Distribution Shifts of Acanthaster solaris Under Climate Change and the Impact on Coral Reef Habitats
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Abiotic and Biotic Variables
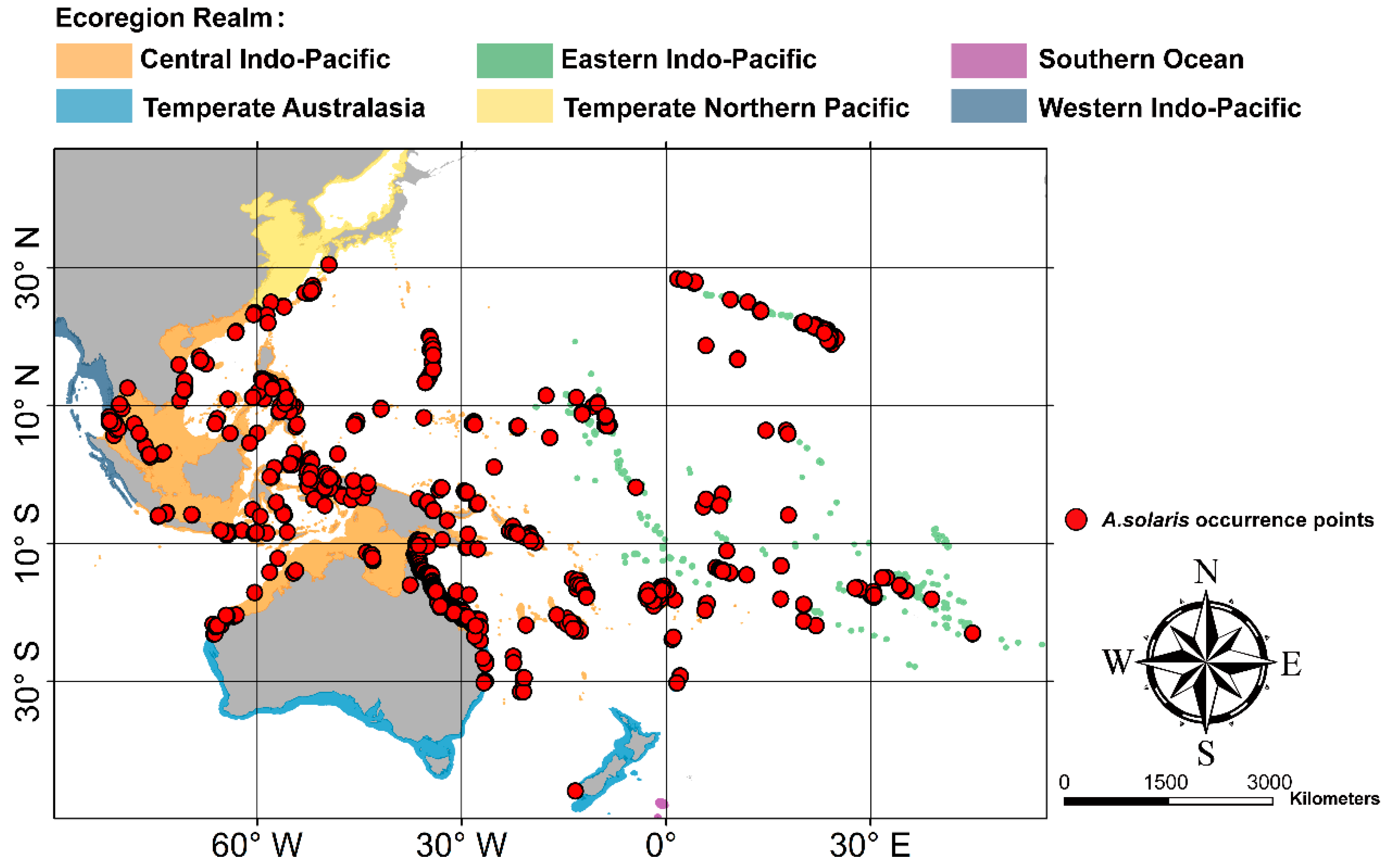
2.3. Species Distribution Data
2.4. SDM Training and Prediction
2.5. Assessing the Impact of A. solaris Distribution on Coral Reef Habitats
3. Results
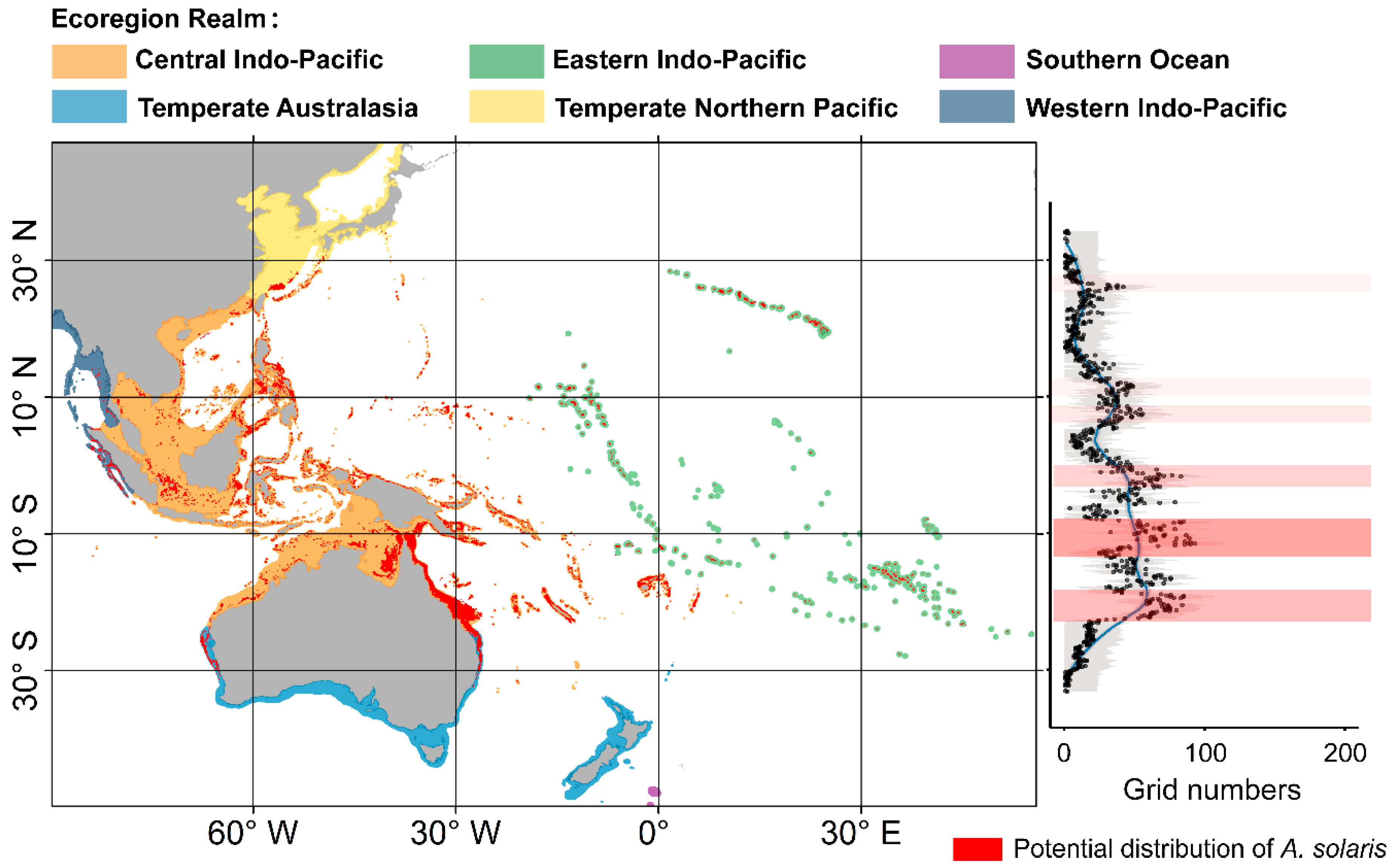
3.1. Current Distribution of A. Solaris and Its Determining Factors
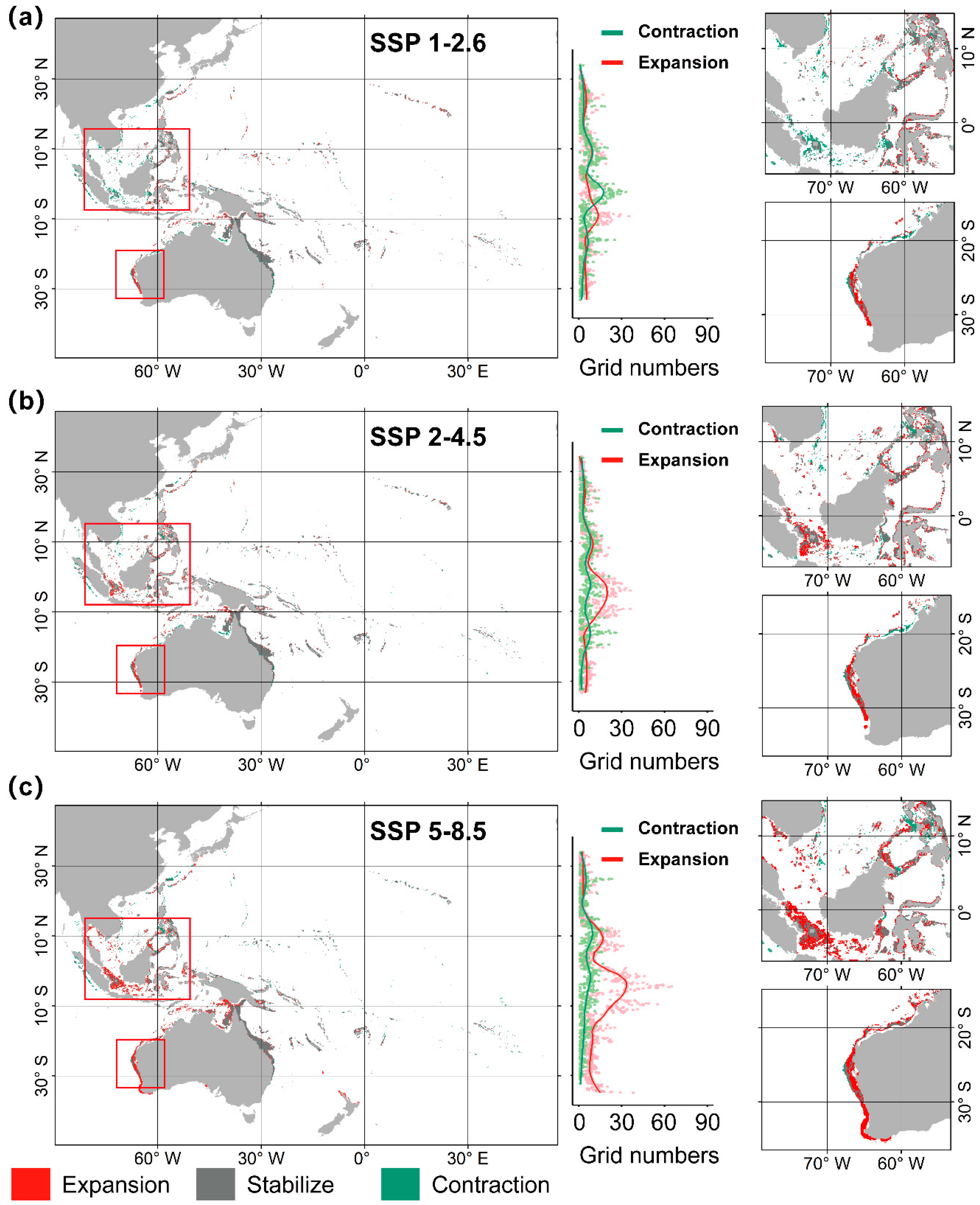
3.2. Distribution of A. solaris Under Climate Change
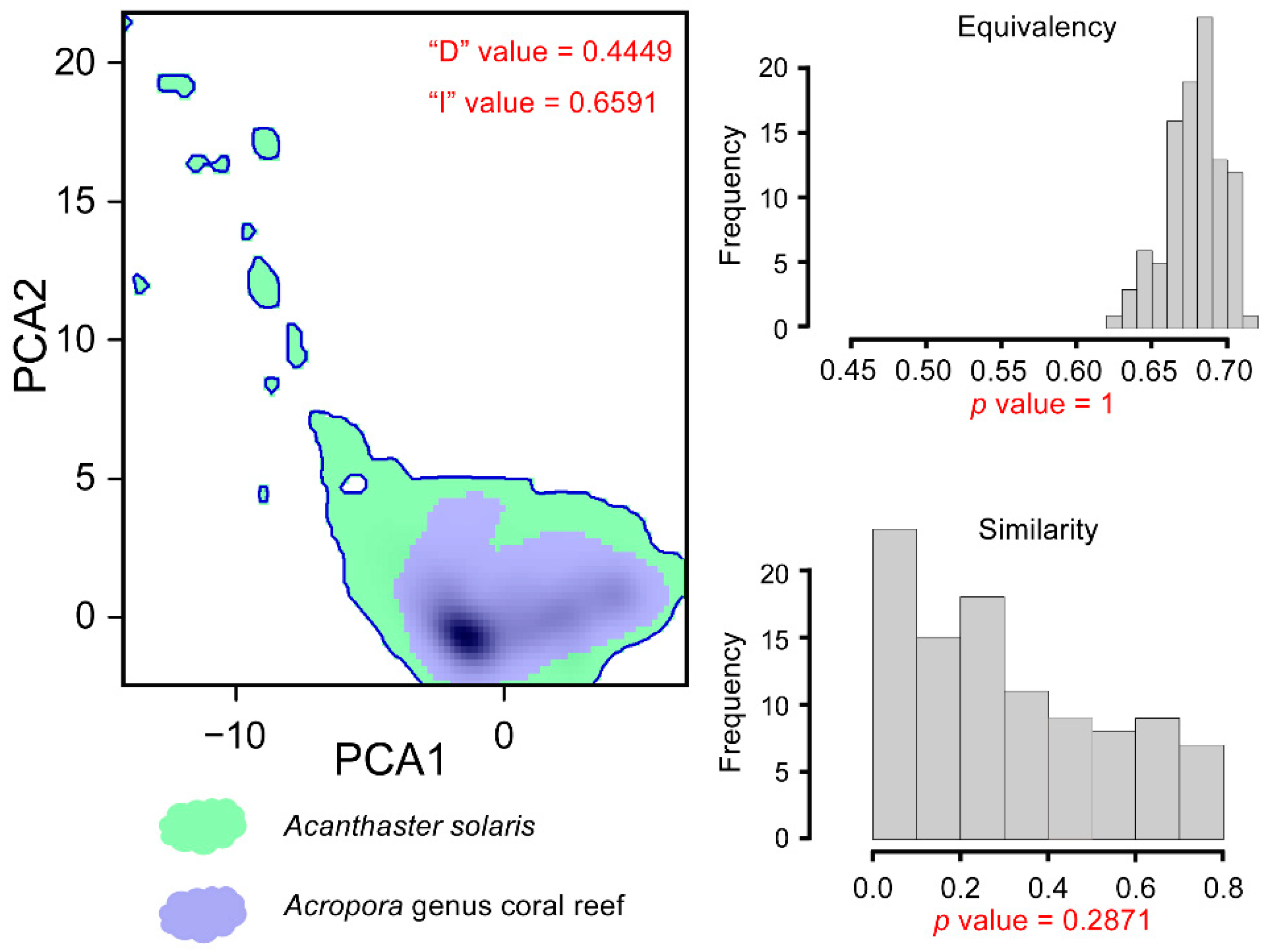
3.3. Impact of A. solaris on Acropora Habitats
4. Discussion
4.1. Distribution and Dynamics of A. solaris
4.2. Risk of A. solaris Under Climate Change
4.3. Study Uncertainty and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fabricius, K.E.; Okaji, K.; De’ath, G. Three lines of evidence to link outbreaks of the crown-of-thorns seastar Acanthaster planci to the release of larval food limitation. Coral Reefs 2010, 29, 593–605. [Google Scholar] [CrossRef]
- Wang, Y.; Gu, Y.-B.; Guo, H.; Cao, L.-Q.; Jin, Y. Advances and perspectives on the research of starfish outbreaks in northern China. Ying Yong Sheng Tai Xue Bao 2023, 34, 1146–1152. [Google Scholar] [CrossRef] [PubMed]
- Vogler, C.; Benzie, J.; Lessios, H.; Barber, P.; Wörheide, G. A threat to coral reefs multiplied? Four species of crown-of-thorns starfish. Biol. Lett. 2008, 4, 696–699. [Google Scholar] [CrossRef]
- Chesher, R.H. Destruction of Pacific Corals by the Sea Star Acanthaster planci. Science 1969, 165, 280–283. [Google Scholar] [CrossRef]
