The Influence of Aspiration Pressure, Follicle Flushing Method and Needle Rotation During Single-Operator OPU Technique on Oocyte Recovery and Embryo Production in the Mare
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
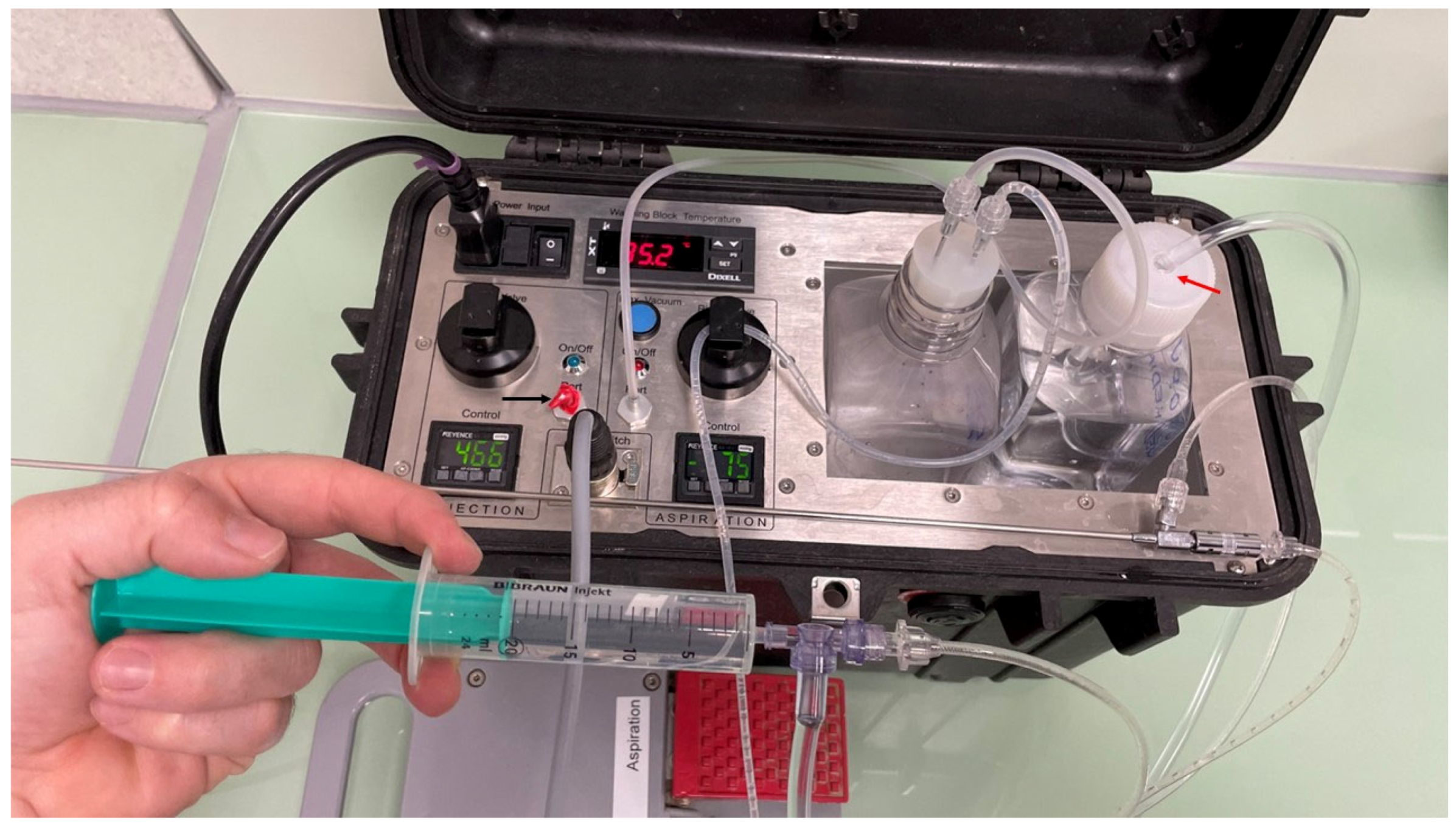
2.2. OPU Technique
2.3. Oocyte Search, Handling and Shipment
2.4. IVM, ICSI and IVC of Shipped Oocytes
2.5. Experimental Design
2.6. Statistical Analyses
3. Results
3.1. Effect of Aspiration Pressure on Oocyte Recovery and Embryo Production (Experiment 1)
3.2. Effect of Follicle Flushing Method on Oocyte Recovery (Experiment 2)
3.3. Effect of Needle Twisting During a Single-Operator OPU Technique on Oocyte Recovery (Experiment 3)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hinrichs, K. In Vitro production of equine embryos: State of the art. Reprod. Domest. Anim. 2010, 45 (Suppl. S2), 3–8. [Google Scholar] [CrossRef] [PubMed]
- Galli, C.; Colleoni, S.; Duchi, R.; Lagutina, I.; Lazzari, G. Developmental competence of equine oocytes and embryos obtained by in vitro procedures ranging from in vitro maturation and ICSI to embryo culture, cryopreservation and somatic cell nuclear transfer. Anim. Reprod. Sci. 2007, 98, 39–55. [Google Scholar] [CrossRef] [PubMed]
- Felix, M.R.; Turner, R.M.; Dobbie, T.; Hinrichs, K. Successful in vitro fertilization in the horse: Production of blastocysts and birth of foals after prolonged sperm incubation for capacitation. Biol. Reprod. 2022, 107, 1551–1564. [Google Scholar] [CrossRef]
- Stout, T.A.E. Clinical Application of in Vitro Embryo Production in the Horse. J. Equine Vet. Sci. 2020, 89, 103011. [Google Scholar] [CrossRef]
- Lazzari, G.; Colleoni, S.; Crotti, G.; Turini, P.; Fiorini, G.; Barandalla, M.; Landriscina, L.; Dolci, G.; Benedetti, M.; Duchi, R.; et al. Laboratory Production of Equine Embryos. J. Equine Vet. Sci. 2020, 89, 103097. [Google Scholar] [CrossRef] [PubMed]
- Cuervo-Arango, J.; Claes, A.N.; Stout, T.A.E. Mare and stallion effects on blastocyst production in a commercial equine ovum pick-up-intracytoplasmic sperm injection program. Reprod. Fertil. Dev. 2019, 31, 1894–1903. [Google Scholar] [CrossRef]
