4. Discussion
SCFAs, defined as saturated fatty acids with six or fewer carbon atoms, are also referred to as volatile fatty acids due to their inherent volatility [
8]. The primary SCFAs include acetic acid, propionic acid, butyric acid, and valeric acid. SCFAs originate from two major pathways: dietary intake of exogenous SCFAs, which are absorbed into the bloodstream and transported to various tissues [
9], and microbial fermentation of carbohydrates in the gastrointestinal tract following carbohydrate consumption by animals [
10,
11]. In herbivores, microbial carbohydrate fermentation serves as the predominant source of SCFAs. In ruminants, the majority of SCFAs are synthesized via rumen fermentation, with a smaller fraction generated by intestinal microbiota. These SCFAs primarily exist in ionic form and are absorbed through the rumen or intestinal epithelium, subsequently entering different tissues to participate in various physiological processes, including energy metabolism and nutrient utilization [
12]. In monogastric herbivores, anaerobic bacteria in the large intestines (cecum and colon) ferment undigested and unabsorbed carbohydrates, producing SCFAs that serve as key nutrients and primary energy substrates [
13]. For instance, propionic acid acts as a key gluconeogenic precursor, contributing to energy metabolism via glucose synthesis, whereas acetic acid and butyric acid function as both substrates for de novo fatty acid synthesis in the mammary gland and signaling molecules regulating mammary fatty acid metabolism, thereby influencing milk fat composition and content [
14]. Urrutia et al. [
15] reported that supplementing dairy cow diets with 2.9% acetate significantly increased milk fat percentage and yield by 0.2% and 90 g/d, respectively, whereas an equimolar butyrate addition did not exert a significant effect on either parameter. Matamoros et al. [
14] reported that dietary supplementation with 3.25% acetate significantly increased milk fat yield in dairy cows, likely due to enhanced acetate supply to the mammary glands, which stimulated de novo fatty acid synthesis. Studies on ruminal SCFA infusion in dairy cows further demonstrated that acetate and butyrate administration significantly elevated milk fat percentage, milk fat yield, and the concentration of de novo-synthesized fatty acids (<C16 and C16), particularly increasing palmitic acid content and yield. A clear dose–response relationship was observed between acetate infusion and milk fat synthesis, with both milk fat percentage and milk fat yield exhibiting linear and quadratic increases in response to acetate supply. These findings suggest that augmenting ruminal acetate and butyrate availability in lactating dairy cows enhances milk fat yield by providing essential substrates for de novo fatty acid synthesis through increased acetate and BHB levels [
16]. In the present study, sodium acetate and sodium butyrate addition over an 8 h period led to an increase in milk yield by lactating mares, as well as higher milk protein, fat, and lactose contents. Notably, sodium acetate addition significantly elevated milk fat content, whereas sodium butyrate addition significantly increased milk protein levels. These findings align with the results reported by Urrutia et al. [
13] and Matamoros et al. [
14] in dairy cows. The capacity of acetic acid and butyric acid to enhance milk fat content is attributed not only to their role as metabolic energy sources and precursors for de novo fatty acid synthesis but also to their regulatory effects on the expression of mammary gland genes involved in fatty acid metabolism. Sun et al. [
17] demonstrated that butyric acid and propionic acid promoted triacylglycerol accumulation and lipid droplet formation in goat mammary epithelial cells while concurrently upregulating key genes associated with fatty acid synthesis, including SCD1, FABP3, and PPARG. These findings indicate that SCFAs improve milk fat composition and mammary gland function by enhancing the fatty acid biosynthesis pathway. Blood biochemical parameters serve as critical indicators of an organism’s health status, providing essential insights into metabolic activity and physiological functions. These parameters reflect key processes such as glucose metabolism, lipid metabolism, and nitrogen metabolism [
18].