- Adams, M.S.; Demmig-Adams, B.; Li, R.; Zarate, D.; Li, J. Coral reef productivity and diversity—Contributions from enhanced photosynthesis via demand for carbohydrate from the host. Mar. Ecol. 2020, 41, e12618. [Google Scholar] [CrossRef]
- Pratchett, M.S.; Caballes, C.F.; Wilmes, J.C.; Matthews, S.; Mellin, C.; Sweatman, H.P.A.; Nadler, L.E.; Brodie, J.; Thompson, C.A.; Hoey, J.; et al. Thirty Years of Research on Crown-of-Thorns Starfish (1986–2016): Scientific Advances and Emerging Opportunities. Diversity 2017, 9, 41. [Google Scholar] [CrossRef]
- Carpenter, K.E.; Abrar, M.; Aeby, G.; Aronson, R.B.; Banks, S.; Bruckner, A.; Chiriboga, A.; Cortés, J.; Delbeek, J.C.; DeVantier, L.; et al. One-Third of Reef-Building Corals Face Elevated Extinction Risk from Climate Change and Local Impacts. Science 2008, 321, 560–563. [Google Scholar] [CrossRef]
- Uthicke, S.; Pratchett, M.S.; Bronstein, O.; Alvarado, J.J.; Wörheide, G. The crown-of-thorns seastar species complex: Knowledge on the biology and ecology of five corallivorous Acanthaster species. Mar. Biol. 2024, 171, 32. [Google Scholar] [CrossRef]
- De’ath, G.; Fabricius, K.E.; Sweatman, H.; Puotinen, M. The 27–year decline of coral cover on the Great Barrier Reef and its causes. Proc. Natl. Acad. Sci. USA 2012, 109, 17995–17999. [Google Scholar] [CrossRef]
- Castro-Sanguino, C.; Bozec, Y.-M.; Condie, S.A.; Fletcher, C.S.; Hock, K.; Roelfsema, C.; Westcott, D.A.; Mumby, P.J. Control efforts of crown-of-thorns starfish outbreaks to limit future coral decline across the Great Barrier Reef. Ecosphere 2023, 14, e4580. [Google Scholar] [CrossRef]
