Simple Summary
High stocking density (HD) adversely affects broiler growth, and previous studies have indicated that aspirin eugenol ester (AEE) improves production performance in HD broilers; however, the specific mechanism of its feeding regulation is unknown. The main objectives of this study were to investigate the effects of AEE addition on the production performance and hypothalamic transcript levels of HD broilers under high-density rearing conditions, and to further analyze the potential link between these two factors. A group of 360 one-day-old male Arbor Acres broilers was randomly divided into four groups: an ND group (14 broilers/m2), HD group (22 broilers/m2), ND-AEE group, and HD-AEE group. AEE increased the average daily feed intake of 22–28-day-old HD broilers and decreased their feed conversion ratio. In addition, AEE upregulated the expression levels of the ingestive genes NPY, AGRP, and GAL mRNA in the hypothalamus of 28-day-old HD broilers. Therefore, these genes may be key factors through which AEE promotes feeding and favors growth in HD broilers. Therefore, the present study provides new insights to improve the growth and development of HD broilers.
Abstract
Broilers grown in a high-density (HD) stocking environment may experience intense competition that may adversely affect their growth relative to animals reared at a normal density (ND). The growth performance of HD broilers is increased by aspirin eugenol ester (AEE), although the mechanism by which this compound modulates hypothalamus-regulated feeding behavior is unclear. The aims of this study were to determine the effects of including AEE in the basal diet on the hypothalamic transcriptome and to examine in parallel the impact of these modifications on broiler production performance in HD conditions. Three hundred sixty one-day-old male Arbor Acres broilers were randomly divided into four groups: an ND group (14 broilers/m2), HD group (22 broilers/m2), ND-AEE group, and HD-AEE group. Each treatment group had 10 replicates, with 7 broilers per replicate in the ND and ND-AEE groups and 11 broilers per replicate in the HD and HD-AEE groups. Broiler growth performance was monitored, and hypothalamus samples were collected for transcriptome analysis on day 28. The HD group exhibited a reduced body weight (p < 0.01) at this timepoint compared to the ND group. However, the addition of AEE significantly improved average daily feed intake, average daily gain, and feed conversion ratio in the HD group from days 22 to 28 compared to the HD group without AEE (p < 0.05). The transcriptome results showed that 20 signaling pathways were commonly enriched among the groups (ND vs. HD, HD vs. HD-AEE). Several potential candidate genes were identified as involved in chicken central nervous system development and regulation of feed intake. Thus, the current study provides new insights into hypothalamic transcription patterns that are associated with the ameliorative effects of AEE in HD broilers.
1. Introduction
The importance of animal welfare and sustainable farming practices is well-established [1]. Raising broilers in a high-density (HD) stocking environment lowers production costs, makes better use of available space, and increases economic efficiency compared to those reared at normal density (ND). However, as large-scale intensive and standardized poultry farming expands, so does the density of breeding, which raises numerous problems. For example, HD environments negatively impact broiler growth [2,3], reducing production efficiency, diminishing feed intake, and increasing feed conversion ratios [4,5,6]. The impact of HD environments on animal health varies, but an increase in broiler density may be associated with higher frequencies of footpad dermatitis and scratches [7,8], reduced leg movement, and increased mortality [9]. Furthermore, HD broilers may have weakened immune systems which may induce heightened inflammatory responses and elevated levels of pro-inflammatory factors that promote physiological damage [10].
The hypothalamus is a key organ in the central nervous system that controls energy homeostasis and regulates diverse physiological functions, including feeding, body temperature, and energy metabolism [11]. The hypothalamus also secretes hormones in response to physiological needs, thereby regulating stress responses and endocrine activity. There are two main types of neurons in the hypothalamus that regulate feeding behavior: pro-feeding neurons that express neuropeptide Y (NPY)/agouti-related peptide (AGRP) and food-suppressing neurons that express proopiomelanocortin (POMC)/cocaine- and amphetamine-regulated transcript (CART) [12,13]. As the hypothalamus integrates multiple signals to influence appetite and change feed intake, and these neuropeptides play a key role in regulating appetite and energy balance, high-density feeding may affect their expression and function. However, it remains unclear how HD influences hypothalamic gene expression.
Aspirin eugenol ester (AEE) is a novel medicinal compound that is synthesized based on the principle of the pharmacological structure colocation of aspirin and eugenol [14]. AEE maintains the activity of both aspirin and eugenol but reduces the gastrointestinal irritation caused by aspirin upon ingestion and overcomes the characteristic odor and instability of eugenol without altering the clinical action of the parental compounds [15]. AEE possesses pharmacological properties that include anti-inflammatory, analgesic, anti-thrombotic, anti-atherosclerotic, anti-vascular endothelial oxidation, and antipyretic effects [15,16]. Our previous study indicated that AEE improves the production of HD broilers [5]. AEE may exert these effects by altering the transcriptional expression of key genes in the hypothalamus.
In this study, we compared the hypothalamic transcripts of ND, HD, and HD broilers that were fed AEE with the aim of investigating the effects of AEE on hypothalamic gene expression in HD broilers, as well as examining the impact of AEE on production performance. Our data provide new insights into the effects of AEE on the hypothalamus transcriptome of HD broilers.
2. Materials and Methods
2.1. Ethical Treatment
Experimental protocols were approved by the Laboratory Animal Management and Use Committee of Henan University of Science and Technology (DWFL36891-2023) on 1 October 2023. Experimental procedures were conducted in accordance with animal ethical guidelines.
