Influence of a Combination of Glycerol Polyethylene Glycol Ricinoleate and Bi-Distilled Oleic Acid in Powder Form on Growth Performance, Nutrient Digestibility, Excreta Nitrogen and Liver Fatty Acid Profile of Broilers Fed Reduced-Energy Diets
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Experimental Design, Diets and Animal Housing
2.3. Growth Performance
2.4. Feed and Excreta Analyses and Apparent Total Tract Nutrient Digestibility (ATTD)
2.5. Lipid Extraction and Fatty Acid Characterization in Hepatic Tissue
2.6. Statistical Evaluations
3. Results
3.1. Growth Performance Evaluation
3.2. Nutrients’ Apparent Total Tract Digestibility and Energy Utilization
3.3. Nitrogen Ammonia Content in Excreta
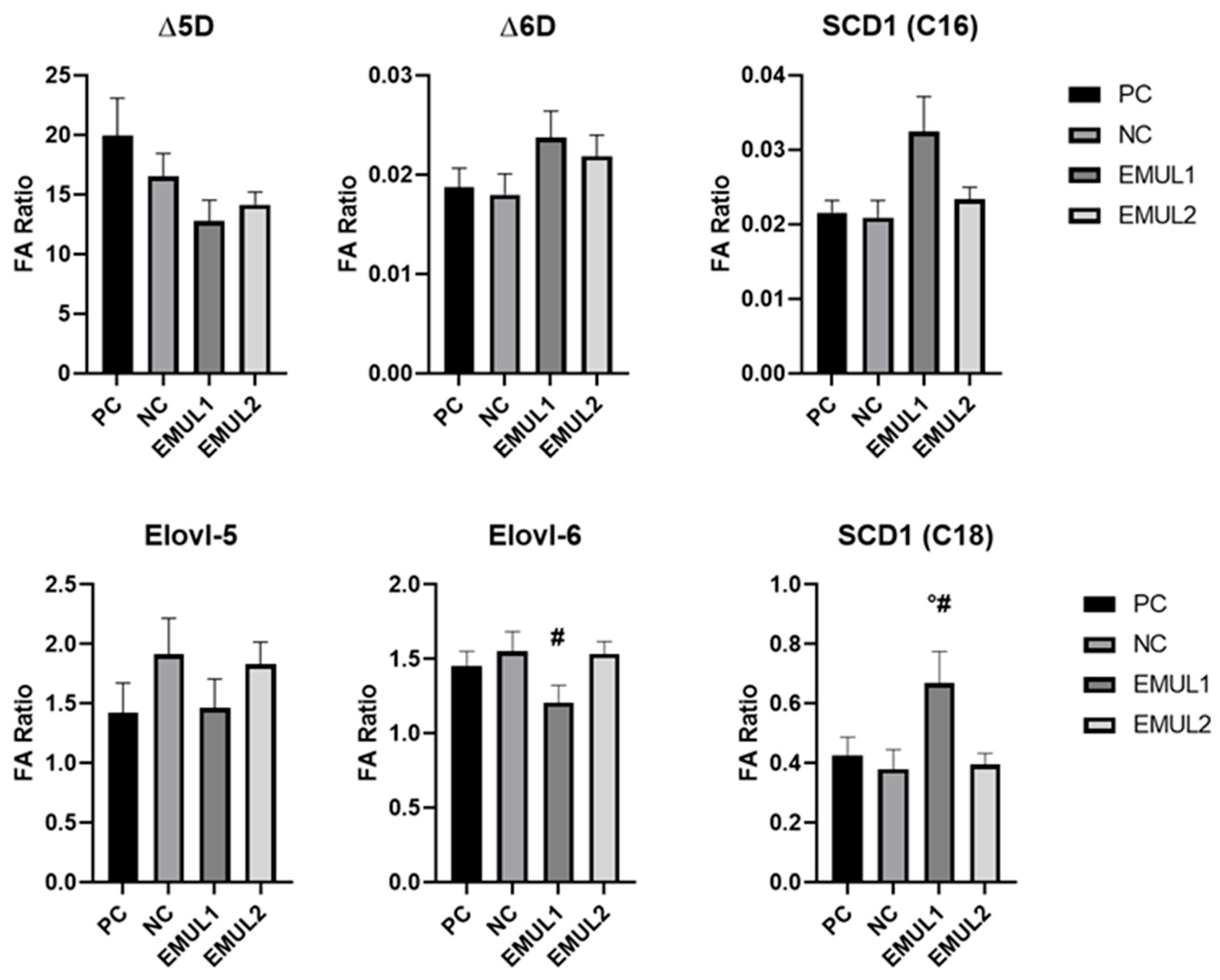
3.4. Hepatic Fatty Acid Profile and Deducted Enzymatic Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shahid, I.; Anwar, U.; Swar, S.O.; Saleem, M.I.; Butt, S.F.; Khan, W.; Bilal, M.B.; Riaz1, M.; Chishti, M.F.A.; Hussain, M.; et al. Effect of emulsifier (lysophospholipid) supplementation in broilers during different phases on growth performance, blood profile, digestibility, economics and meat quality. Pak. J. Agric. Sci. 2021, 58, 1033–1040. [Google Scholar]
- Shahid, I.; Sharif, M.; Yousaf, M.; Ahmad, F.; Anwar, U.; Ali, A.; Rahman, M.A. Emulsifier supplementation response in ross 308 broilers at 1–10 days. Braz. J. Poult. Sci. 2020, 22, 001–006. [Google Scholar] [CrossRef]
- Kamran, J.; Mehmood, S.; Mahmud, A. Effect of fat sources and emulsifier levels in broiler diets on performance, nutrient digestibility, and carcass parameters. Braz. J. Poult. Sci. 2020, 22, 001–010. [Google Scholar] [CrossRef]
- Kamran, J.; Mehmood, S.; Rahman, M.A.; Mahmud, A.; Hussain, M.; Rehman, A.; Qamar, S.H. Effect of fat sources and emulsifier supplementation in broiler starter, grower and finisher diets on performance, nutrient digestibility, and carcass parameters. Braz. J. Poult. Sci. 2020, 22, 001–010. [Google Scholar] [CrossRef]
- Arshad, M.A.; Bhatti, S.A.; Hassan, I.; Rahman, M.A.; Rehman, M.S. Effects of bile acids and lipase supplementation in low-energy diets on growth performance, fat digestibility and meat quality in broiler chickens. Braz. J. Poult. Sci. 2020, 22, 001–008. [Google Scholar] [CrossRef]
- Anwar, U.; El-Kott, A.F.; Bilal, M.Q.; Riaz, M.; Khalid, M.F.; Mustafa, R.; Rahman, M.A.U. Supplementation of xylanase levels in lower energy diets on digesta viscosity, blood metabolites and gut health of broiler. Pak. Vet. J. 2023, 43, 351–355. [Google Scholar]
- Brenda, V.D.; Zain, M.; Agustin, F. Strategy to reduce methane to increase feed efficiency in ruminants through adding essential oils as feed additives. Int. J. Vet. Sci. 2024, 13, 195–201. [Google Scholar]
- Sultanayeva, L.; Balji, Y.; Korotkiy, V.; Shantyz, A.; Issabekova, S.; Borovskiy, A.; Abakanova, G. The effect of extruded feed additives with balsamic poplar buds on productivity of dairy goats. Int. J. Vet. Sci. 2023, 12, 114–119. [Google Scholar]
- Anwar, U.; Yousaf, M.; Mirza, M.A.; Aziz-ur-Rahman, M. Impact of stored wheat-based feed on gut morphology, digesta viscosity and blood metabolites of broiler chickens. Pak. Vet. J. 2023, 43, 179–183. [Google Scholar]