- Keesing, J.K.; Thomson, D.P.; Haywood, M.D.E.; Babcock, R.C. Two time losers: Selective feeding by crown-of-thorns starfish on corals most affected by successive coral-bleaching episodes on western Australian coral reefs. Mar. Biol. 2019, 166, 72. [Google Scholar] [CrossRef]
- Baird, A.H.; Pratchett, M.S.; Hoey, A.S.; Herdiana, Y.; Campbell, S.J. Acanthaster planci is a major cause of coral mortality in Indonesia. Coral Reefs 2013, 32, 803–812. [Google Scholar] [CrossRef]
- Foo, S.A.; Millican, H.R.; Byrne, M. Crown-of-thorns seastar (Acanthaster spp.) feeding ecology across species and regions. Sci. Total Environ. 2024, 930, 172691. [Google Scholar] [CrossRef] [PubMed]
- Neil, R.C.; Gomez Cabrera, M.; Uthicke, S. Juvenile age and available coral species modulate transition probability from herbivory to corallivory in Acanthaster cf. Solaris (Crown-of-Thorns Seastar). Coral Reefs 2022, 41, 843–848. [Google Scholar] [CrossRef]
- Chansang, H.; Boonyanate, P.; Pongsuwan, N.; Charuchinda, M.; Wungboonkong, G. Infestation of Acanthaster-Planci in the Andaman Sea. Bull. Mar. Sci. 1987, 41, 634. [Google Scholar]
- De’ath, G.; Moran, P.J. Factors affecting the behaviour of crown-of-thorns starfish (Acanthaster planci L.) on the Great Barrier Reef: 2: Feeding preferences. J. Exp. Mar. Biol. Ecol. 1998, 220, 107–126. [Google Scholar] [CrossRef]
- Hunt, J. Great Barrier Reef coral loss and crown-of-thorns starfish. Aust. Canegrower 2013, 12–13. Available online: https://search.informit.org/doi/10.3316/informit.215362437817151 (accessed on 6 April 2024).
