Effect of Vachellia tortilis Leaf Meal and Sunflower Oil Inclusion in Supplementary Diets of Lambs on In Vitro Short-Chain Fatty Acid and Gas Production and In Vivo Growth Performance
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site and Ethics
2.2. Leaf Meal Collection and Preparation
2.3. Experimental Design, Experimental Diets, and Growth Performance
2.4. Chemical Analyses of Dietary Treatments and Fecal Samples
2.5. In Vitro Digestion
2.6. Gas Chromatography Analysis of Short-Chain Fatty Acids
2.7. Calculation of Carbon Dioxide and Methane Production from the Proportion of Short-Chain Fatty Acids
2.8. Statistical Analysis
3. Results
3.1. Chemical Composition
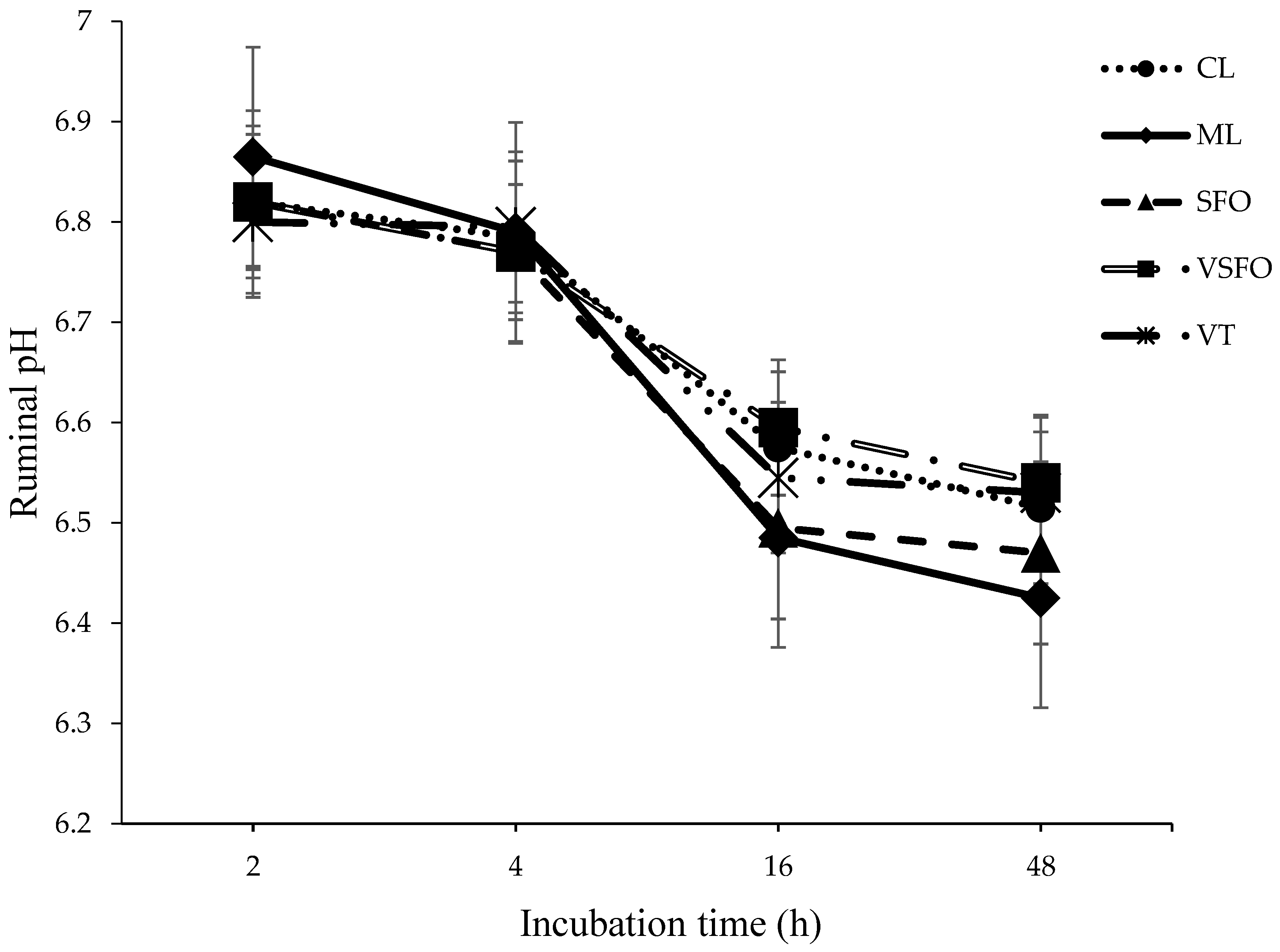
3.2. In Vitro Fermentation Parameters
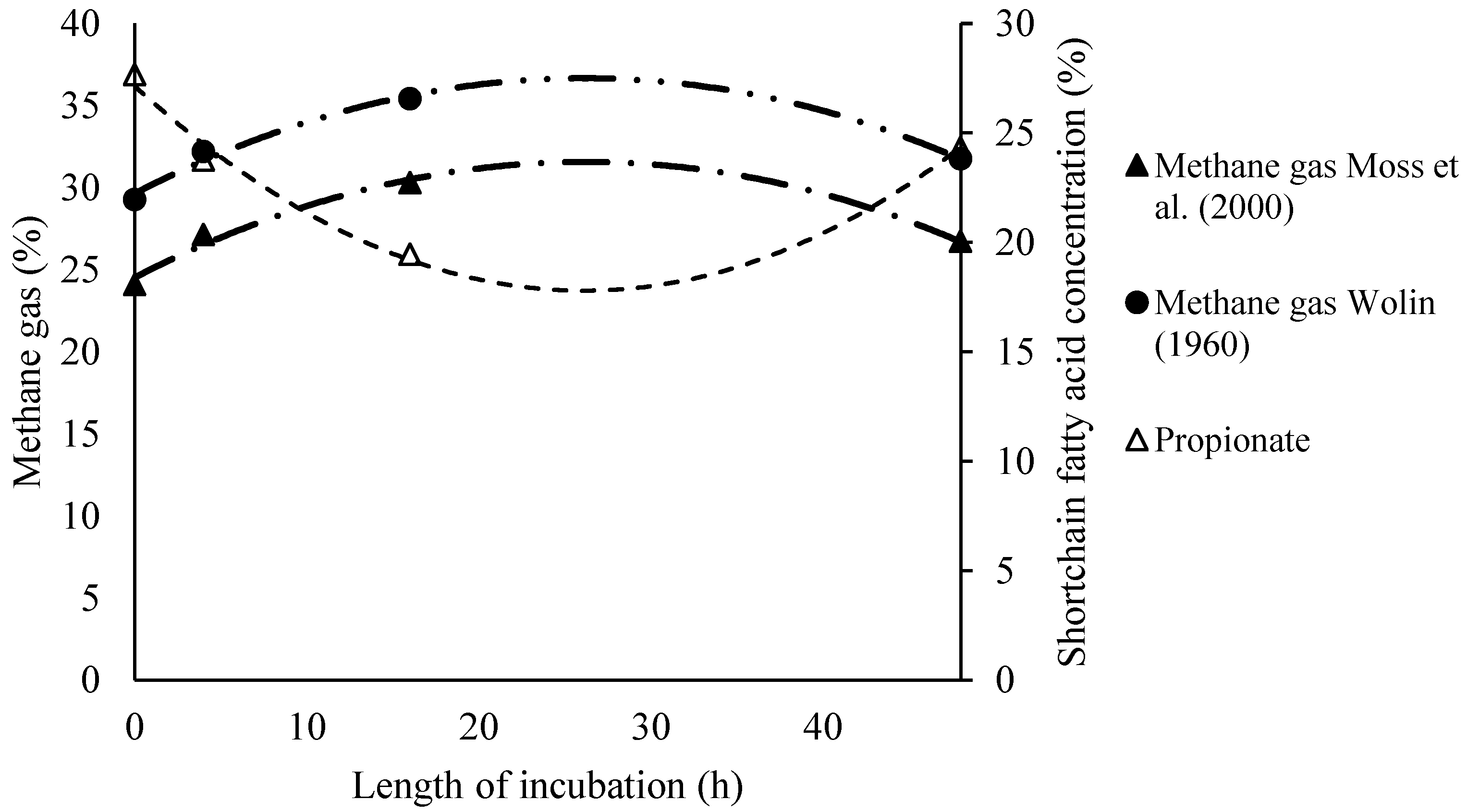
3.3. Regression Equations of the Relationship Between Short-Chain Fatty Acid and Methane Production and Length of Incubation Time
3.4. Growth Performance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alemu, A.W.; Vyas, D.; Manafiazar, G.; Basarab, J.A.; Beauchemin, K.A. Enteric Methane Emissions from Low– and High–Residual Feed Intake Beef Heifers Measured Using GreenFeed and Respiration Chamber Techniques. J. Anim. Sci. 2017, 95, 3727. [Google Scholar] [CrossRef] [PubMed]
- Fernández, C.; Pérez-Baena, I.; Marti, J.V.; Palomares, J.L.; Jorro-Ripoll, J.; Segarra, J.V. Use of Orange Leaves as a Replacement for Alfalfa in Energy and Nitrogen Partitioning, Methane Emissions and Milk Performance of Murciano-Granadina Goats. Anim. Feed Sci. Technol. 2019, 247, 103–111. [Google Scholar] [CrossRef]
- Gemeda, B.S. The Potential of Tropical Tannin Rich Browses in Reduction of Enteric Methane. Approaches Poult. Dairy Vet. Sci. 2018, 2, 154–162. [Google Scholar] [CrossRef]
- Lima, P.R.; Apdini, T.; Freire, A.S.; Santana, A.S.; Moura, L.M.L.; Nascimento, J.C.S.; Rodrigues, R.T.S.; Dijkstra, J.; Garcez Neto, A.F.; Queiroz, M.A.Á.; et al. Dietary Supplementation with Tannin and Soybean Oil on Intake, Digestibility, Feeding Behavior, Ruminal Protozoa and Methane Emission in Sheep. Anim. Feed Sci. Technol. 2019, 249, 10–17. [Google Scholar] [CrossRef]
- Ungerfeld, E.M. Shifts in Metabolic Hydrogen Sinks in the Methanogenesis-Inhibited Ruminal Fermentation: A Meta-Analysis. Front. Microbiol. 2015, 6, 37. [Google Scholar] [CrossRef]