- Murugesan, G.R. Understanding the effectiveness of blended fats and oils in poultry diets. Indian J. Anim. Reprod. 2013, 659, 55. [Google Scholar]
- Bauer, E.; Jakob, S.; Mosenthin, R. Principles of physiology of lipid digestion. Asian-Australas. J. Anim. Sci. 2005, 18, 282–295. [Google Scholar] [CrossRef]
- Singh, H.; Ye, A.; Horne, D. Structuring food emulsions in the gastrointestinal tract to modify lipid digestion. Prog. Lipid Res. 2009, 48, 92–100. [Google Scholar] [CrossRef]
- Arshad, M.A.; Bhatti, S.A.; Rehman, M.S.U.; Yousaf, W.; Younus, G.; Sizmaz, O.; Bilal, M.Q. Supplementation of bile acids and lipase in broiler diets for better nutrient utilization and performance: Potential effects and future implications–A review. Ann. Anim. Sci. 2021, 21, 757–787. [Google Scholar] [CrossRef]
- Acevedo-Fani, A.; Singh, H. Biophysical insights into modulating lipid digestion in food emulsions. Prog. Lipid Res. 2022, 85, 101129. [Google Scholar] [CrossRef] [PubMed]
- Siyal, F.A.; Babazadeh, D.; Wang, C.; Arain, M.A.; Saeed, M.; Ayasan, T.; Zhang, L.; Wang, T. Emulsifiers in the poultry industry. World’s Poult. Sci. J. 2017, 73, 611–620. [Google Scholar] [CrossRef]
- McClements, D.J.; Bai, L.; Chung, C. Recent advances in the utilization of natural emulsifiers to form and stabilize emulsions. Annu. Rev. Food Sci. Technol. 2017, 8, 205–236. [Google Scholar] [CrossRef]
- McClements, D.J.; Jafari, S.M. Improving emulsion formation, stability and performance using mixed emulsifiers: A review. Adv. Colloid Interface Sci. 2018, 251, 55–79. [Google Scholar] [CrossRef] [PubMed]
- Majdolhosseini, L.; Ghasemi, H.; Hajkhodadadi, I.; Moradi, M. Nutritional and physiological responses of broiler chickens to dietary supplementation with de-oiled soyabean lecithin at different metabolisable energy levels and various fat sources. Br. J. Nutr. 2019, 122, 863–872. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi-Sefat, A.A.; Taherpour, K.; Ghasemi, H.A.; Gharaei, M.A.; Shirzadi, H.; Rostami, F. Effects of an emulsifier blend supplementation on growth performance, nutrient digestibility, intestinal morphology, and muscle fatty acid profile of broiler chickens fed with different levels of energy and protein. Poult. Sci. 2022, 101, 102145. [Google Scholar] [CrossRef]
- Hasenhuettl, G.L. Overview of food emulsifiers. In Food Emulsifiers and their Applications; Springer: New York, NY, USA, 2019; pp. 1–9. [Google Scholar]
- Giermanska-Kahn, J.; Laine, V.; Arditty, S.; Schmitt, V.; Leal-Calderon, F. Particle-stabilized emulsions comprised of solid droplets. Langmuir 2005, 21, 4316–4323. [Google Scholar] [CrossRef]
- Bontempo, V.; Comi, M.; Jiang, X.R.; Rebucci, R.; Caprarulo, V.; Giromini, C.; Gottardo, D.; Fusi, E.; Stella, S.; Tirloni, E.; et al. Evaluation of a synthetic emulsifier product supplementation on broiler chicks. Anim. Feed Sci. Technol. 2018, 240, 157–164. [Google Scholar] [CrossRef]
- Wealleans, A.L.; Desbruslais, A.; Goncalves, R.; Scholey, D.; Gonzalez-Sanchez, D.; Burton, E.; Spaepen, R.; Elliot, A.; Currie, D. Research Note: Comparative effects of liquid and dry applications of a combination of lysolecithin, synthetic emulsifier, and monoglycerides on growth performance, nutrient digestibility, and litter moisture in broilers fed diets of differing energy density. Poult. Sci. 2024, 103, 103345. [Google Scholar] [CrossRef] [PubMed]