- Munday, P.L. Habitat loss, resource specialization, and extinction on coral reefs. Glob. Change Biol. 2004, 10, 1642–1647. [Google Scholar] [CrossRef]
- Osborne, K.; Dolman, A.M.; Burgess, S.C.; Johns, K.A. Disturbance and the Dynamics of Coral Cover on the Great Barrier Reef (1995–2009). PLoS ONE 2011, 6, e17516. [Google Scholar] [CrossRef]
- Pratchett, M.S.; Caballes, C.F.; Rivera-Posada, J.A.; Sweatman, H.P.A. Limits to understanding and managing outbreaks of crown-of-thorns starfish (Acanthaster spp.). Oceanogr. Mar. Biol. Annu. Rev. 2014, 52, 133–200. [Google Scholar]
- Uthicke, S.; Robson, B.; Doyle, J.R.; Logan, M.; Pratchett, M.S.; Lamare, M. Developing an effective marine eDNA monitoring: eDNA detection at pre-outbreak densities of corallivorous seastar (Acanthaster cf. Solaris). Sci. Total Environ. 2022, 851, 158143. [Google Scholar] [CrossRef]
- Peng, C.; Wang, K.; Wang, W.; Kuang, F.; Gao, Y.; Jiang, R.; Sun, X.; Dong, X.; Chen, B.; Lin, H. Phytoplankton community structure and environmental factors during the outbreak of Crown-of-Thorns Starfish in Xisha Islands, South China Sea. Environ. Res. 2023, 235, 116568. [Google Scholar] [CrossRef]
- Yan, Z.; Xing, J.; Cai, W.; Zhang, K.; Wu, Z.; Li, Y.; Tang, J.; Zhou, Z. Study on the population distribution of Acanthaster planci in the reef area of the Xisha Islands based on environmental DNA technology. Haiyang Xuebao 2023, 45, 76–83. [Google Scholar] [CrossRef]
- Tkachenko, K.S.; Hoang, D.T. Concurrent effect of crown-of-thorns starfish outbreak and thermal anomaly of 2020 on coral reef communities of the Spratly Islands (South China Sea). Mar. Ecol. 2022, 43, e12717. [Google Scholar] [CrossRef]
- Trapon, M.L.; Pratchett, M.S.; Penin, L. Comparative Effects of Different Disturbances in Coral Reef Habitats in Moorea, French Polynesia. J. Mar. Sci. 2011, 2011, 807625. [Google Scholar] [CrossRef]
- Plass-Johnson, J.G.; Schwieder, H.; Heiden, J.; Weiand, L.; Wild, C.; Jompa, J.; Ferse, S.C.A.; Teichberg, M. A recent outbreak of crown-of-thorns starfish (Acanthaster planci) in the Spermonde Archipelago, Indonesia. Reg. Environ. Change 2015, 15, 1157–1162. [Google Scholar] [CrossRef]
- Chak, S.T.C.; Dumont, C.P.; Adzis, K.-A.A.; Yewdall, K. Effectiveness of the removal of coral-eating predator Acanthaster planci in Pulau Tioman Marine Park, Malaysia. J. Mar. Biol. Assoc. UK 2018, 98, 183–189. [Google Scholar] [CrossRef]
- Birkeland, C.; Lucas, J. Acanthaster planci: Major Management Problem of Coral Reefs; CRC Press: Boca Raton, FL, USA, 1990. [Google Scholar]
- Pratchett, M.S.; Lang, B.J.; Matthews, S. Culling crown-of-thorns starfish (Acanthaster cf. Solaris) on Australia’s Great Barrier Reef: Rationale and effectiveness. Aust. Zool. 2019, 40, 13–24. [Google Scholar] [CrossRef]
- Kamya, P.Z.; Dworjanyn, S.A.; Hardy, N.; Mos, B.; Uthicke, S.; Byrne, M. Larvae of the coral eating crown-of-thorns starfish, Acanthaster planci, in a warmer–high CO2 ocean. Glob. Change Biol. 2014, 20, 3365–3376. [Google Scholar] [CrossRef]
- Kamya, P.Z.; Byrne, M.; Graba-Landry, A.; Dworjanyn, S.A. Near-future ocean acidification enhances the feeding rate and development of the herbivorous juveniles of the crown-of-thorns starfish, Acanthaster planci. Coral Reefs. 2016, 35, 1241–1251. [Google Scholar] [CrossRef]
- Yasuda, N. Distribution Expansion and Historical Population Outbreak Patterns of Crown-of-Thorns Starfish, Acanthaster planci Sensu Lato, in Japan from 1912 to 2015; Iguchi, A., Hongo, C., Eds.; Coral Reef Studies of Japan; Springer: Singapore, 2018; pp. 125–148. [Google Scholar] [CrossRef]
- Lane, D.J.W. Acanthaster planci impact on coral communities at permanent transect sites on Bruneian reefs, with a regional overview and a critique on outbreak causes. J. Mar. Biol. Assoc. UK 2012, 92, 803–809. [Google Scholar] [CrossRef]
- Tkachenko, K.S.; Huan, N.H.; Thanh, N.H.; Britayev, T.A. Extensive coral reef decline in Nha Trang Bay, Vietnam: Acanthaster planci outbreak: The final event in a sequence of chronic disturbances. Mar. Freshw. Res. 2021, 72, 186–199. [Google Scholar] [CrossRef]
- Tkachenko, K.S. Degradation of Coral Reefs under Complex Impact of Natural and Anthropogenic Factors with Nha Trang Bay (Vietnam) as an Example. Biol. Bull. Rev. 2023, 13, 442–459. [Google Scholar] [CrossRef]
- Glynn, P.W. Mass mortalities of echinoids and other reef flat organisms coincident with midday, low water exposures in Puerto Rico. Mar. Biol. 1968, 1, 226–243. [Google Scholar] [CrossRef]
- Dahlke, F.T.; Wohlrab, S.; Butzin, M.; Pörtner, H.-O. Thermal bottlenecks in the life cycle define climate vulnerability of fish. Science 2020, 369, 65–70. [Google Scholar] [CrossRef]
- Sill, S.R.; Dawson, T.P. Climate change impacts on the ecological dynamics of two coral reef species, the humphead wrasse (Cheilinus undulatus) and crown-of-thorns starfish (Ancanthaster planci). Ecol. Inform. 2021, 65, 101399. [Google Scholar] [CrossRef]
- Hue, T.; Chateau, O.; Lecellier, G.; Marin, C.; Coulombier, N.; Le Dean, L.; Gossuin, H.; Adjeroud, M.; Dumas, P. Impact of near-future ocean warming and acidification on the larval development of coral-eating starfish Acanthaster Solaris after parental exposure. J. Exp. Mar. Biol. Ecol. 2022, 548, 151685. [Google Scholar] [CrossRef]
- Simon, T.N.; Levitan, D.R. Measuring Fertilization Success of Broadcast-Spawning Marine Invertebrates Within Seagrass Meadows. Biol. Bull. 2011, 220, 32–38. [Google Scholar] [CrossRef]
- Yamano, H.; Sugihara, K.; Nomura, K. Rapid poleward range expansion of tropical reef corals in response to rising sea surface temperatures: Poleward Range Expansion of Corals. Geophys. Res. Lett. 2011, 38, L04601. [Google Scholar] [CrossRef]
- Xu, Y.; Ma, L.; Sui, J.; Li, X.; Wang, H.; Zhang, B. Potential impacts of climate change on the distribution of echinoderms in the Yellow Sea and East China Sea. Mar. Pollut. Bull. 2023, 194, 115246. [Google Scholar] [CrossRef]
- Koop, K.; Booth, D.; Dennison, W.; Erdmann, M.; Jones, G.B.; Larkum, A.W.D.; O’Neil, J.; Steven, A.; Tentori, E.; Ward, S.; et al. Encore: The Effect of Nutrient Enrichment on Coral Reefs. Synthesis of Results and Conclusions. Mar. Pollut. Bull. 2001, 42, 91–120. [Google Scholar] [CrossRef] [PubMed]
- Anthony, K.R.N.; Kline, D.I.; Diaz-Pulido, G.; Dove, S.; Hoegh-Guldberg, O. Ocean acidification causes bleaching and productivity loss in coral reef builders. Proc. Natl. Acad. Sci. USA 2008, 105, 17442–17446. [Google Scholar] [CrossRef]
- Despalatović, M.; Grubelić, I.; Piccinetti, C.; Cvitković, I.; Antolić, B.; Žuljević, A.; Nikolić, V. Distribution of echinoderms on continental shelf in open waters of the northern and middle Adriatic Sea. J. Mar. Biol. Assoc. UK 2009, 89, 585–591. [Google Scholar] [CrossRef]
- Stuart-Smith, R.D.; Bates, A.E.; Lefcheck, J.S.; Duffy, J.E.; Baker, S.C.; Thomson, R.J.; Stuart-Smith, J.F.; Hill, N.A.; Kininmonth, S.J.; Airoldi, L.; et al. Integrating abundance and functional traits reveals new global hotspots of fish diversity. Nature 2013, 501, 539–542. [Google Scholar] [CrossRef]
- Pyle, R.L.; Copus, J.M. Mesophotic Coral Ecosystems: Introduction and Overview; Loya, Y., Puglise, K., Bridge, T., Eds.; Mesophotic Coral Ecosystems. Coral Reefs of the World; Springer: Cham, Switzerland, 2019; Volume 12. [Google Scholar] [CrossRef]
- Barnes, M. The Acanthaster Phenomenon. Oceanogr. Mar. Biol. 1986, 24, 379. [Google Scholar]
- Lucas, J.S. Crown-of-thorns starfish. Curr. Biol. 2013, 23, R945–R946. [Google Scholar] [CrossRef] [PubMed]
- Assis, J.; Fernández Bejarano, S.J.; Salazar, V.W.; Schepers, L.; Gouvêa, L.; Fragkopoulou, E.; Leclercq, F.; Vanhoorne, B.; Tyberghein, L.; Serrão, E.A.; et al. Bio-ORACLE v3.0. Pushing marine data layers to the CMIP6 Earth System Models of climate change research. Glob. Ecol. Biogeogr. 2024, 33, e13813. [Google Scholar] [CrossRef]
- The GEBCO 2024 Grid—A Continuous Terrain Model of the Global Oceans and Land; NERC EDS British Oceanographic Data Centre NOC: Liverpool, UK, 2024. [CrossRef]
- Tyberghein, L.; Verbruggen, H.; Pauly, K.; Troupin, C.; Mineur, F.; De Clerck, O. Bio-ORACLE: A global environmental dataset for marine species distribution modelling. Glob. Ecol. Biogeogr. 2012, 21, 272–281. [Google Scholar] [CrossRef]
- O’Neill, B.C.; Tebaldi, C.; Van Vuuren, D.P.; Eyring, V.; Friedlingstein, P.; Hurtt, G.; Knutti, R.; Kriegler, E.; Lamarque, J.-F.; Lowe, J.; et al. The Scenario Model Intercomparison Project (ScenarioMIP) for CMIP6. Geosci. Model Dev. 2016, 9, 3461–3482. [Google Scholar] [CrossRef]
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; Marquéz, J.R.G.; Gruber, B.; Lafourcade, B.; Leitão, P.J.; et al. Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]
- Owens, H.; Barve, V.; Chamberlain, S. spocc: Interface to Species Occurrence Data Sources. R Package Version 1.2.3. 2024. Available online: https://docs.ropensci.org/spocc/ (user manual); https://github.com/ropensci/spocc (devel) (accessed on 6 April 2024).