- Ungerfeld, E.M. Metabolic Hydrogen Flows in Rumen Fermentation: Principles and Possibilities of Interventions. Front. Microbiol. 2020, 11, 589. [Google Scholar] [CrossRef]
- Hariadi, B.T.; Santoso, B. Evaluation of Tropical Plants Containing Tannin on in Vitro Methanogenesis and Fermentation Parameters Using Rumen Fluid. J. Sci. Food Agric. 2010, 90, 456–461. [Google Scholar] [CrossRef]
- Jiao, H.P.; Dale, A.J.; Carson, A.F.; Murray, S.; Gordon, A.W.; Ferris, C.P. Effect of Concentrate Feed Level on Methane Emissions from Grazing Dairy Cows. J. Dairy Sci. 2014, 97, 7043–7053. [Google Scholar] [CrossRef] [PubMed]
- Goel, G.; Makkar, H.P.S. Methane Mitigation from Ruminants Using Tannins and Saponins. Trop. Anim. Health Prod. 2012, 44, 729–739. [Google Scholar] [CrossRef]
- Goel, G.; Makkar, H.P.S.; Becker, K. Effects of Sesbania Sesban and Carduus Pycnocephalus Leaves and Fenugreek (Trigonella Foenum-graecum L.) Seeds and Their Extracts on Partitioning of Nutrients from Roughage- and Concentrate-Based Feeds to Methane. Anim. Feed Sci. Technol. 2008, 147, 72–89. [Google Scholar] [CrossRef]
- Newton, E.E.; Pétursdóttir, Á.H.; Ríkharðsson, G.; Beaumal, C.; Desnica, N.; Giannakopoulou, K.; Juniper, D.; Ray, P.; Stergiadis, S. Effect of Dietary Seaweed Supplementation in Cows on Milk Macrominerals, Trace Elements and Heavy Metal Concentrations. Foods 2021, 10, 1526. [Google Scholar] [CrossRef] [PubMed]
- Beauchemin, K.A.; Ungerfeld, E.M.; Eckard, R.J.; Wang, M. Review: Fifty Years of Research on Rumen Methanogenesis: Lessons Learned and Future Challenges for Mitigation. Animal 2020, 14, S2–S16. [Google Scholar] [CrossRef]
- Hlatini, V.A.; Ncobela, C.N.; Zindove, T.J.; Chiminyo, M.; Hlatini, V.A. Use of Polyethylene Glycol to Improve the Utilisation of Leguminous Leaf Meals in Pigs: A Review. South Afr. J. Anim. Sci. 2018, 48, 609–620. [Google Scholar] [CrossRef]
- Hristov, A.N.; Melgar, A.; Wasson, D.; Arndt, C. Symposium Review: Effective Nutritional Strategies to Mitigate Enteric Methane in Dairy Cattle. J. Dairy Sci. 2022, 105, 8543–8557. [Google Scholar] [CrossRef] [PubMed]
- Patra, A.K. A Meta-Analysis of the Effect of Dietary Fat on Enteric Methane Production, Digestibility and Rumen Fermentation in Sheep, and a Comparison of These Responses between Cattle and Sheep. Livest. Sci. 2014, 162, 97–103. [Google Scholar] [CrossRef]