- Setyahadi, S. Animal Feed from Oil Producing Plants. In Biorefinery of Oil Producing Plants for Value-Added Products; John Wiley & Sons: Hoboken, NJ, USA, 2022; Volume 2, pp. 631–651. [Google Scholar] [CrossRef]
- Lavenburg, V.M.; Rosentrater, K.A.; Jung, S. Extraction methods of oils and phytochemicals from seeds and their environmental and economic impacts. Processes 2021, 9, 1839. [Google Scholar] [CrossRef]
- Liu, J.J.; Gasmalla, M.A.A.; Li, P.; Yang, R. Enzyme-assisted extraction processing from oilseeds: Principle, processing and application. Innov. Food Sci. Emerg. Technol. 2016, 35, 184–193. [Google Scholar] [CrossRef]
- Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32007L0043&from=EN (accessed on 4 February 2025).
- Scott, T.A.; Boldaji, F. Comparison of inert markers [chromic oxide or insoluble ash (Celite)] for determining apparent metabolizable energy of wheat-or barley-based broiler diets with or without enzymes. Poult. Sci. 1997, 76, 594–598. [Google Scholar] [CrossRef] [PubMed]
- Zampiga, M.; Meluzzi, A.; Sirri, F. Effect of dietary supplementation of lysophospholipids on productive performance, nutrient digestibility and carcass quality traits of broiler chickens. Ital. J. Anim. Sci. 2016, 15, 521–528. [Google Scholar] [CrossRef]
- Lopez, G.; Leeson, S. Nitrogen content of manure from older broiler breeders fed varying quantities of crude protein. J. Appl. Poult. Res. 1995, 4, 390–394. [Google Scholar] [CrossRef]
- Thiex, N.J.; Anderson, S.; Gildemeister, B. Crude fat, diethyl ether extraction, in feed, cereal grain, and forage (Randall/Soxtec/submersion method): Collaborative study. J. AOAC Int. 2003, 86, 888–898. [Google Scholar] [CrossRef]
- Thiex, N.; Novotny, L.; Crawford, A. Determination of ash in animal feed: AOAC official method 942.05 revisited. J. AOAC Int. 2012, 95, 1392–1397. [Google Scholar] [CrossRef]
- Maharjan, P.; Mayorga, M.; Hilton, K.; Weil, J.; Beitia, A.; Caldas, J.; England, J.; Coon, C. Non-cellulosic polysaccharide content in feed ingredients and ileal and total tract non-cellulosic polysaccharide digestibility in 21-and 42-day-old broilers fed diets with and without added composite enzymes. Poult. Sci. 2019, 98, 4048–4057. [Google Scholar] [CrossRef]
- Jimenez-Moya, B.; Barroeta, A.C.; Guardiola, F.; Soler, M.D.; Rodriguez-Sanchez, R.; Sala, R. Replacement of palm oil with soybean acid oil in broiler chicken diet: Fat digestibility and lipid class content along the intestinal tract. Animals 2021, 11, 2586. [Google Scholar] [CrossRef] [PubMed]
- Maharjan, P.; Rahimi, A.; Harding, K.L.; Vu, T.C.; Malheiros, R.; Oviedo-Rondon, E.O.; Mian, R.; Joseph, M.; Dean, L.; Anderson, K.E.; et al. Effects of full-fat high-oleic soybean meal in layer diets on nutrient digestibility and egg quality parameters of a white laying hen strain. Poult. Sci. 2023, 102, 102486. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Schuchardt, F.; Shen, Y.; Li, G.; Li, C. Impact of struvite crystallization on nitrogen losses during composting of pig manure and cornstalk. Waste Manag. 2010, 30, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Serini, S.; Cassano, R.; Corsetto, P.A.; Rizzo, A.M.; Calviello, G.; Trombino, S. Omega-3 PUFA loaded in resveratrol-based solid lipid nanoparticles: Physicochemical properties and antineoplastic activities in human colorectal cancer cells in vitro. Int. J. Mol. Sci. 2018, 19, 586. [Google Scholar] [CrossRef]