- Mammola, S.; Goodacre, S.L.; Isaia, M. Climate change may drive cave spiders to extinction. Ecography 2018, 41, 233–243. [Google Scholar] [CrossRef]
- Hu, W.; Su, S.; Mohamed, H.F.; Xiao, J.; Kang, J.; Krock, B.; Xie, B.; Luo, Z.; Chen, B. Assessing the global distribution and risk of harmful microalgae: A focus on three toxic Alexandrium dinoflagellates. Sci. Total Environ. 2024, 948, 174767. [Google Scholar] [CrossRef] [PubMed]
- Spalding, M.D.; Fox, H.E.; Allen, G.R.; Davidson, N.; Ferdaña, Z.A.; Finlayson, M.; Halpern, B.S.; Jorge, M.A.; Lombana, A.; Lourie, S.A.; et al. Marine Ecoregions of the World: A Bioregionalization of Coastal and Shelf Areas. BioScience 2007, 57, 573–583. [Google Scholar] [CrossRef]
- Zhang, Z.; Ma, S.; Bede-Fazekas, Á.; Mammola, S.; Qu, M.; Zhou, J.; Lin, Q. Considering biotic interactions exacerbates the predicted impacts of climate change on coral-dwelling species. J. Biogeogr. 2024, 51, 769–782. [Google Scholar] [CrossRef]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudík, M.; Chee, Y.E.; Yates, C.J. A statistical explanation of MaxEnt for ecologists: Statistical explanation of MaxEnt. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Dudík, M.; Schapire, R.E.; Blair, M.E. Opening the black box: An open-source release of Maxent. Ecography 2017, 40, 887–893. [Google Scholar] [CrossRef]
- Melo-Merino, S.M.; Reyes-Bonilla, H.; Lira-Noriega, A. Ecological niche models and species distribution models in marine environments: A literature review and spatial analysis of evidence. Ecol. Model. 2020, 415, 108837. [Google Scholar] [CrossRef]
- Barve, N.; Barve, V.; Jiménez-Valverde, A.; Lira-Noriega, A.; Maher, S.P.; Peterson, A.T.; Soberón, J.; Villalobos, F. The crucial role of the accessible area in ecological niche modeling and species distribution modeling. Ecol. Model. 2011, 222, 1810–1819. [Google Scholar] [CrossRef]
- Thuiller, W.; Guéguen, M.; Renaud, J.; Karger, D.N.; Zimmermann, N.E. Uncertainty in ensembles of global biodiversity scenarios. Nat. Commun. 2019, 10, 1446. [Google Scholar] [CrossRef]
- Shipley, B.R.; Bach, R.; Do, Y.; Strathearn, H.; McGuire, J.L.; Dilkina, B. megaSDM: Integrating dispersal and time-step analyses into species distribution models. Ecography 2022, 2022, ecog.05450. [Google Scholar] [CrossRef]
- De Kort, H.; Baguette, M.; Lenoir, J.; Stevens, V.M. Toward reliable habitat suitability and accessibility models in an era of multiple environmental stressors. Ecol. Evol. 2020, 10, 10937–10952. [Google Scholar] [CrossRef] [PubMed]
- Swets, J.A. Measuring the Accuracy of Diagnostic Systems. Science 1988, 240, 1285–1293. [Google Scholar] [CrossRef] [PubMed]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Kass, J.M.; Muscarella, R.; Galante, P.J.; Bohl, C.L.; Pinilla-Buitrago, G.E.; Boria, R.A.; Soley-Guardia, M.; Anderson, R.P. ENMeval 2.0: Redesigned for customizable and reproducible modeling of species’ niches and distributions. Methods Ecol. Evol. 2021, 12, 1602–1608. [Google Scholar] [CrossRef]
- Zurell, D.; Franklin, J.; König, C.; Bouchet, P.J.; Dormann, C.F.; Elith, J.; Fandos, G.; Feng, X.; Guillera-Arroita, G.; Guisan, A.; et al. A standard protocol for reporting species distribution models. Ecography 2020, 43, 1261–1277. [Google Scholar] [CrossRef]
- Broennimann, O.; Fitzpatrick, M.C.; Pearman, P.B.; Petitpierre, B.; Pellissier, L.; Yoccoz, N.G.; Guisan, A. Measuring ecological niche overlap from occurrence and spatial environmental data. Glob. Ecol. Biogeogr. 2012, 21, 481–497. [Google Scholar] [CrossRef]
- Schoener, T.W. Nonsynchronous spatial overlap of lizards in patchy habitats. Ecology 1970, 51, 408–418. [Google Scholar] [CrossRef]
- Warren, D.L.; Glor, R.E.; Turelli, M. Environmental Niche Equivalency Versus Conservatism: Quantitative Approaches To Niche Evolution. Evolution 2008, 62, 2868–2883. [Google Scholar] [CrossRef]
- Encarnación-Luévano, A.; Escoto-Moreno, J.A.; Villalobos-Jiménez, G. Evaluating Potential Distribution and Niche Divergence among Populations of the World’s Largest Living Damselfly, Megaloprepus caerulatus (Drury, 1782). Diversity 2022, 14, 84. [Google Scholar] [CrossRef]
- Smyčka, J.; Roquet, C.; Boleda, M.; Alberti, A.; Boyer, F.; Douzet, R.; Perrier, C.; Rome, M.; Valay, J.-G.; Denoeud, F.; et al. Tempo and drivers of plant diversification in the European mountain system. Nat. Commun. 2022, 13, 2750. [Google Scholar] [CrossRef]
- Tong, R.; Davies, A.J.; Yesson, C.; Yu, J.; Luo, Y.; Zhang, L.; Burgos, J.M. Environmental drivers and the distribution of cold-water corals in the global ocean. Front. Mar. Sci. 2023, 10, 1217851. [Google Scholar] [CrossRef]
- Zhou, Y.; Lu, X.; Zhang, G. Potentially differential impacts on niche overlap between Chinese endangered Zelkova schneideriana and its associated tree species under climate change. Front. Ecol. Evol. 2023, 11, 1218149. [Google Scholar] [CrossRef]
- Aguirre-Gutiérrez, J.; Serna-Chavez, H.M.; Villalobos-Arambula, A.R.; Pérez de la Rosa, J.A.; Raes, N. Similar but not equivalent: Ecological niche comparison across closely—Related M exican white pines. Divers. Distrib. 2015, 21, 245–257. [Google Scholar] [CrossRef]
- Broennimann, O.; Di Cola, V.; Guisan, A.; Ecospat: Spatial Ecology Miscellaneous Methods. R Package Version 4.0.0. 2023. Available online: https://CRAN.R-project.org/package=ecospat (accessed on 1 July 2024).