Carbohydrate metabolism encompasses glucose synthesis, utilization, and storage, directly influencing an organism’s energy balance and expenditure [
19]. These metabolic processes affect not only growth and development but also overall metabolic efficiency, production performance, and health status in animals [
20]. Blood glucose serves as a critical biomarker of energy homeostasis, reflecting an organism’s energy requirements and regulatory mechanisms. Its concentration is modulated by dietary intake, insulin secretion, and hepatic gluconeogenesis, impacting growth, metabolism, and nutrient utilization efficiency [
21,
22]. Fluctuations in blood glucose levels not only affect physiological homeostasis but also have direct implications for production performance and animal health [
23]. In the present study, plasma glucose concentrations in mares supplemented with sodium acetate were significantly higher than in the control group and the sodium butyrate group. This finding is consistent with the results reported by Cheng Yating et al. [
24] in sows, suggesting that SCFAs may enhance glucose metabolism by activating G protein-coupled receptors and stimulating insulin secretion, thereby increasing plasma glucose levels in the supplemented groups. Additionally, sodium acetate and sodium butyrate, as rapidly absorbed SCFAs, can be efficiently metabolized in the intestine and utilized as an immediate energy source. Their rapid conversion into ATP supports glucose synthesis and metabolism, improving overall energy homeostasis and contributing to elevated plasma glucose levels in mares [
12].
Lipid metabolism encompasses the synthesis, storage, and breakdown of lipid molecules, including fatty acids, triglycerides, and cholesterol, playing a pivotal role in energy homeostasis, cell membrane integrity, and hormone biosynthesis [
25]. Cholesterol and triglycerides serve as key biomarkers of lipid metabolism, with fluctuations in their levels reflecting metabolic activity and lipid homeostasis in animals [
26]. This study revealed that triglyceride levels in the sodium butyrate group were significantly higher than those in the control group, aligning with the findings of Chen et al. [
27], who reported that sodium butyrate enhances triglyceride synthesis by upregulating the enzymes involved in fatty acid biosynthesis. Additionally, total cholesterol levels were significantly elevated in the sodium acetate group compared to the control, possibly due to its regulatory influence on hepatic cholesterol synthesis and fatty acid metabolism. Previous studies suggest that sodium acetate promotes lipid metabolism by enhancing cholesterol biosynthesis, further supporting its role in lipid homeostasis [
28]. Furthermore, total bilirubin levels were significantly higher in the sodium acetate group than in the control. Given that bilirubin is a metabolic byproduct of hemoglobin degradation following erythrocyte turnover [
29], its increased concentration may be associated with enhanced hepatic metabolic function and bile acid secretion. Sodium acetate likely contributes to elevated bilirubin levels by modulating hepatic lipid metabolism and promoting bile acid synthesis, further highlighting its metabolic regulatory effects.
Total protein, albumin, and globulin serve as critical biochemical markers for assessing nitrogen metabolism and immune function. In this study, mares given sodium acetate and sodium butyrate exhibited an upward trend in these protein levels compared to the control group, suggesting that SCFAs may enhance protein synthesis and immune function by modulating nitrogen metabolism. This observation aligns with findings by Wang Yaling [
5], who reported that dietary sodium acetate addition in dairy goats significantly increased blood total protein, albumin, and globulin concentrations, thereby improving nitrogen metabolism and immune function. Additionally, urea levels in the sodium butyrate group were significantly higher than in the sodium acetate and control groups, a result consistent with the findings of Tan [
30], who demonstrated that dietary sodium acetate addition in perinatal dairy cows significantly elevated plasma urea levels, promoting protein catabolism and nitrogen excretion. These findings further support the regulatory role of SCFAs in nitrogen metabolism and protein turnover.
The contents of total protein, albumin, and globulin are important biochemical indicators for evaluating the nitrogen metabolism level of the body and are closely related to the body’s immune function. In this experiment, the contents of total protein, albumin, and globulin in the blood of mares supplemented with sodium acetate and sodium butyrate showed a certain upward trend compared with the control group. This result indicates that short-chain fatty acids may promote protein synthesis and improve immune function by regulating nitrogen metabolism. This finding is consistent with the research results of Wang Yaling [
5], who found that adding sodium acetate to the basic diet of dairy goats could significantly increase the levels of total protein, albumin, and globulin in the blood, thereby improving nitrogen metabolism and immune function. In addition, this experiment also found that the urea content in the sodium butyrate group was significantly higher than that in the sodium acetate group and the control group. This result is consistent with the research results of Tan [
30], who pointed out that adding sodium acetate in the diet of perinatal dairy cows could significantly increase the urea level in plasma, further promoting protein catabolism and nitrogen excretion.