- Patra, A.K.; Saxena, J. The Effect and Mode of Action of Saponins on the Microbial Populations and Fermentation in the Rumen and Ruminant Production. Nutr. Res. Rev. 2009, 22, 204–219. [Google Scholar] [CrossRef]
- Hassanp, S.; Maheri-Sis, N.; Eshratkhah, B.; Baghbani, F.; Hassanpour, S.; Mehmandar, F.B. Plants and Secondary Metabolites (Tannins): A Review. Int. J. For. Soil Eros. 2011, 1, 47–53. [Google Scholar]
- AFRC Energy and Protein Requirements of Ruminants. Advisory Manual Prepared by AFRC Technical Committee on Response to Nutrients; CAB International: Walingford, UK, 2003; Volume 14. [Google Scholar]
- Mathobela, R.M. Effect of Acacia Species Leaf Meal Inclusion on Methane Emission and Productivity of Yearling Male Boer Goats Fed an Avena Sativa Hay-Based Diet. Ph.D. Thesis, University of Limpopo, Polokwane, South Africa, 2018. [Google Scholar]
- Bitende, S.N.; Ledin, I. Effect of Doubling the Amount of Low Quality Grass Hay Offered and Supplementation with Acacia Tortilis Fruits or Sesbania Sesban Leaves, on Intake and Digestibility by Sheep in Tanzania. Livest. Prod. Sci. 1996, 45, 39–48. [Google Scholar] [CrossRef]
- Hussien, B.; Bekele, B. Effects of Supplementing Borana Dairy Cows with Local (Vachelliatortilis Pods) and Conventional Feeds on Milk Yield and Milk Composition. Glob. J. Anim. Sci. Res. 2022, 10, 79–89. [Google Scholar]
- Araya, M.R.; Ngugi, R.K.; Musimba, N.K.R.; Nyariki, D.M. Effect of Acacia Tortilis Pods on Intake, Digestibility and Nutritive Quality of Goat Diets in Southwestern Eritrea. Afr. J. Range Forage Sci. 2003, 20, 59–62. [Google Scholar] [CrossRef]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci 1991, 74, 3583–3597. [Google Scholar] [CrossRef] [PubMed]
- Reed, J.D. Nutritional Toxicology of Tannins and Related Polyphenols in Forage Legumes. J. Anim. Sci. 1995, 73, 1516–1528. [Google Scholar] [CrossRef] [PubMed]
- Tilley, J.M.A.; Terry, R.A. A Two-Stage Technique for The In Vitro Digestion of Forage Crops. Grass Forage Sci. 1963, 18, 104–111. [Google Scholar] [CrossRef]
- Akanmu, A.M.; Hassen, A. The Use of Certain Medicinal Plant Extracts Reduced in vitro Methane Production While Improving in Vitro Organic Matter Digestibility. Anim. Prod. Sci. 2018, 58, 900–908. [Google Scholar] [CrossRef]