- Claes, A.; Stout, T.A.E. Success rate in a clinical equine in vitro embryo production program. Theriogenology 2022, 187, 215–218. [Google Scholar] [CrossRef]
- Hinrichs, K.; Kenney, R.M. A colpotomy procedure to increase oocyte recovery rates on aspiration of equine preovulatory follicles. Theriogenology 1987, 27, 237. [Google Scholar] [CrossRef]
- Vogelsang, M.M.; Kreider, J.L.; Bowen, M.J.; Potter, G.D.; Forrest, D.W.; Kraemer, D.C. Methods for collecting follicular oocytes from mares. Theriogenology 1988, 29, 1007–1018. [Google Scholar] [CrossRef]
- Hawley, L.R.; Enders, A.C.; Hinrichs, K. Comparison of Equine and Bovine Oocyte-Cumulus Morphology within the Ovarian Follicle. Biol. Reprod. 1995, 52, 243–252. [Google Scholar] [CrossRef]
- Márquez-Moya, A.; Sala-Ayala, L.; Carreras-Vico, N.; Martínez-Boví, R.; Cuervo-Arango, J. Factors influencing oocyte recovery during ultrasound-guided follicle aspiration in mares: A postmortem study. Theriogenology 2025, 235, 39–45. [Google Scholar] [CrossRef]
- Sasamoto, Y.; Sakaguchi, M.; Katagiri, S.; Yamada, Y.; Takahashi, Y. The effects of twisting and type of aspiration needle on the efficiency of transvaginal ultrasound-guided ovum pick-up in cattle. J. Vet. Med. Sci. 2003, 65, 1083–1086. [Google Scholar] [CrossRef] [PubMed]
- Bols, P.E.; Van Soom, A.; Ysebaert, M.T.; Van den heede, J.M.; de Kruif, A. Effects of aspiration vacuum and needle diameter on cumulus oocyte complex morphology and developmental capacity of bovine oocytes. Theriogenology 1996, 45, 1001–1014. [Google Scholar] [CrossRef] [PubMed]
- Bols, P.E.; Ysebaert, M.T.; Van Soom, A.; de Kruif, A. Effects of needle tip bevel and aspiration procedure on the morphology and developmental capacity of bovine compact cumulus oocyte complexes. Theriogenology 1997, 47, 1221–1236. [Google Scholar] [CrossRef]
- Ritchie, W.A. Nuclear transfer in sheep. Methods Mol. Biol. 2006, 325, 11–23. [Google Scholar]
- Galli, C.; Duchi, R.; Colleoni, S.; Lagutina, I.; Lazzari, G. Ovum pick up, intracytoplasmic sperm injection and somatic cell nuclear transfer in cattle, buffalo and horses: From the research laboratory to clinical practice. Theriogenology 2014, 81, 138–151. [Google Scholar] [CrossRef] [PubMed]