Flavor is a comprehensive manifestation of the intrinsic quality of dairy products and an important part of the sensory evaluation of fresh milk [
31]. Corresponding to human olfaction, taste, and touch, the flavor characteristics of milk can be analyzed from the dimensions of odor, taste, and texture [
32]. Volatile compounds in milk are the main manifestation of its flavor. Volatile compounds are not only the main source of flavor but also the main substances that produce the odor of milk. Only some volatile compounds in raw milk are helpful for flavor formation [
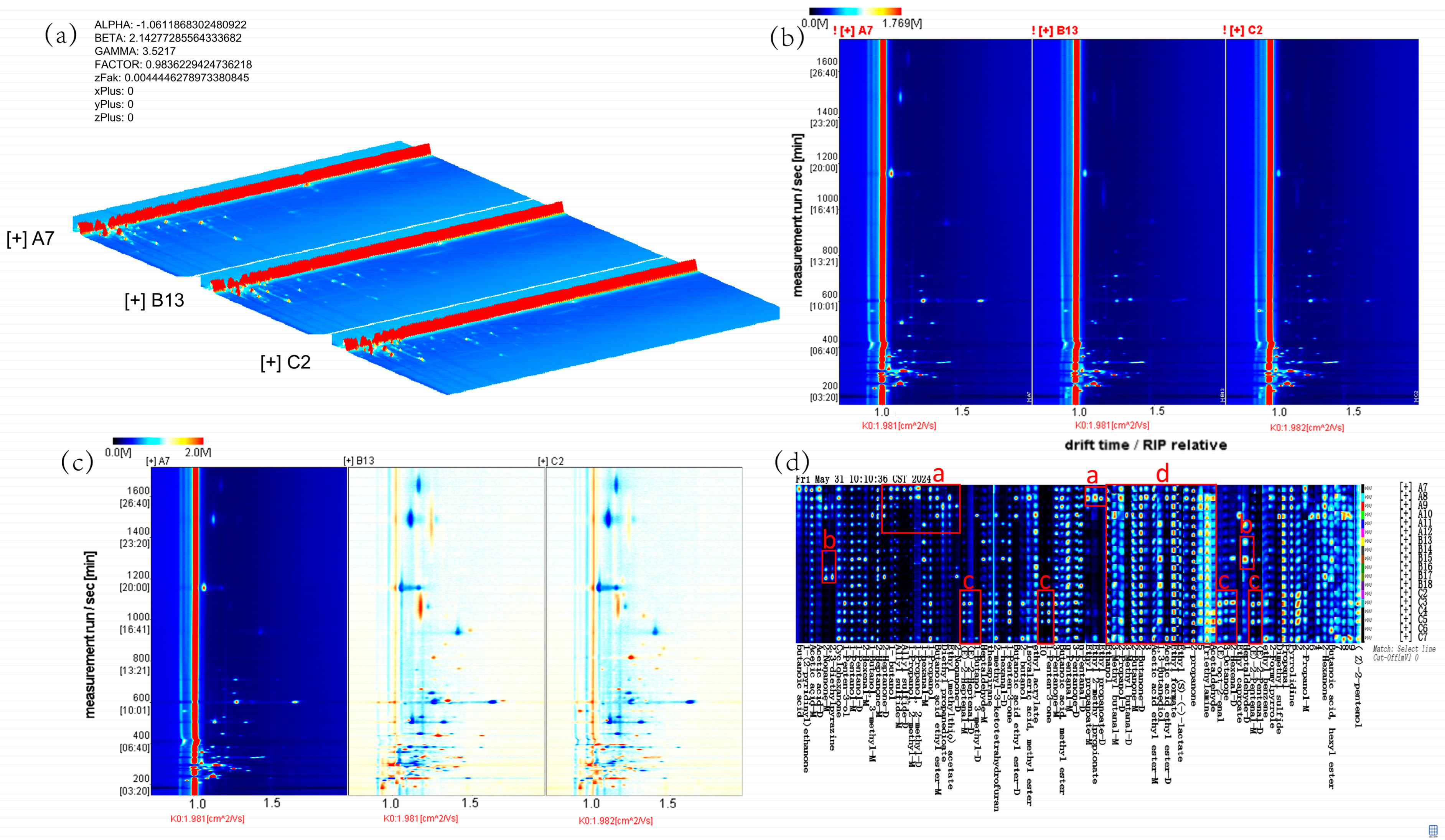
33]. The main volatile compounds in raw milk include terpenoids, acids (such as C4-C12 fatty acids), ketones (such as methyl ketones), aldehydes (such as nonenal, heptenal), esters (such as γ-lactone), phenols (such as
p-cresol), and sulfides (such as dimethyl sulfide) [
34]. Milk flavor is closely related to many factors, such as breed, diet, and feeding management [
35]. Currently, diet is considered the most important and sensitive factor affecting the flavor of milk and dairy products. Nutrients such as carbohydrates, fats, and proteins in the diet can affect the flavor substances in milk through different pathways, thereby changing the flavor of milk [
36]. Tu Xiaoting et al. [
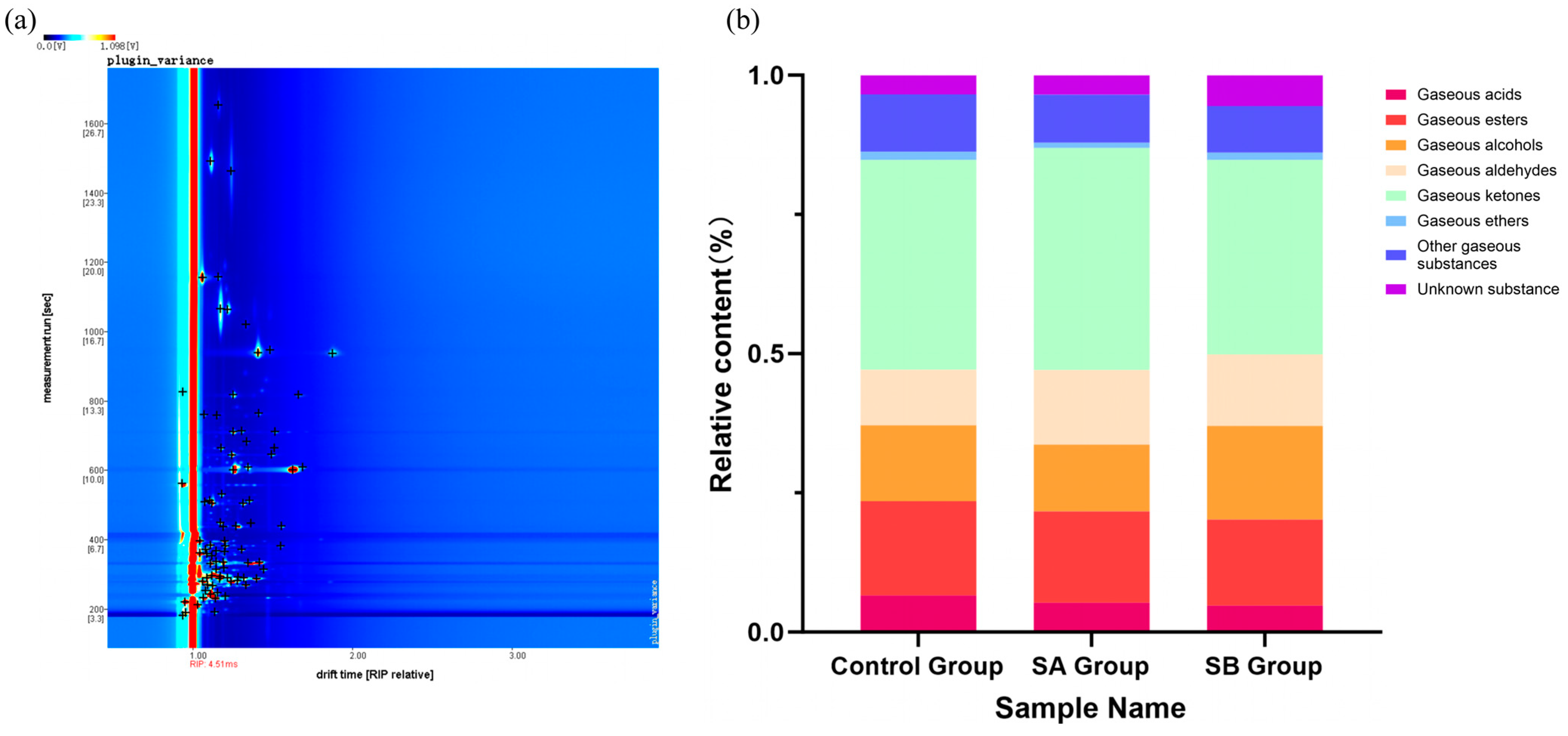
37] found that after short-chain fatty acids are ingested by the body, some are absorbed through the intestine and enter the mitochondrial citric acid cycle for energy supply, while others reach the liver through the portal vein and are ultimately converted into acetyl-CoA through β-oxidation in the mitochondria as the substrate for fatty acid synthesis in liver cells or participate in