- Ungaro, F.; Tacconi, C.; Massimino, L.; Corsetto, P.A.; Correale, C.; Fonteyne, P.; Danese, S. MFSD2A promotes endothelial generation of inflammation-resolving lipid mediators and reduces colitis in mice. Gastroenterology 2017, 153, 1363–1377. [Google Scholar] [CrossRef]
- Drąg, J.; Goździalska, A.; Knapik-Czajka, M.; Gawędzka, A.; Gawlik, K.; Jaśkiewicz, J. Effect of high carbohydrate diet on elongase and desaturase activity and accompanying gene expression in rat’s liver. Genes Nutr. 2017, 12, 2. [Google Scholar] [CrossRef]
- Zhao, P.Y.; Kim, I.H. Effect of diets with different energy and lysophospholipids levels on performance, nutrient metabolism, and body composition in broilers. Poult. Sci. 2017, 96, 1341–1347. [Google Scholar] [CrossRef]
- Collett, S.R. Nutrition and wet litter problems in poultry. Anim. Feed. Sci. Technol. 2012, 173, 65–75. [Google Scholar] [CrossRef]
- Boontiam, W.; Hyun, Y.K.; Jung, B.; Kim, Y.Y. Effects of lysophospholipid supplementation to reduced energy, crude protein, and amino acid diets on growth performance, nutrient digestibility, and blood profiles in broiler chickens. Poult. Sci. 2019, 98, 6693–6701. [Google Scholar] [CrossRef]
- Oketch, E.O.; Lee, J.W.; Yu, M.; Hong, J.S.; Kim, Y.B.; Chiu, J.W.; Nawarathne, S.R.; Heo, J.M. Physiological responses of broiler chickens fed reduced-energy diets supplemented with emulsifiers. Anim. Biosci. 2022, 35, 1929–1939. [Google Scholar] [CrossRef]
- Upadhaya, S.D.; Park, J.W.; Park, J.H.; Kim, I.H. Efficacy of 1, 3-diacylglycerol as a fat emulsifier in low-density diet for broilers. Poult. Sci. 2017, 96, 1672–1678. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Choi, H.; Kim, W.K. Effects of dietary energy level and 1, 3-diacylglycerol on growth performance and carcass yield in broilers. J. Appl. Poult. Res. 2020, 29, 665–672. [Google Scholar] [CrossRef]
- Massuquetto, A.; Panisson, J.C.; Schramm, V.G.; Surek, D.; Krabbe, E.L.; Maiorka, A. Effects of feed form and energy levels on growth performance, carcass yield and nutrient digestibility in broilers. Animal 2020, 14, 1139–1146. [Google Scholar] [CrossRef] [PubMed]
- Jalal, M.P.; Sharifi, S.D.; Honarbakhsh, S.; Rouhanipour, H. Effects of low energy diets supplemented with emulsifier on growth performance, nutrient digestibility, and intestinal morphology of broiler chickens. Livest. Sci. 2024, 289, 105581. [Google Scholar] [CrossRef]
- Bontempo, V.; Comi, M.; Jiang, X.R. The effects of a novel synthetic emulsifier product on growth performance of chickens for fattening and weaned piglets. Animal 2016, 10, 592–597. [Google Scholar] [CrossRef]
- Wang, Y.; Zeng, D.; Wei, L.; Chen, J.; Li, H.; Wen, L.; Huang, G.; Dai, Z.; Luo, J.; Sun, J.; et al. Effects of emulsifiers on lipid metabolism and performance of yellow-feathered broilers. BMC Vet. Res. 2024, 20, 246. [Google Scholar] [CrossRef]