- Brown, J.L. SDM toolbox: A python-based GIS toolkit for landscape genetic, biogeographic and species distribution model analyses. Methods Ecol. Evol. 2014, 5, 694–700. [Google Scholar] [CrossRef]
- Pratchett, M.S.; Dworjanyn, S.; Mos, B.; Caballes, C.F.; Thompson, C.A.; Blowes, S. Larval Survivorship and Settlement of Crown-of-Thorns Starfish (Acanthaster cf. Solaris) at Varying Algal Cell Densities. Diversity 2017, 9, 2. [Google Scholar] [CrossRef]
- Hue, T.; Chateau, O.; Lecellier, G.; Kayal, M.; Lanos, N.; Gossuin, H.; Adjeroud, M.; Dumas, P. Temperature affects the reproductive outputs of coral-eating starfish Acanthaster spp. After adult exposure to near-future ocean warming and acidification. Mar. Environ. Res. 2020, 162, 105164. [Google Scholar] [CrossRef]
- Lang, B.J.; Donelson, J.M.; Caballes, C.F.; Uthicke, S.; Doll, P.C.; Pratchett, M.S. Effects of elevated temperature on the performance and survival of pacific crown-of-thorns starfish (Acanthaster cf. Solaris). Mar. Biol. 2022, 169, 43. [Google Scholar] [CrossRef]
- Espinel-Velasco, N.; Hoffmann, L.; Agüera, A.; Byrne, M.; Dupont, S.; Uthicke, S.; Webster, N.S.; Lamare, M. Effects of ocean acidification on the settlement and metamorphosis of marine invertebrate and fish larvae: A review. Mar. Ecol. Prog. Ser. 2018, 606, 237–257. [Google Scholar] [CrossRef]
- Lang, B.J.; Caballes, C.F.; Uthicke, S.; Doll, P.C.; Donelson, J.M.; Pratchett, M.S. Impacts of ocean warming on the settlement success and post-settlement survival of Pacific crown-of-thorns starfish (Acanthaster cf. Solaris). Coral Reefs 2023, 42, 143–155. [Google Scholar] [CrossRef]
- Baine, M.S.P. A major outbreak of crown-of-thorns starfish in Bootless Bay, Central Province, Papua New Guinea. Coral Reefs 2006, 25, 607. [Google Scholar] [CrossRef]
- Pratchett, M.S.; Schenk, T.J.; Baine, M.; Syms, C.; Baird, A.H. Selective coral mortality associated with outbreaks of Acanthaster planci L. in Bootless Bay 2009, Papua New Guinea. Mar. Environ. Res. 2009, 67, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Pratchett, M.S. Changes in coral assemblages during an outbreak of Acanthaster planci at Lizard Island, northern Great Barrier Reef (1995–1999). Coral Reefs 2010, 29, 717–725. [Google Scholar] [CrossRef]
- Jones, M.C.; Cheung, W.W.L. Multi-model ensemble projections of climate change effects on global marine biodiversity. ICES J. Mar. Sci. 2015, 72, 741–752. [Google Scholar] [CrossRef]
- Randall, C.J.; Szmant, A.M. Elevated Temperature Affects Development, Survivorship, and Settlement of the Elkhorn Coral, Acropora palmata (Lamarck 1816). Biol. Bull. 2009, 217, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Foster, T.; Gilmour, J.P.; Chua, C.M.; Falter, J.L.; McCulloch, M.T. Effect of ocean warming and acidification on the early life stages of subtropical Acropora spicifera. Coral Reefs 2015, 34, 1217–1226. [Google Scholar] [CrossRef]
- Pratchett, M.S.; Caballes, C.F.; Cvitanovic, C.; Raymundo, M.L.; Babcock, R.C.; Bonin, M.C.; Bozec, Y.-M.; Burn, D.; Byrne, M.; Castro-Sanguino, C.; et al. Knowledge Gaps in the Biology, Ecology, and Management of the Pacific Crown-of-Thorns Sea Star Acanthaster sp. On Australia’s Great Barrier Reef. Biol. Bull. 2021, 241, 330–346. [Google Scholar] [CrossRef]
- Deaker, D.J.; Byrne, M. Crown of thorns starfish life-history traits contribute to outbreaks, a continuing concern for coral reefs. Emerg. Top. Life Sci. 2022, 6, 67–79. [Google Scholar] [CrossRef]
- Kayal, M.; Vercelloni, J.; Lison de Loma, T.; Bosserelle, P.; Chancerelle, Y.; Geoffroy, S.; Stievenart, C.; Michonneau, F.; Penin, L.; Planes, S.; et al. Predator Crown-of-Thorns Starfish (Acanthaster planci) Outbreak, Mass Mortality of Corals, and Cascading Effects on Reef Fish and Benthic Communities. PLoS ONE 2012, 7, e47363. [Google Scholar] [CrossRef]
- Uthicke, S.; Logan, M.; Liddy, M.; Francis, D.; Hardy, N.; Lamare, M. Climate change as an unexpected co-factor promoting coral eating seastar (Acanthaster planci) outbreaks. Sci. Rep. 2015, 5, 8402. [Google Scholar] [CrossRef]
- Chivers, D.P.; McCormick, M.I.; Allan, B.J.M.; Ferrari, M.C.O. Risk assessment and predator learning in a changing world: Understanding the impacts of coral reef degradation. Sci. Rep. 2016, 6, 32542. [Google Scholar] [CrossRef]
- Harvey, B.J.; Nash, K.L.; Blanchard, J.L.; Edwards, D.P. Ecosystem-based management of coral reefs under climate change. Ecol. Evol. 2018, 8, 6354–6368. [Google Scholar] [CrossRef] [PubMed]
- Renzi, J.J.; Shaver, E.C.; Burkepile, D.E.; Silliman, B.R. The role of predators in coral disease dynamics. Coral Reefs 2022, 41, 405–422. [Google Scholar] [CrossRef]