- Suinyuy, T.N.; Donaldson, J.S.; Johnson, S.D. Patterns of Odour Emission, Thermogenesis and Pollinator Activity in Cones of an African Cycad: What Mechanisms Apply? Ann. Bot. 2013, 112, 891–902. [Google Scholar] [CrossRef]
- Wolin, M.J. A Theoretical Rumen Fermentation Balance. J. Dairy Sci. 1960, 43, 1452–1459. [Google Scholar] [CrossRef]
- Moss, A.R.; Jouany, J.-P.; Newbold, J. Methane Production by Ruminants: Its Contribution to Global Warming. Ann. Zootech. 2000, 49, 231–253. [Google Scholar] [CrossRef]
- Molina-Botero, I.C.; Arroyave-Jaramillo, J.; Valencia-Salazar, S.; Barahona-Rosales, R.; Aguilar-Pérez, C.F.; Ayala Burgos, A.; Arango, J.; Ku-Vera, J.C. Effects of Tannins and Saponins Contained in Foliage of Gliricidia Sepium and Pods of Enterolobium Cyclocarpum on Fermentation, Methane Emissions and Rumen Microbial Population in Crossbred Heifers. Anim. Feed Sci. Technol. 2019, 251, 1–11. [Google Scholar] [CrossRef]
- Paya, H.; Author, C.; Akbar, T.; Hamid, P.; Akbar, T.; Hossein, J.; Gholam Ali, M. Nutrient Digestibility and Gas Production of Some Tropical Feeds Used in Ruminant Diets Estimated by the in Vivo and in Vitro Gas Production Techniques. Am. J. Anim. Vet. Sci. 2007, 2, 108–113. [Google Scholar]
- da Silva, J.J.M.; Campanharo, S.C.; Paschoal, J.A.R. Ethnoveterinary for Food-Producing Animals and Related Food Safety Issues: A Comprehensive Overview about Terpenes. Compr. Rev. Food Sci. Food Saf. 2021, 20, 48–90. [Google Scholar] [CrossRef]
- Camero, A.; Franco, M. Improving Rumen Fermentation and Milk Production with Legume-Tree Fodder in the Tropics. Agrofor. Syst. 2001, 51, 157–166. [Google Scholar] [CrossRef]
- Śliwiński, B.J.; Kreuzer, M.; Wettstein, H.-R.; Machmüller, A. Rumen Fermentation and Nitrogen Balance of Lambs Fed Diets Containing Plant Extracts Rich in Tannins and Saponins, and Associated Emissions of Nitrogen and Methane. Arch. Anim. Nutr. 2002, 56, 379–392. [Google Scholar] [CrossRef]
- Muhlisin; Yusiati, L.M.; Hanim, C.; Anas, M.A.; Muktiari, B.N. Effect of Leucaena Leucocephala Substitution on in Vitro Rumen Fermentation and Methane Emission in Thin Tailed-Sheep. IOP Conf. Ser. Earth Environ. Sci. 2019, 387, 012124. [Google Scholar] [CrossRef]
- Animut, G.; Puchala, R.; Goetsch, A.L.; Patra, A.K.; Sahlu, T.; Varel, V.H.; Wells, J. Methane Emission by Goats Consuming Different Sources of Condensed Tannins. Anim. Feed Sci. Technol. 2008, 144, 228–241. [Google Scholar] [CrossRef]