other metabolic pathways. Acetyl-CoA is an important intermediate product in biochemical pathways such as fatty acid synthesis and cholesterol synthesis. Through a series of enzymatic reactions, acetyl-CoA can participate in the synthesis of esters, acids, ketones, aldehydes, and other substances [
38]. Further, Han Yaping et al. [
39] found that substances such as alcohols, aldehydes, acids, esters, and ketones, which are the main volatile flavor components in milk, have a significant impact on the flavor of milk. Xue Haiyan et al. [
40] identified 56 volatile substances in full-fat cow milk powder and 63 volatile substances in full-fat goat milk powder, including acids, alcohols, carbonyls, esters, aromatic compounds, and heterocyclic compounds. The results of this experiment indicate that a total of 86 volatile components were identified in the three groups of samples, including 16 esters, 15 alcohols, 15 ketones, three acids, 18 aldehydes, and nine others. Combining the GC-IMS three-dimensional, two-dimensional, difference, and fingerprint spectra of the volatile components in mare milk showed that the volatile substances in the control group, sodium acetate group, and sodium butyrate group have similar composition, but their signal intensities are different. The types of volatile substances in the sodium acetate group and sodium butyrate group are similar, and both are significantly higher than those in the control group, with the sodium butyrate group having the highest concentration. This indicates that supplementing mares with sodium acetate and sodium butyrate can increase the types and concentrations of volatile substances in mare milk compared to the control group. The rich volatile compounds in milk