- Colleoni, S.; Lagutina, I.; Lazzari, G.; Rodriguez-Martinez, H.; Galli, C.; Morrell, J.M. New methods for selecting stallion spermatozoa for assisted reproduction. J. Equine Vet. Sci. 2011, 31, 536–541. [Google Scholar] [CrossRef]
- Rodríguez, C.; Anel, L.; Alvarez, M.; Anel, E.; Boixo, J.C.; Chamorro, C.A.; de Paz, P. Ovum pick-up in sheep: A comparison between different aspiration devices for optimal oocyte retrieval. Reprod. Domest. Anim. 2006, 41, 106–113. [Google Scholar] [CrossRef]
- Sá, M.A.F.; Paiva, S.O.; Dutra, G.A.; Barbosa, C.G.; Mello, M.R.B.; Jacob, J.C.F. Use of different pressures for transvaginal follicular aspiration in mares. Arq. Bras. Med. Vet. Zootec. 2017, 69, 529–534. [Google Scholar] [CrossRef]
- Brüssow, K.P.; Torner, H.; Rátky, J.; Hunter, M.G.; Nürnberg, G. Ovum pick up in swine: The influence of aspiration vacuum pressure on oocyte recovery from preovulatory follicles. Acta Vet. Hung. 1997, 45, 189–196. [Google Scholar]
- Ferré, L.B.; Alvarez-Gallardo, H.; Romo, S.; Fresno, C.; Stroud, T.; Stroud, B.; Lindsey, B.; Kjelland, M.E. Transvaginal ultrasound-guided oocyte retrieval in cattle: State-of-the-art and its impact on the in vitro fertilization embryo production outcome. Reprod. Domest. Anim. 2023, 58, 363–378. [Google Scholar] [CrossRef] [PubMed]
- Foss, R.; Ortis, H.; Hinrichs, K. Effect of potential oocyte transport protocols on blastocyst rates after intracytoplasmic sperm injection in the horse. Equine Vet. J. Suppl. 2013, 45, 39–43. [Google Scholar] [CrossRef]
- Sala-Ayala, L.; Pytel, A.T.; Stychno, K.; Cuervo-Arango, J. Use of excised ovaries for oocyte recovery by ultrasound guided follicular aspiration—Validation of an experimental model for research purposes in live mares ovum pick up. Theriogenology 2024, 228, 9–16. [Google Scholar] [CrossRef]
- Claes, A.; Cuervo-Arango, J.; van den Broek, J.; Galli, C.; Colleoni, S.; Lazzari, G.; Deelen, C.; Beitsma, M.; Stout, T.A. Factors affecting the likelihood of pregnancy and embryonic loss after transfer of cryopreserved in vitro produced equine embryos. Equine Vet. J. 2019, 51, 446–450. [Google Scholar] [CrossRef]