- Lourey, M.J.; Ryan, D.A.J.; Miller, I.R. Rates of decline and recovery of coral cover on reefs impacted by, recovering from and unaffected by crown-of-thorns starfish Acanthaster planci: A regional perspective of the Great Barrier Reef. Mar. Ecol. Prog. Ser. 2000, 196, 179–186. [Google Scholar] [CrossRef]
- Jackson, J.; Donovan, M.; Cramer, K.; Lam, V. Status and Trends of Caribbean Coral Reefs: 1970–2012; Global Coral Reef Monitoring Network; International Union for the Conservation of Nature (IUCN): Gland, Switzerland, 2014. [Google Scholar]
- Hughes, T.P.; Barnes, M.L.; Bellwood, D.R.; Cinner, J.E.; Cumming, G.S.; Jackson, J.B.C.; Kleypas, J.; van de Leemput, I.A.; Lough, J.M.; Morrison, T.H.; et al. Coral reefs in the Anthropocene. Nature 2017, 546, 82–90. [Google Scholar] [CrossRef]
- Hoegh-Guldberg, O.; Skirving, W.; Dove, S.G.; Spady, B.L.; Norrie, A.; Geiger, E.F.; Liu, G.; De La Cour, J.L.; Manzello, D.P. Coral reefs in peril in a record-breaking year. Science 2023, 382, 1238–1240. [Google Scholar] [CrossRef]
- Haywood, M.D.E.; Thomson, D.P.; Babcock, R.C.; Pillans, R.D.; Keesing, J.K.; Miller, M.; Rochester, W.A.; Donovan, A.; Evans, R.D.; Shedrawi, G.; et al. Crown-of-thorns starfish impede the recovery potential of coral reefs following bleaching. Mar. Biol. 2019, 166, 99. [Google Scholar] [CrossRef]
- Cowan, Z.-L.; Dworjanyn, S.A.; Caballes, C.F.; Pratchett, M. Benthic Predators Influence Microhabitat Preferences and Settlement Success of Crown-of-Thorns Starfish (Acanthaster cf. Solaris). Diversity 2016, 8, 27. [Google Scholar] [CrossRef]
- Kroon, F.J.; Lefèvre, C.D.; Doyle, J.R.; Patel, F.; Milton, G.; Severati, A.; Kenway, M.; Johansson, C.L.; Schnebert, S.; Thomas-Hall, P.; et al. DNA-based identification of predators of the corallivorous Crown-of-Thorns Starfish (Acanthaster cf. Solaris) from fish faeces and gut contents. Sci. Rep. 2020, 10, 8184. [Google Scholar] [CrossRef]
- Caballes, C.F.; Pratchett, M.S.; Raymundo, M.L.; Rivera-Posada, J.A. Environmental Tipping Points for Sperm Motility, Fertilization, and Embryonic Development in the Crown-of-Thorns Starfish. Diversity 2017, 9, 10. [Google Scholar] [CrossRef]
- Pratchett, M.S.; Cowan, Z.-L.; Nadler, L.E.; Caballes, C.F.; Hoey, A.S.; Messmer, V.; Fletcher, C.S.; Westcott, D.A.; Ling, S.D. Body size and substrate type modulate movement by the western Pacific crown-of-thorns starfish, Acanthaster solaris. PLoS ONE 2017, 12, e0180805. [Google Scholar] [CrossRef]
- Uthicke, S.; Pratchett, M.S.; Messmer, V.; Harrison, H. Limited genetic signal from potential cloning and selfing within wild populations of coral-eating crown-of-thorns seastars (Acanthaster cf. Solaris). Coral Reefs 2021, 40, 131–138. [Google Scholar] [CrossRef]
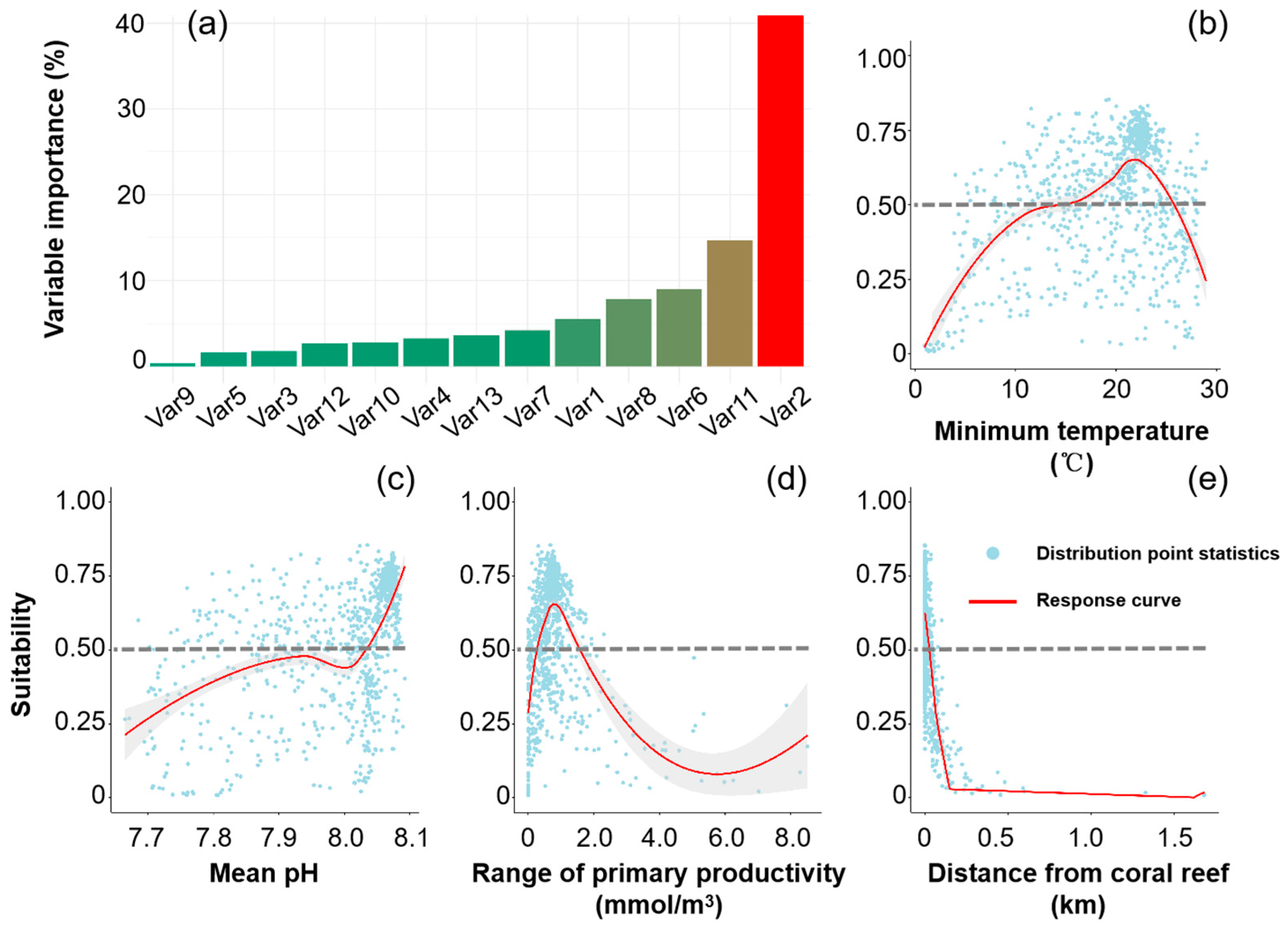

| Variable | Type | Acronym | Unit | ID | Source |
|---|---|---|---|---|---|
| Bathymetry | Abiotic | Bathymetry | meter | Var1 | gebco.net |
| Distance from coral reef | Biotic | Dis | meter | Var2 | SDM outputs |
| Mean dissolved oxygen | Abiotic | DO_mean | mmol/m3 | Var3 | Bio-ORACLE v3.0 (https://www.bio-oracle.org/, accessed on 15 July 2024 [50]) |
| Mean diffuse attenuation | Abiotic | Kdpar_mean | m−1 | Var4 | |
| Range of nitrate | Abiotic | N_range | mmol/m3 | Var5 | |
| Mean pH | Abiotic | PH_mean | - | Var6 | |
| Minimum primary productivity | Abiotic | PP_mean | mmol/m3 | Var7 | |
| Range primary productivity | Abiotic | PP_range | mmol/m3 | Var8 | |
| Maximum salinity | Abiotic | S_max | mmol/m3 | Var9 | |
| Range of salinity | Abiotic | S_range | mmol/m3 | Var10 | |
| Minimum temperature | Abiotic | T_min | °C | Var11 | |
| Range of temperature | Abiotic | T_range | °C | Var12 | |
| Mean current velocity | Abiotic | Velocity_mean | m/s | Var13 |
| Realm | Province | Area (km2) | Percentage |
|---|---|---|---|
| Western Indo-Pacific | Andaman | 46,887 | 2.99% |
| Western Indo-Pacific | Bay of Bengal | 252 | 0.02% |
| Eastern Indo-Pacific | Central Polynesia | 7310 | 0.47% |
| Temperate Northern Pacific | Cold Temperate Northwest Pacific | 0 | 0.00% |
| Temperate Australasia | East Central Australian Shelf | 41,173 | 2.62% |
| Central Indo-Pacific | Eastern Coral Triangle | 121,252 | 7.72% |
| Eastern Indo-Pacific | Hawaii | 27,729 | 1.77% |
| Central Indo-Pacific | Java Transitional | 3361 | 0.21% |
| Central Indo-Pacific | Lord Howe and Norfolk Islands | 1764 | 0.11% |
| Eastern Indo-Pacific | Marquesas | 2016 | 0.13% |
| Eastern Indo-Pacific | Marshall, Gilbert, and Ellis Islands | 35,123 | 2.24% |
| Central Indo-Pacific | Northeast Australian Shelf | 264,855 | 16.87% |
| Temperate Australasia | Northern New Zealand | 84 | 0.01% |
| Central Indo-Pacific | Northwest Australian Shelf | 37,980 | 2.42% |
| Central Indo-Pacific | Sahul Shelf | 182,424 | 11.62% |
| Central Indo-Pacific | South China Sea | 41,089 | 2.62% |