- Gholami, M.; Shirzadi, H.; Taherpour, K.; Rahmatnejad, E.; Shokri, A.; Khatibjoo, A. Effect of emulsifier on growth performance, nutrient digestibility, intestinal morphology, faecal microbiology and blood biochemistry of broiler chickens fed low-energy diets. Vet. Med. Sci. 2024, 10, e1437. [Google Scholar] [CrossRef]
- Dierick, N.A.; Decuypere, J.A. Influence of lipase and/or emulsifier addition on the ileal and faecal nutrient digestibility in growing pigs fed diets containing 4% animal fat. J. Sci. Food Agric. 2004, 84, 1443–1450. [Google Scholar] [CrossRef]
- Mun, S.; Decker, E.A.; McClements, D.J. Influence of emulsifier type on in vitro digestibility of lipid droplets by pancreatic lipase. Food Res. Int. 2007, 40, 770–781. [Google Scholar] [CrossRef]
- Oketch, E.O.; Wickramasuriya, S.S.; Oh, S.; Choi, J.S.; Heo, J.M. Physiology of lipid digestion and absorption in poultry: An updated review on the supplementation of exogenous emulsifiers in broiler diets. J. Anim. Physiol. Anim. Nutr. 2023, 107, 1429–1443. [Google Scholar] [CrossRef]
- Roy, A.; Haldar, S.; Mondal, S.; Ghosh, T.K. Effects of supplemental exogenous emulsifier on performance, nutrient metabolism, and serum lipid profile in broiler chickens. Vet. Med. Int. 2010, 2010, 262604. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.Y.; Li, H.L.; Hossain, M.M.; Kim, I.H. Effect of emulsifier (lysophospholipids) on growth performance, nutrient digestibility and blood profile in weanling pigs. Anim. Feed Sci. Technol. 2015, 207, 190–195. [Google Scholar] [CrossRef]
- Zou, L.; Liu, W.; Liu, C.; Xiao, H.; McClements, D.J. Designing excipient emulsions to increase nutraceutical bioavailability: Emulsifier type influences curcumin stability and bioaccessibility by altering gastrointestinal fate. Food Funct. 2015, 6, 2475–2486. [Google Scholar] [CrossRef]
- Zhang BingKun, Z.B.; Li HaiTao, L.H.; Zhao DongQin, Z.D.; Guo YuMing, G.Y.; Barri, A. Effect of fat type and lysophosphatidylcholine addition to broiler diets on performance, apparent digestibility of fatty acids, and apparent metabolizable energy content. Anim. Feed. Sci. Technol. 2011, 163, 177–184. [Google Scholar] [CrossRef]
- Gass, J.; Vora, H.; Hofmann, A.F.; Gray, G.M.; Khosla, C. Enhancement of dietary protein digestion by conjugated bile acids. Gastroenterology 2007, 133, 16–23. [Google Scholar] [CrossRef]
- Mackie, A.; Macierzanka, A. Colloidal aspects of protein digestion. Curr. Opin. Colloid Interface Sci. 2010, 15, 102–108. [Google Scholar] [CrossRef]
- San Tan, H.; Zulkifli, I.; Farjam, A.S.; Goh, Y.M.; Croes, E.; Partha, S.K.; Tee, A.K. Effect of exogenous emulsifier on growth performance, fat digestibility, apparent metabolisable energy in broiler chickens. J. Biochem. Microbiol. Biotechnol. 2016, 4, 7–10. [Google Scholar] [CrossRef]
- Oliveira, M.V.G.D.; Leandro, N.S.M.; Café, M.B.; Santos, R.R.D.; Jacob, D.V.; Pires, M.F. Effect of emulsifier addition on metabolizable energy reduction in broiler diets. Ciênc. Anim. Bras. 2023, 24, e-75526E. [Google Scholar] [CrossRef]
- Giannakis, E.; Kushta, J.; Bruggeman, A.; Lelieveld, J. Costs and benefits of agricultural ammonia emission abatement options for compliance with European air quality regulations. Environ. Sci. Eur. 2019, 31, 93. [Google Scholar] [CrossRef]