- Cortez, J.V.; Hardwicke, K.; Cuervo-Arango, J.; Grupen, C.G. Cloning horses by somatic cell nuclear transfer: Effects of oocyte source on development to foaling. Theriogenology 2023, 203, 99–108. [Google Scholar] [CrossRef]
- Cuervo-Arango, J.; Claes, A.N.; Beitsma, M.; Stout, T.A.E. The Effect of Different Flushing Media Used to Aspirate Follicles on the Outcome of a Commercial Ovum Pickup-ICSI Program in Mares. J. Equine Vet. Sci. 2019, 75, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Lazzari, G.; Colleoni, S.; Barandalla, M.; Benedetti, M.; Duchi, R.; Galli, C. 57° Influence of donor mare age on pre- and postimplanation embryo development within an equine ovum pick-up-intracytoplasmic sperm injection-embryo transfer (OPU-ICSI-ET) program over a three-year period. Reprod. Fertil. Dev. 2021, 34, 264. [Google Scholar] [CrossRef] [PubMed]
- Papas, M.; Govaere, J.; Peere, S.; Gerits, I.; Van de Velde, M.; Angel-Velez, D.; De Coster, T.; Van Soom, A.; Smits, K. Anti-Müllerian Hormone and OPU-ICSI Outcome in the Mare. Animals 2021, 11, 2004. [Google Scholar] [CrossRef]
- Ramírez-Agámez, L.; Hernández-Avilés, C.; Whitfield-Cargile, C.M.; Coleman, M.C.; Love, C.C. Treatment of mares with the non-steroidal anti-inflammatory drug (NSAID) phenylbutazone transiently affects in vitro maturation of equine oocytes and blastocyst development after Intracytoplasmic Sperm Injection (ICSI). Theriogenology 2024, 223, 53–58. [Google Scholar] [CrossRef]
- Walbornn, S.R.; Felix, M.; Schnobrich, M.R.; Bradecamp, E.A.; Scoggin, C.F.; Stefanovski, D.; Hinrichs, K. Effect of day of estrus cycle at time of transvaginal follicle aspiration for oocyte recovery on rates of in vitro maturation and blastocyst production after intracytoplasmic sperm injection. J. Am. Vet. Med. Assoc. 2022, 260, 1683–1689. [Google Scholar] [CrossRef]