| Central Indo-Pacific | South Kuroshio | 19,914 | 1.27% |
| Temperate Australasia | Southeast Australian Shelf | 0 | 0.00% |
| Eastern Indo-Pacific | Southeast Polynesia | 31,930 | 2.03% |
| Temperate Australasia | Southern New Zealand | 0 | 0.00% |
| Temperate Australasia | Southwest Australian Shelf | 0 | 0.00% |
| Southern Ocean | Subantarctic New Zealand | 0 | 0.00% |
| Central Indo-Pacific | Sunda Shelf | 122,932 | 7.83% |
| Central Indo-Pacific | Tropical Northwestern Pacific | 39,913 | 2.54% |
| Central Indo-Pacific | Tropical Southwestern Pacific | 134,192 | 8.55% |
| Temperate Northern Pacific | Warm Temperate Northwest Pacific | 31,594 | 2.01% |
| Temperate Australasia | West Central Australian Shelf | 35,711 | 2.27% |
| Central Indo-Pacific | Western Coral Triangle | 340,312 | 21.68% |
| Total | \ | 1,569,806 | 100.00% |
| Scenario | North Latitude | South Latitude | Northward Shift | Southward Shift |
|---|---|---|---|---|
| Present | 34.31° | 33.26° | \ | \ |
| SSP1-2.6 | 34.31° | 33.34° | 0° | 0.08° |
| SSP2-4.5 | 34.41° | 33.34° | 0.08° | 0.08° |
| SSP5-8.5 | 35.08° | 38.09° | 0.77° | 4.84° |
| REALM | PROVINC | SSP1-2.6 | SSP2-4.5 | SSP5-8.5 |
|---|---|---|---|---|
| Western Indo-Pacific | Andaman | 10,503 | 17,394 | 14,033 |
| Western Indo-Pacific | Bay of Bengal | 84 | 0 | 0 |
| Eastern Indo-Pacific | Central Polynesia | 7478 | 6386 | 4790 |
| Temperate Northern Pacific | Cold Temperate Northwest Pacific | 0 | 0 | 0 |
| Temperate Australasia | East Central Australian Shelf | 30,670 | 37,308 | 40,081 |
| Central Indo-Pacific | Eastern Coral Triangle | 121,336 | 126,378 | 127,722 |
| Eastern Indo-Pacific | Hawaii | 28,990 | 27,897 | 27,057 |
| Central Indo-Pacific | Java Transitional | 2605 | 7731 | 4790 |
| Central Indo-Pacific | Lord Howe and Norfolk Islands | 2353 | 2269 | 4537 |
| Eastern Indo-Pacific | Marquesas | 2101 | 1512 | 588 |
| Eastern Indo-Pacific | Marshall, Gilbert and Ellis Islands | 32,519 | 34,872 | 30,166 |
| Central Indo-Pacific | Northeast Australian Shelf | 256,621 | 264,772 | 280,989 |
| Temperate Australasia | Northern New Zealand | 0 | 0 | 8991 |
| Central Indo-Pacific | Northwest Australian Shelf | 35,292 | 37,897 | 80,835 |
| Central Indo-Pacific | Sahul Shelf | 196,541 | 195,197 | 300,063 |
| Central Indo-Pacific | South China Sea | 28,149 | 40,417 | 50,249 |
| Central Indo-Pacific | South Kuroshio | 24,536 | 25,376 | 25,208 |
| Temperate Australasia | Southeast Australian Shelf | 0 | 0 | 0 |
| Eastern Indo-Pacific | Southeast Polynesia | 33,359 | 30,670 | 28,317 |
| Temperate Australasia | Southern New Zealand | 0 | 0 | 3949 |
| Temperate Australasia | Southwest Australian Shelf | 0 | 2017 | 39,157 |
| Southern Ocean | Subantarctic New Zealand | 0 | 0 | 0 |
| Central Indo-Pacific | Sunda Shelf | 52,769 | 174,106 | 340,901 |
| Central Indo-Pacific | Tropical Northwestern Pacific | 43,526 | 38,569 | 33,779 |
| Central Indo-Pacific | Tropical Southwestern Pacific | 140,578 | 136,377 | 139,486 |
| Temperate Northern Pacific | Warm Temperate Northwest Pacific | 26,049 | 31,258 | 26,973 |
| Temperate Australasia | West Central Australian Shelf | 66,046 | 74,701 | 86,633 |
| Central Indo-Pacific | Western Coral Triangle | 362,748 | 418,038 | 443,415 |
| Scenario | Habitat Area (A. solaris) | Habitat Area (Acropora) | Overlap | Occupancy Rate of A. solaris in Acropora Habitats |
|---|---|---|---|---|
| Present | 1,569,807 | 1,407,884 | 1,135,600 | 80.66% |
| SSP1-2.6 | 1,504,853 | 1,423,597 | 1,032,678 | 72.54% |
| SSP2-4.5 | 1,731,142 | 1,321,755 | 1,136,182 | 85.96% |
| SSP5-8.5 | 2,142,710 | 1,203,780 | 1,120,119 | 93.05% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, S.; Liu, J.; Chen, B.; Wang, W.; Xiao, J.; Li, Y.; Du, J.; Kang, J.; Hu, W.; Zhang, J. Distribution Shifts of Acanthaster solaris Under Climate Change and the Impact on Coral Reef Habitats. Animals 2025, 15, 858. https://doi.org/10.3390/ani15060858
Su S, Liu J, Chen B, Wang W, Xiao J, Li Y, Du J, Kang J, Hu W, Zhang J. Distribution Shifts of Acanthaster solaris Under Climate Change and the Impact on Coral Reef Habitats. Animals. 2025; 15(6):858. https://doi.org/10.3390/ani15060858
Chicago/Turabian StyleSu, Shangke, Jinquan Liu, Bin Chen, Wei Wang, Jiaguang Xiao, Yuan Li, Jianguo Du, Jianhua Kang, Wenjia Hu, and Junpeng Zhang. 2025. "Distribution Shifts of Acanthaster solaris Under Climate Change and the Impact on Coral Reef Habitats" Animals 15, no. 6: 858. https://doi.org/10.3390/ani15060858
APA StyleSu, S., Liu, J., Chen, B., Wang, W., Xiao, J., Li, Y., Du, J., Kang, J., Hu, W., & Zhang, J. (2025). Distribution Shifts of Acanthaster solaris Under Climate Change and the Impact on Coral Reef Habitats. Animals, 15(6), 858. https://doi.org/10.3390/ani15060858