- Poudel, S.; Zeller, W.E.; Fike, J.; Pent, G. Condensed Tannins Attributes: Potential Solution to Fescue Toxicosis? Agriculture 2023, 13, 672. [Google Scholar] [CrossRef]
- Animut, G.; Puchala, R.; Goetsch, A.L.; Patra, A.K.; Sahlu, T.; Varel, V.H.; Wells, J. Methane Emission by Goats Consuming Diets with Different Levels of Condensed Tannins from Lespedeza. Anim. Feed Sci. Technol. 2008, 144, 212–227. [Google Scholar] [CrossRef]
- Naumann, H.D.; Tedeschi, L.O.; Zeller, W.E.; Huntley, N.F. The Role of Condensed Tannins in Ruminant Animal Production: Advances, Limitations and Future Directions. Rev. Bras. Zootec. 2017, 46, 929–949. [Google Scholar] [CrossRef]
- Puchala, R.; Min, B.R.; Goetsch, A.L.; Sahlu, T. The Effect of a Condensed Tannin-Containing Forage on Methane Emission by Goats. J. Anim. Sci. 2005, 83, 182–186. [Google Scholar] [CrossRef]
- Tedeschi, L.O.; Muir, J.P.; Naumann, H.D.; Norris, A.B.; Ramírez-Restrepo, C.A.; Mertens-Talcott, S.U. Nutritional Aspects of Ecologically Relevant Phytochemicals in Ruminant Production. Front. Vet. Sci. 2021, 8, 628445. [Google Scholar] [CrossRef]
- Króliczewska, B.; Pecka-Kiełb, E.; Bujok, J. Strategies Used to Reduce Methane Emissions from Ruminants: Controversies and Issues. Agriculture 2023, 13, 602. [Google Scholar] [CrossRef]
- Sutton, J.D.; Knight, R.; McAllan, A.B.; Smith, R.H. Digestion and Synthesis in the Rumen of Sheep given Diets Supplemented with Free and Protected Oils. Br. J. Nutr. 1983, 49, 419–432. [Google Scholar] [CrossRef] [PubMed]
- Lunsin, R. Effect of Oil Palm Meal on Nutrient Utilization and Milk Production in Lactating Dairy Cows Fed with Urea-Treated Rice Straw. Agric. Nat. Resour. 2018, 52, 285–289. [Google Scholar] [CrossRef]

| Dietary Treatments (g/kg) 1 | |||||
|---|---|---|---|---|---|
| Ingredient Composition | CL | VT | VSFO | SFO | SEM |
| Vachellia tortilis | 0.0 | 121.500 | 63.400 | 0.0 | - |
| Sunflower oil | 0.0 | 0.0 | 19.500 | 40.810 | - |
| Maize grain | 297.901 | 261.700 | 273.200 | 285.704 | - |
| Soybean meal | 212.801 | 186.910 | 195.101 | 204.100 | - |
| Lucerne | 276.601 | 243.0 | 253.700 | 265.300 | - |
| Wheat bran | 148.913 | 130.8 | 136.600 | 142.900 | - |
| Sunflower cake | 63.832 | 56.104 | 58.500 | 61.200 | - |
| Chemical Composition (g/kg) 2 | |||||
| DM | 894 | 894 | 899 | 901 | ±0.001 |
| ASH | 54.332 | 67.8 | 61.628 | 55.183 | ±2.38 |