can provide a more diverse taste experience. Compared to the control group, the characteristic peak signals of various substances in mare milk after addition of sodium acetate were all enhanced, mainly concentrated in 2,3-diethylpyrazine, 2-nonanone, and heptanal. Different from the sodium acetate group, the characteristic peak signals of various substances in mare milk after addition of sodium butyrate were all enhanced, especially in aldehyde and ketone compounds, mainly concentrated in 2-heptenal, trans-2-octenal, 2-hexenal, trans-2-pentenal, 1-penten-3-one, and 3-octanone. Aldehydes and ketones are key compounds involved in regulating the flavor of dairy products and play a decisive role in the formation of dairy product flavor [
41].
The unique flavor characteristics of milk are derived from the combined effects of various flavor substances, which are mainly composed of volatile organic compounds such as free fatty acids, alcohols, esters, and lactones [
42]. Among them, acid compounds mainly come from the decomposition process of fats and biological metabolic pathways such as microbial fermentation. Acetic acid gives dairy products a vinegar-like taste and a slightly irritating odor. In this experiment, after supplementing mares with sodium butyrate compared to the control group, the relative content of acetic acid in mare milk was significantly reduced, further improving the flavor of mare milk. The reduction in the relative content of acetic acid may be due to the addition of short-chain fatty acids, which increased the energy level of the body, promoting more acetic acid to participate in energy metabolism and milk fat synthesis, thereby reducing the relative content of acetic acid in mare milk [
43].