- Such, N.; Pál, L.; Strifler, P.; Horváth, B.; Koltay, I.A.; Rawash, M.A.; Farkas, V.; Mezőlaki, A.; Wagner, L.; Dublecz, K. Effect of feeding low protein diets on the production traits and the nitrogen composition of excreta of broiler chickens. Agriculture 2021, 11, 781. [Google Scholar] [CrossRef]
- Ahmadipour, B.; Hassanpour, H.; Khajali, F. Evaluation of hepatic lipogenesis and antioxidant status of broiler chickens fed mountain celery. BMC Vet. Res. 2018, 13, 234. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Luo, W.; Liu, H.; Zhang, K.; Wang, J.; Ding, X.; Zeng, Q.; Peng, H.; Bai, J.; Xuan, Y.; et al. Effects of high dietary iron on the lipid metabolism in the liver and adipose tissue of male broiler chickens. Anim. Feed Sci. Technol. 2021, 282, 115131. [Google Scholar] [CrossRef]
- Boschetti, E.; Bordoni, A.; Meluzzi, A.; Castellini, C.; Dal Bosco, A.; Sirri, F. Fatty acid composition of chicken breast meat is dependent on genotype-related variation of FADS1 and FADS2 gene expression and desaturating activity. Animal 2016, 10, 700–708. [Google Scholar] [CrossRef] [PubMed]
- Baião, N.C.; Lara, L.J.C. Oil and fat in broiler nutrition. Braz. J. Poult. Sci. 2005, 7, 129–141. [Google Scholar] [CrossRef]
- Wang, D.; Li, X.; Zhang, P.; Cao, Y.; Zhang, K.; Qin, P.; Guo, Y.; Li, Z.; Tian, Y.; Kang, X.; et al. ELOVL gene family plays a virtual role in response to breeding selection and lipid deposition in different tissues in chicken (Gallus gallus). BMC Genom. 2022, 23, 705. [Google Scholar] [CrossRef]
- Tallima, H.; El Ridi, R. Arachidonic acid: Physiological roles and potential health benefits–a review. J. Adv. Res. 2018, 11, 33–41. [Google Scholar] [CrossRef]
- den Besten, G.; Lange, K.; Havinga, R.; van Dijk, T.H.; Gerding, A.; van Eunen, K.; Müller, M.; Groen, A.K.; Hooiveld, G.J.; Bakker, B.M.; et al. Gut-derived short-chain fatty acids are vividly assimilated into host carbohydrates and lipids. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 305, G900–G910. [Google Scholar] [CrossRef]
- D’Andre, H.C.; Paul, W.; Shen, X.; Jia, X.; Zhang, R.; Sun, L.; Zhang, X. Identification and characterization of genes that control fat deposition in chickens. J. Anim. Sci. Biotechnol. 2013, 4, 43. [Google Scholar] [CrossRef]


| Starter | Grower | Finisher | ||||
|---|---|---|---|---|---|---|
| Ingredients, % as Fed | PC | NC, EMUL1, EMUL2 | PC | NC, EMUL1, EMUL2 | PC | NC, EMUL1, EMUL2 |
| Maize meal | 52.17 | 49.00 | 47.51 | 44.51 | 53.28 | 53.28 |
| Soybean meal (46% CP) | 37.50 | 38.00 | 34.00 | 33.00 | 31.30 | 32.00 |
| Wheat | 2.00 | 5.30 | 10.00 | 15.20 | 5.00 | 5.50 |
| Soybean oil | 4.00 | 2.97 | 5.00 | 3.80 | 6.20 | 5.00 |
| Sodium chloride | 0.40 | 0.40 | 0.35 | 0.35 | 0.25 | 0.25 |
| Calcium carbonate | 1.20 | 1.20 | 1.00 | 1.00 | 0.95 | 0.95 |
| Dicalcium phosphate | 1.50 | 1.50 | 1.00 | 1.00 | 1.00 | 1.00 |
| DL-methionine | 0.34 | 0.34 | 0.30 | 0.30 | 0.25 | 0.25 |
| L-threonine | 0.11 | 0.11 | 0.09 | 0.09 | 0.04 | 0.04 |
| L-lysine HCL | 0.28 | 0.25 | 0.25 | 0.25 | 0.23 | 0.23 |
| Vitamins + trace elements 1 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| Celite | - | - | - | - | 0.50 | 0.50 |
| Chemical components, % as fed (calculated) | ||||||