- Dobbie, T.; Felix, M.R.; Gleason, K.; Hinrichs, K. Transvaginal aspiration, oocyte maturation and production of blastocysts and a pregnancy via ICSI in an endangered breed: Baudet du Poitou donkey. Theriogenology 2024, 224, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Martin-Pelaez, S.; Fuente, A.; Takahashi, K.; Perez, I.T.; Orozco, J.; Okada, C.T.C.; Neto, C.R.; Meyers, S.; Dini, P. IVF with frozen-thawed sperm after prolonged capacitation yields comparable results to ICSI in horses: A morphokinetics study. Theriogenology 2025, 232, 39–45. [Google Scholar] [CrossRef] [PubMed]

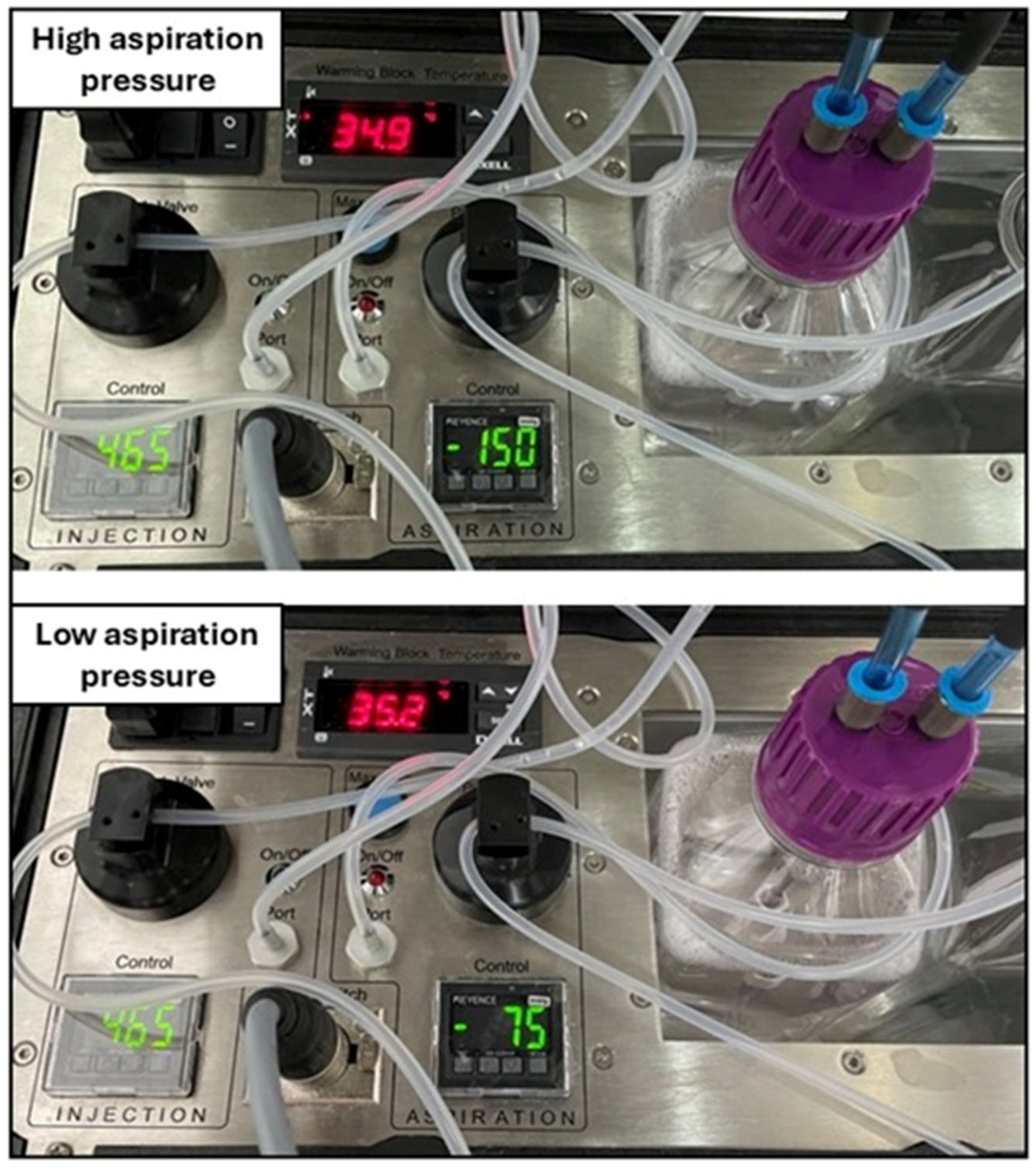
| Vacuum Pressure | Mares (n) | Donor Age (Years) | Flow Rate (mL/s) | Aspirated Follicles | Follicles < 10 mm (%) | Recovered Oocytes | Oocyte Recovery (%) | Overall Oocytes per Follicle | MII Rate (%) | Cleavage Rate (%) | Blastocyst Rate (%) | Embryos per Session | Success (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 150 mmHg | 18 | 12.1 ± 5.7 (4–24) | 1.33 | 25.1 ± 7.6 (12–38) | 70.4 ± 22.1 (10.5–100) | 14.2 ± 5.7 (5–26) | 57.3 ± 16.7 (25–66.7) | 256/451 (0.57) | 51.6 ± 12.1 a (25.3–70.4) | 77.2 ± 15.7 (40–100) | 20.6 ± 16.4 a (0–50) | 1.61 ± 1.3 (0–4) | 72.2 |
| 75 mmHg | 18 | 12.7 ± 5.8 (5–25) | 0.75 | 21.1 ± 8.2 (13–45) | 66.8 ± 20.2 (37–100) | 11.2 ± 6.1 (2–25) | 51.6 ± 18.9 (14.3–88.2) | 201/379 (0.53) | 64.4 ± 18.5 b (33.3–100) | 85.8 ± 16.8 (50–100) | 31.4 ± 28.9 b (0–100) | 1.89 ± 1.8 (0–7) | 77.7 |
| Injection System | Mares (n) | Donor Age (Years) | Aspirated Follicles | Follicles <10 mm (%) | Recovered Oocytes | Oocyte Recovery (%) | Overall Oocytes per Follicle | MII Rate (%) | Cleavage Rate (%) | Blastocyst Rate (%) | Embryos per Session | Success (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pump pedal | 18 | 13.4 ± 5.3 (3–26) | 23.4 ± 7.7 (8–37) | 60.9 ± 21.2 (12.1–95.5) | 12.2 ± 4.4 (6–19) | 53.4 ± 14.0 (31.6–77.8) | 220/421 (0.52) | 61.6 ± 12.4 (37.5–83.3) | 85.0 ± 17.6 (50–100) | 29.1 ± 26 (0–100) | 2.05 ± 1.8 (0–7) | 80.0 |
| Syringe | 18 | 14.0 ± 5.9 (5–25) | 23.2 ± 6.7 (12–36) | 69.7 ± 21.9 (41.2–100) | 12.3 ± 4.2 (5–20) | 54.0 ± 18.9 (25–86.7) | 222/417 (0.53) | 60.5 ± 16.5 (33.3–100) | 79.6 ± 19.9 (43–100) | 30.1 ± 22.2 (0–80) | 2.05 ± 1.4 (0–4) | 75.0 |