Ester compounds are an important component of the neutral volatile components in milk, formed by the esterification of short-chain fatty acids and alcohols. These substances emit pleasant fruit and floral aromas and are important sources of volatile flavor substances in milk [
44]. Moio et al. [
45] found that ethyl butyrate is a key flavor substance in fresh cow’s milk, goat’s milk, and sheep’s milk, with a unique fresh fruit aroma, which is crucial for improving the flavor of milk. In addition, ethyl L-lactate is also an important flavor substance in milk and food, with a slight fruity and wine-like odor, enhancing consumers’ olfactory experience of dairy products. This experiment found that after adding sodium acetate and sodium butyrate, the relative contents of ethyl butyrate and ethyl L-lactate in mare milk were significantly increased, improving the flavor and quality of mare milk. On the one hand, short-chain fatty acids can increase the activity of enzymes related to the synthesis of ester compounds by regulating the inflammatory response process of the body, promoting the synthesis of esters [
46]; on the other hand, short-chain fatty acids participate in the synthesis and metabolism of milk fat, enriching the free fatty acids in the mammary gland through de novo synthesis or elongation of carbon chains, thereby affecting the metabolism of flavor substances in milk [
47]. Alcohols are mostly formed by the oxidation of polyunsaturated fatty acids and have a sweet and mellow flavor characteristic. They account for a large proportion of the volatile flavor substances in milk [
48]. Ji Quan et al. [
49] demonstrated that abundant alcohols and ketones are the key volatile substances that affect the flavor of yak milk and cow milk products. In this study, mare milk with sodium acetate and sodium butyrate was rich in 1-penten-3-ol, pentanol, butanol, and cis-2-penten-1-ol. Through aroma characteristic retrieval, it was found that 1-penten-3-ol has a buttery aroma, pentanol has a grassy and fruity aroma, n-butanol has an alcohol aroma and a unique mature fruit aroma, and cis-2-penten-1-ol is a major component of fruity odors [
50,
51,
52]. These flavor substances give mare milk a fresher flavor and a richer taste. The increase in alcohols may be related to the metabolism of short-chain fatty acids. After absorption in the intestine, short-chain fatty acids can be metabolized through gluconeogenesis. During gluconeogenesis, some intermediate products (such as pyruvic acid) may be further converted into alcohol compounds. Additionally, the increase in alcohols may be related to the potential ability of certain intestinal microorganisms to convert short-chain fatty acids into alcohols [
53]. These microorganisms may convert short-chain fatty acids into corresponding alcohol compounds through specific enzymatic reactions [
54]. This speculation may partially explain the effect of adding sodium acetate and sodium butyrate on mare milk flavor, but further research is needed for verification.
Most aldehydes in milk are derived from fat oxidation [
55], while ketones are mainly produced through the oxidation and decomposition of unsaturated fatty acids, thermal decomposition processes, and the degradation of amino acids [
56]. Both aldehydes and ketones play important roles in flavor formation. Zhang Lin et al. [
57] demonstrated that the peak area of ketone components in volatile substances of cow milk is greater than that of other volatile components. Bai Xi et al. [
58] showed that donkey milk powder has a rich variety of volatile flavor compounds, with a total of 54 volatile substances identified, among which aldehydes account for eight types. Studies have shown that 2-hexenal has a sweet almond and grassy aroma, often described as a “Green” aroma type, and is commonly used as a flavor enhancer in food [
59]. Hexanal has a green, fruity, woody, and oily and grassy aroma, along with an apple-like scent; trans-2-octenal has a strong floral and fruity aroma; and propanal has a strong fruity aroma, such as apple and pear, and makes a significant contribution to the overall aroma of food. Gu Saiqi et al. [
60] demonstrated that ketones have a certain effect in reducing fishy odors. For example, 3-pentanone is a characteristic flavor component of fresh fruits [
61], 2-methyltetrahydrofuran-3-one has a sweet, nutty, and creamy aroma [
62], 1-penten-3-one belongs to the fruity type of compounds [
63], and 3-octanone as a volatile flavor substance can contribute a rich cheese-like aroma [
64]. In this study, the relative contents of aldehyde flavor substances such as 2-hexenal, hexanal, 2-heptenal, heptaldehyde-M, pentanal, and 3-methylbutanal in mare milk with added sodium acetate and sodium butyrate were significantly increased; the relative contents of ketone flavor substances such as 2-methyltetrahydrofuran-3-one, 1-penten-3-one, 3-pentanone, and 3-octanone were all extremely significantly higher, and sodium butyrate addition significantly increased the ketone flavor substances in mare milk. Additionally, the relative content of 2-acetylpyridine in milk increased extremely significantly with the addition of sodium butyrate. 2-Acetylpyridine has a popcorn aroma, a prominent nutty and sweet aroma, and a fruity aroma [
65], and is one of the key components for enhancing the flavor of mare milk. In addition, the relative content of dimethyl sulfide was significantly or extremely significantly higher than that of the control group. Dimethyl sulfide is not only a key aroma component of cooked garlic but also integrates flavors such as cabbage, vegetables, seafood, and cream, significantly enhancing the flavor of dairy products [
66,
67]. In this study, adding sodium acetate and sodium butyrate to the mares’ diet significantly increased the content of dimethyl sulfide and improved the flavor of the mare’s milk. In summary, after adding sodium acetate and sodium butyrate to the diet of mares, the relative content of aldehydes and ketones in the mare’s milk was increased, thereby generating aldehydes and ketones with different flavor characteristics, especially in the sodium butyrate group, where the effect was more significant.