| OM | 946 | 932 | 938 | 945 | ±2.38 |
| CP | 229 | 241 | 257 | 253 | ±4.84 |
| EE | 18.401 | 20.3 | 37.802 | 55.8 | ±0.17 |
| NDF | 314 | 311 | 302 | 346 | ±1.17 |
| ADF | 143 | 149 | 145 | 147 | ±0.73 |
| ADL | 34 | 36 | 38 | 32 | ±0.35 |
| Condensed tannin | 0.0 | 7.101 | 5.672 | 0.0 | ±1.69 |
| Dietary Treatments 1 | ||||||
|---|---|---|---|---|---|---|
| Parameters 2 | CL | VT | VSFO | SFO | RMSE 3 | p-Value |
| Total SCFAs (µmol/L) | 222.237 | 132.053 | 159.264 | 140.580 | 75.789 | 0.161 |
| Acetate | 63.761 | 60.361 | 57.636 | 62.330 | 7.341 | 0.547 |
| Propionate | 23.873 | 24.732 | 25.65 | 23.790 | 3.999 | 0.837 |
| Butyrate | 10.749 b | 13.810 ba | 14.871 a | 13.870 ba | 2.23 | 0.052 |
| A: P ratio | 2.745 | 2.581 | 2.371 | 2.711 | 0.589 | 0.750 |
| %CH4 (M) | 27.072 | 26.346 | 25.570 | 27.061 | 2.656 | 0.778 |
| %CH4 (W) | 32.090 | 31.476 | 30.765 | 32.155 | 2.498 | 0.888 |
| %CO2 (W) | 56.399 | 58.821 | 60.313 | 57.933 | 3.192 | 0.223 |
| IVDMD (g/kg) | 44.170 ba | 34.333 b | 53.5710 a | 37.511 b | 12.458 | 0.026 |
| Parameters 1 | Incubation Time (h) | Multiple Comparisons | Regression Coefficient | |||||
|---|---|---|---|---|---|---|---|---|
| 2 | 4 | 16 | 48 | RMSE 2 | p-Value | Linear | Quadratic | |
| Total SCFAs (mol/L) | 153.742 | 199.931 | 162.013 | 134.300 | 71.174 | 0.252 | −0.44 NS | −0.009 NS |
| Acetate (%) | 55.120 b | 61.545 ba | 66.010 a | 61.7201 ba | 7.342 | 0.034 | 0.98 NS | −0.02 ** |
| Propionate (%) | 28.302 a | 24.891 b | 21.390 b | 23.803 b | 3.399 | 0.002 | −0.64 NS | 0.01 *** |
| Butyrate (%) | 16.580 | 13.596 | 12.610 | 14.482 | 4.051 | 0.190 | −0.33 NS | 0.006 NS |
| A:P ratio | 2.000 b | 2.566 ba | 3.144 a | 2.661 ba | 0.589 | 0.003 | 0.11 NS | −0.002 *** |
| % CH4 (M) | 23.65 b | 26.290 a | 28.857 a | 27.023 a | 2.656 | 0.003 | 0.48 * | −0.009 *** |
| % CH4 (W) | 28.773 b | 31.352 a | 33.964 a | 32.153 a | 2.549 | 0.002 | 0.48 * | −0.009 *** |
| % CO2 (W) | 59.500 | 57.382 | 57.261 | 58.533 | 3.262 | 0.390 | −0.18 NS | 0.004 NS |
| Variables | Parameter Estimates | Regression Analysis | Turning Point | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Sunflower Oil Inclusion | Intercept | Linear | Quadratic | RMSE | R2-Value | p-Value | Type 1 | X (h) | Y (%) |
| Propionate (%) | 28.1 ± 1.840 | −0.79 ± 2.669 | 0.01 ± 0.005 | 2.665 | 0.635 | 0.034 | Min | 39.50 | 12.50 |
