; Duc, M.; Iezhova, T. First experimental observation on biology of the avian malaria parasite Plasmodium (Novyella) homonucleophilum (lineage pSW2), with remarks on virulence and distribution. Acta Trop. 2024, 253, 107174. [Google Scholar] [CrossRef] [PubMed]


| Name | Sequences (5′ to 3′) | Target Gene | Length of Amplicon (bp) |
|---|---|---|---|
| 18sPlasm7 | AGC CTG AGA AAT AGC TAC CAC ATC TA | 18s rDNA | 60 |
| 18sPlasm8 | TGT TAT TTC TTG TCA CTA CCT CTC TTC TTT | ||
| Plasm Hyb2 | FAM-CAG CAG GCG CGT AAA TTA CCC AAT TC-BHQ1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.; Wut Hmohn, Z.Z.; Han, J.-I. The Prevalence and Genetic Diversity of Avian Malaria in Wild Birds in the Republic of Korea. Animals 2025, 15, 957. https://doi.org/10.3390/ani15070957
Kim M, Wut Hmohn ZZ, Han J-I. The Prevalence and Genetic Diversity of Avian Malaria in Wild Birds in the Republic of Korea. Animals. 2025; 15(7):957. https://doi.org/10.3390/ani15070957
Chicago/Turabian StyleKim, Myeongsu, Zun Zun Wut Hmohn, and Jae-Ik Han. 2025. "The Prevalence and Genetic Diversity of Avian Malaria in Wild Birds in the Republic of Korea" Animals 15, no. 7: 957. https://doi.org/10.3390/ani15070957
APA StyleKim, M., Wut Hmohn, Z. Z., & Han, J.-I. (2025). The Prevalence and Genetic Diversity of Avian Malaria in Wild Birds in the Republic of Korea. Animals, 15(7), 957. https://doi.org/10.3390/ani15070957