| ME, kcal/kg | 3050 | 2980 | 3100 | 3030 | 3200 | 3130 |
| Crude protein, % | 21.80 | 21.80 | 20.20 | 20.20 | 19.40 | 19.40 |
| Ether extract, % | 6.30 | 5.30 | 6.20 | 5.00 | 8.00 | 7.20 |
| Lysine, % | 1.33 | 1.33 | 1.20 | 1.20 | 1.13 | 1.13 |
| Calcium, % | 0.90 | 0.90 | 0.75 | 0.75 | 0.70 | 0.70 |
| Available phosphorus, % | 0.60 | 0.60 | 0.55 | 0.55 | 0.50 | 0.50 |
| Chemical Components, % as Fed 1 | Starter | Grower | Finisher | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PC | NC | EMUL1 | EMUL2 | PC | NC | EMUL1 | EMUL2 | PC | NC | EMUL1 | EMUL2 | |
| DM | 89.34 ± 3.81 | 87.59 ± 2.97 | 89.23 ± 2.88 | 88.40 ± 2.71 | 87.62 ± 3.22 | 88.37 ± 3.30 | 88.12 ± 3.44 | 89.61 ± 3.27 | 89.17 ± 3.71 | 88.56 ± 3.49 | 89.04 ± 3.44 | 87.92 ± 3.31 |
| CP | 22.20 ± 0.71 | 22.00 ± 0.63 | 22.43 ± 68 | 22.67 ± 0.65 | 20.58 ± 0.78 | 20.32 ± 0.73 | 20.12 ± 0.70 | 20.27 ± 0.76 | 19.58 ± 0.62 | 19.81 ± 0.64 | 19.29 ± 0.67 | 19.77 ± 0.74 |
| EE | 6.00 ± 0.35 | 4.93 ± 0.58 | 4.90 ± 0.55 | 4.84 ± 0.59 | 6.88 ± 0.37 | 5.12 ± 0.31 | 5.19 ± 0.42 | 5.24 ± 0.39 | 7.89 ± 0.36 | 6.90 ± 0.33 | 6.85 ± 0.41 | 6.82 ± 0.40 |
| Ash | 4.00 ± 0.20 | 3.98 ± 0.23 | 3.80 ± 0.28 | 4.08 ± 0.26 | 4.12 ± 0.21 | 4.19 ± 0.25 | 3.91 ± 0.34 | 3.78 ± 0.31 | 5.41 ± 0.35 | 5.34 ± 0.29 | 5.62 ± 0.32 | 5.79 ± 0.33 |
| GE, kcal/kg | 4038 ± 54 | 3964 ± 51 | 3957 ± 56 | 3961 ± 53 | 4126 ± 52 | 4040 ± 57 | 4059 ± 54 | 4063 ± 50 | 4233 ± 52 | 4129 ± 55 | 4127 ± 49 | 4124 ± 58 |
| Parameters | PC | NC | EMUL1 | EMUL2 | SEM | p-Value |
|---|---|---|---|---|---|---|
| BW (g) | ||||||
| 1 d | 42.42 | 42.48 | 42.52 | 42.14 | 0.22 | 0.87 |
| 10 d | 261.14 b | 266.83 ab | 260.49 b | 274.62 a | 3.16 | <0.05 |
| 21 d | 840 B | 828 B | 870 AB | 898 A | 13 | <0.01 |
| 42 d | 2609 B | 2558 B | 2846 A | 2774 A | 30 | <0.01 |
| ADG (g/d) | ||||||
| 1–10 d | 23.74 b | 24.26 ab | 23.68 b | 24.96 a | 0.29 | <0.05 |
| 11–21 d | 57.92 AB | 56.11 B | 60.64 AB | 62.08 A | 1.26 | <0.01 |
| 22–42 d | 83.76 B | 82.12 B | 92.58 A | 89.07 A | 1.28 | <0.01 |
| 1–42 d | 62.60 B | 61.34 B | 68.36 A | 66.63 A | 0.74 | <0.01 |
| ADFI (g/d) | ||||||
| 1–10 d | 26.73 | 26.56 | 27.01 | 27.14 | 0.32 | 0.91 |
| 11–21 d | 79.06 | 75.96 | 77.91 | 79.35 | 1.18 | 0.89 |
| 22–42 d | 151.11 ab | 144.32 b | 153.35 ab | 154.32 a | 2.54 | <0.05 |
| 1–42 d | 103.85 ab | 99.57 b | 104.80 a | 105.68 a | 1.33 | <0.05 |
| FCR | ||||||
| 1–10 d | 1.12 | 1.09 | 1.14 | 1.08 | 0.01 | 0.82 |
| 11–21 d | 1.37 | 1.35 | 1.29 | 1.28 | 0.03 | 0.75 |
| 22–42 d | 1.80 b | 1.76 ab | 1.65 a | 1.73 ab | 0.03 | <0.05 |
| 1–42 d | 1.66 B | 1.62 B | 1.53 A | 1.59 AB | 0.02 | <0.01 |
| Mortality% | ||||||
| 1–42 d | 2.22 | 1.66 | 2.22 | 1.66 | 0.01 | 0.68 |
| Parameters | PC | NC | EMUL1 | EMUL2 | SEM | p-Value |
|---|---|---|---|---|---|---|
| DM | ||||||
| 24 d | 0.934 b | 0.941 ab | 0.954 a | 0.953 a | 0.004 | <0.05 |
| 42 d | 0.941 B | 0.943 AB | 0.956 A | 0.961 A | 0.004 | <0.01 |
| Ash | ||||||
| 24 d | 0.431 | 0.497 | 0.523 | 0.477 | 0.020 | 0.62 |
| 42 d | 0.508 B | 0.534 B | 0.624 A | 0.556 AB | 0.023 | <0.01 |
| CP | ||||||
| 24 d | 0.760 ab | 0.794 b | 0.811 ab | 0.832 a | 0.013 | <0.05 |
| 42 d | 0.723 B | 0.755 B | 0.774 AB | 0.803 A | 0.011 | <0.01 |
| EE | ||||||
| 24 d | 0.936 ab | 0.917 b | 0.952 a | 0.950 a | 0.009 | <0.05 |