| A:P | 1.9 ± 0.280 | 0.14 ± 0.040 | −0.003 ± 0.001 | 0.399 | 0.718 | 0.017 | Max | 23.30 | 3.53 |
| Methane gas production | |||||||||
| Sunflower oil inclusion | |||||||||
| % CH4 (M) | 23.8 ± 1.410 | 0.59 ± 0.206 | −0.01 ± 0.004 | 2.043 | 0.623 | 0.037 | Max | 29.50 | 32.50 |
| % CH4 (W) | 29.0 ± 1.380 | 0.59 ± 0.202 | −0.01 ± 0.004 | 1.999 | 0.635 | 0.034 | Max | 29.50 | 37.70 |
| Parameters 2 | Dietary Treatments (g/kg) 1 | |||||
|---|---|---|---|---|---|---|
| CL | VT | VSFO | SFO | RMSE 3 | p-Value | |
| Concentrate DMI (g DM) | 433 | 433 | 431 | 433 | 0.006 | 0.342 |
| Basal diet DMI (g DM) | 548 | 583 | 568 | 534 | 0.081 | 0.245 |
| Total DMI (g DM) | 981 | 1016 | 998 | 968 | 0.083 | 0.233 |
| ADG (g) | 181 | 170 | 173 | 161 | 0.071 | 0.621 |
| FCR (g feed/g gain) | 4.10 | 4.80 | 4.90 | 4.70 | 2.594 | 0.457 |
| APD (g/kg DM) | 570 a | 480 ba | 440 b | 410 b | 82 | 0.033 |
| ttNDFd (g/kg DM) | 500 | 450 | 450 | 470 | 32 | 0.069 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serumula, M.D.; Pepeta, B.N.; Moyo, M.; Suinyuy, T.N.; Nsahlai, I.V. Effect of Vachellia tortilis Leaf Meal and Sunflower Oil Inclusion in Supplementary Diets of Lambs on In Vitro Short-Chain Fatty Acid and Gas Production and In Vivo Growth Performance. Animals 2025, 15, 863. https://doi.org/10.3390/ani15060863
Serumula MD, Pepeta BN, Moyo M, Suinyuy TN, Nsahlai IV. Effect of Vachellia tortilis Leaf Meal and Sunflower Oil Inclusion in Supplementary Diets of Lambs on In Vitro Short-Chain Fatty Acid and Gas Production and In Vivo Growth Performance. Animals. 2025; 15(6):863. https://doi.org/10.3390/ani15060863
Chicago/Turabian StyleSerumula, Mahlogonolo Daniel, Bulelani Nangamso Pepeta, Mehluli Moyo, Terence Nkwanwir Suinyuy, and Ignatius Verla Nsahlai. 2025. "Effect of Vachellia tortilis Leaf Meal and Sunflower Oil Inclusion in Supplementary Diets of Lambs on In Vitro Short-Chain Fatty Acid and Gas Production and In Vivo Growth Performance" Animals 15, no. 6: 863. https://doi.org/10.3390/ani15060863
APA StyleSerumula, M. D., Pepeta, B. N., Moyo, M., Suinyuy, T. N., & Nsahlai, I. V. (2025). Effect of Vachellia tortilis Leaf Meal and Sunflower Oil Inclusion in Supplementary Diets of Lambs on In Vitro Short-Chain Fatty Acid and Gas Production and In Vivo Growth Performance. Animals, 15(6), 863. https://doi.org/10.3390/ani15060863