| Needle Twisting | Mares (n) | Donor Age (Years) | Aspirated Follicles | Follicles <10 mm (%) | Recovered Oocytes | Oocyte Recovery (%) | Overall Oocytes per Follicle | MII Rate (%) | Cleavage Rate (%) | Blastocyst Rate (%) | Embryos per Session | Success (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes | 16 | 14.4 ± 4.9 (3–23) | 24.2 ± 7.6 (12–35) | 69.4 ± 20.9 (35.3–100) | 13.4 ± 6.4 (4–27) | 54.4 ± 16.8 (22.2–76.5) | 214/387 (0.55) | 60.9 ± 13.2 (32.1–76.9) | 82.4 ± 18.6 (47.5–100) | 23.8 ± 21.3 (0–66.7) | 1.94 ± 1.8 (0–5) | 75.0 |
| No | 16 | 13.9 ± 5.1 (3–22) | 23.7 ± 9.3 (13–42) | 62.3 ± 19 (15.3–88.2) | 13.1 ± 6.6 (3–25) | 54.6 ± 20.5 (14.3–80.5) | 210/379 (0.55) | 68.1 ± 15.5 (41.2–100) | 81.9 ± 19.4 (45–93.7) | 25.2 ± 17.4 (0–50) | 1.86 ± 1.2 (0–4) | 81.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuervo-Arango, J.; Sala-Ayala, L.; Márquez-Moya, A.; Martínez-Boví, R. The Influence of Aspiration Pressure, Follicle Flushing Method and Needle Rotation During Single-Operator OPU Technique on Oocyte Recovery and Embryo Production in the Mare. Animals 2025, 15, 832. https://doi.org/10.3390/ani15060832
Cuervo-Arango J, Sala-Ayala L, Márquez-Moya A, Martínez-Boví R. The Influence of Aspiration Pressure, Follicle Flushing Method and Needle Rotation During Single-Operator OPU Technique on Oocyte Recovery and Embryo Production in the Mare. Animals. 2025; 15(6):832. https://doi.org/10.3390/ani15060832
Chicago/Turabian StyleCuervo-Arango, Juan, Laura Sala-Ayala, Adrián Márquez-Moya, and Rebeca Martínez-Boví. 2025. "The Influence of Aspiration Pressure, Follicle Flushing Method and Needle Rotation During Single-Operator OPU Technique on Oocyte Recovery and Embryo Production in the Mare" Animals 15, no. 6: 832. https://doi.org/10.3390/ani15060832
APA StyleCuervo-Arango, J., Sala-Ayala, L., Márquez-Moya, A., & Martínez-Boví, R. (2025). The Influence of Aspiration Pressure, Follicle Flushing Method and Needle Rotation During Single-Operator OPU Technique on Oocyte Recovery and Embryo Production in the Mare. Animals, 15(6), 832. https://doi.org/10.3390/ani15060832