| 42 d | 0.877 B | 0.889 B | 0.924 AB | 0.936 A | 0.015 | <0.01 |
| GE | ||||||
| 24 d | 0.746 b | 0.789 ab | 0.781 ab | 0.807 a | 0.013 | <0.05 |
| 42 d | 0.769 B | 0.779 B | 0.814 AB | 0.827 A | 0.011 | <0.01 |
| AME (kcal/kg) | ||||||
| 24 d | 3094 | 3166 | 3139 | 3237 | 53 | 0.54 |
| 42 d | 3190 ab | 3130 b | 3274 ab | 3318 a | 46 | <0.05 |
| AMEn (kcal/kg) | ||||||
| 24 d | 3088 | 3140 | 3116 | 3212 | 59 | 0.46 |
| 42 d | 3174 ab | 3099 b | 3241 a | 3285 a | 45 | <0.05 |
| Parameters | PC | NC | EMUL1 | EMUL2 | SEM | p-Value |
|---|---|---|---|---|---|---|
| C16:0 (Palmitic) | 18.90 | 18.60 | 21.02 | 18.46 | 0.60 | 0.08 |
| C16:1 (Palmitoleic) | 0.42 | 0.41 | 0.72 | 0.44 | 0.08 | 0.07 |
| C18:0 (Stearic) | 26.75 ab | 27.97 a | 24.39 b | 27.98 a | 0.84 | <0.05 |
| C18:1 (Oleic) | 11.11 ab | 10.15 b | 15.63 a | 10.81 b | 1.25 | <0.05 |
| C18:2 (Linoleic) | 27.59 a | 26.38 ab | 25.48 ab | 25.59 b | 0.49 | <0.05 |
| C18:3γ (γ-linolenic) | 0.52 | 0.48 | 0.60 | 0.57 | 0.03 | 0.67 |
| C18:3α (α-linolenic) | 0.93 | 0.85 | 0.79 | 0.78 | 0.04 | 0.90 |
| C20:3 (Dihomo-γ-linolenic) | 0.64 b | 0.81 ab | 0.76 b | 0.94 a | 0.04 | <0.05 |
| C20:4 (Arachidonic) | 11.81 ab | 12.99 ab | 9.59 b | 13.08 a | 0.81 | <0.05 |
| C20:5 (Eicosapentaenoic) | 0.08 | 0.09 | 0.08 | 0.09 | 0.003 | 0.22 |
| C22:5 (Docosapentaenoic) | 0.41 | 0.45 | 0.35 | 0.46 | 0.02 | 0.15 |
| C22:6 (Docosahexaenoic) | 0.85 | 0.82 | 0.59 | 0.82 | 0.06 | 0.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marchetti, L.; Rebucci, R.; Piantoni, C.; Corsetto, P.A.; Rizzo, A.M.; Zhang, H.; Jiang, X.; Bontempo, V. Influence of a Combination of Glycerol Polyethylene Glycol Ricinoleate and Bi-Distilled Oleic Acid in Powder Form on Growth Performance, Nutrient Digestibility, Excreta Nitrogen and Liver Fatty Acid Profile of Broilers Fed Reduced-Energy Diets. Animals 2025, 15, 827. https://doi.org/10.3390/ani15060827
Marchetti L, Rebucci R, Piantoni C, Corsetto PA, Rizzo AM, Zhang H, Jiang X, Bontempo V. Influence of a Combination of Glycerol Polyethylene Glycol Ricinoleate and Bi-Distilled Oleic Acid in Powder Form on Growth Performance, Nutrient Digestibility, Excreta Nitrogen and Liver Fatty Acid Profile of Broilers Fed Reduced-Energy Diets. Animals. 2025; 15(6):827. https://doi.org/10.3390/ani15060827
Chicago/Turabian StyleMarchetti, Luca, Raffaella Rebucci, Caterina Piantoni, Paola Antonia Corsetto, Angela Maria Rizzo, Haijun Zhang, Xianren Jiang, and Valentino Bontempo. 2025. "Influence of a Combination of Glycerol Polyethylene Glycol Ricinoleate and Bi-Distilled Oleic Acid in Powder Form on Growth Performance, Nutrient Digestibility, Excreta Nitrogen and Liver Fatty Acid Profile of Broilers Fed Reduced-Energy Diets" Animals 15, no. 6: 827. https://doi.org/10.3390/ani15060827
APA StyleMarchetti, L., Rebucci, R., Piantoni, C., Corsetto, P. A., Rizzo, A. M., Zhang, H., Jiang, X., & Bontempo, V. (2025). Influence of a Combination of Glycerol Polyethylene Glycol Ricinoleate and Bi-Distilled Oleic Acid in Powder Form on Growth Performance, Nutrient Digestibility, Excreta Nitrogen and Liver Fatty Acid Profile of Broilers Fed Reduced-Energy Diets. Animals, 15(6), 827. https://doi.org/10.3390/ani15060827