2.2. Animals and Experimental Design
A total of 360 healthy 1-day-old Arbor Acres male broilers (Henan Quan Da Poultry Breeding Co, Ltd., Hebi, China) of similar body weight were randomly selected and fed either a basal diet or a basal diet supplemented with AEE. The birds were divided into ND and HD groups at the beginning of the study, with 14 and 22 broilers/m2 in the ND and HD groups, respectively, with a total of four treatment groups (ND, HD, ND-AEE, and HD-AEE). Each group comprised 10 replicates and was raised for 42 days. Chickens were fed a basal diet (Table 1) for both the starter (days 1–21) and grower (days 22–42) phases. Their diets were supplemented with AEE purchased from the Lanzhou Institute of Husbandry and Pharmaceutical Sciences of CAAS (Lanzhou, China). The concentration of AEE (0.01%) was selected based on previous studies [5] and our unpublished data. The temperature was maintained at 32 to 34 °C for the first week of the trial, and then it decreased by 1 °C every two days to 23 to 25 °C. The temperature then remained constant until the end of the trial. Housing areas were well-ventilated, and relative humidity was maintained at 55–70%. Lighting was provided for 23 h daily, with lights off for 1 h at a fixed time.

Table 1.
Composition and content of broiler basal diets.
2.3. Growth Performance and Sample Collection
Feed intake was measured daily, and the birds were weighed at days 7, 14, 21, 28, 35, and 42 in order to determine the average daily feed intake, average daily gain, body weight, and feed conversion ratio. On days 21, 28, 35, and 42 of the experiment, 24 chickens (6 per group) were randomly selected from each of the four treatment groups. After euthanasia by cervical dislocation, their hypothalami were removed and placed on ice, promptly submerged in liquid nitrogen, and stored at −80 °C for further examination.
2.4. RNA Isolation and Sequencing
The Trizol method was used to extract total hypothalamic RNA. The concentration and purity of RNA were determined using a Nanodrop 2000 spectrophotometer (Thermo Fisher Scientific, Wilmington, DE, USA), and RNA integrity was detected by RNA-specific agarose electrophoresis or by using an Agilent 2100 Bioanalyzer (Agilent Technologies Inc, Santa Clara, CA, USA) (RIN scores greater than seven were considered high-quality RNA). PolyA-RNA in the total RNA was enriched using oligo (dT) magnetic beads, and random breaks were introduced to fragment the RNA to approximately 300 bp. We synthesized cDNA with reverse transcriptase using RNA as a template, followed by performing cDNA purification and PCR amplification to obtain the final library. After quality checking, the libraries were subjected to pair-end sequencing using second-generation sequencing technology on the Illumina NovaSeq 6000 platform (Illumina, San Diego, CA, USA), with a sequencing read length of 150 bp.
2.5. Original Data Processing, Alignment Analysis, and Quality Control
The sequencing data were transformed, examined, and statistically filtered to remove sequences, including low-quality and spliced sequences, which may have interfered with subsequent analysis. The HISAT2 (v2.1.0) software was used to align the filtered clean reads to the reference genome, obtaining the alignment efficiency. Quality control of the alignment results was performed based on gene coverage uniformity, saturation analysis, and alignment region distribution statistics.
2.6. Differentially Expressed Genes
Gene expression normalization was performed using FPKM based on original expression, and genes with FPKM values > 1 generally were considered as expressed genes. Gene expression was differentially analyzed using DESeq (v1.38.3) software. The conditions for screening differentially expressed genes (DEGs) were |log2FoldChange| ≥ 0.585 and p < 0.05, and the numbers were statistically compared among groups (ND vs. HD, ND vs. ND-AEE, HD vs. HD-AEE).
2.7. Analysis for Functional Pathway Enrichment
Gene Ontology (GO) enrichment analysis was performed using topGO (v2.50.0) to annotate the functions of the DEGs, and the results were categorized as molecular function, cellular component, and biological process. Clusterprofiler was used for Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis to find significantly enriched entries of DEGs and identify the main functions they exercise.
2.8. Determination of Protein-to-Protein Network Interactions
The STRING database was used to determine links between genes and to visualize the network of candidate genes with Cytoscape version 3.9.1.
2.9. Quantitative Real-Time PCR (qRT-PCR) Analysis
Total RNA was extracted by adding hypothalamus tissue to grinding beads and lysing the cells with Trizol reagent (Invitrogen Inc., Carlsbad, CA, USA). RNA concentration and purity were assessed using a UV spectrophotometer (Agilent Technologies Inc., Santa Clara, CA, USA), and OD260/280 values in the range of 1.8–2.0 were used to indicate high purity. After the genomic DNA was removed, the RNA was reverse transcribed to cDNA using the M-MLV Reverse Transcription Kit (Accurate Bio, Changsha, Hunan, China). We added the following reagents to 20 µL of qRT-PCR reaction mixture: 2 µL of cDNA, 0.4 µL each of forward and reverse primers (Table 2), 10 µL of 2X SYBR Green Pro Taq HS Premix (Accurate Bio, Changsha, Hunan, China), and 7.2 µL of RNase-free water. The qRT-PCR reaction was carried out using the SYBR Green fluorescence method, and amplification and lysis curves were confirmed at the conclusion of the reaction. The reaction was carried out in triplicate, and the following conditions were set: an initial denaturation at 95 °C for 30 s, followed by 40 cycles of 10 s and 30 s at 95 °C and 60 °C, respectively. The qRT-PCR data were normalized using the housekeeping gene GAPDH. Data were analyzed using 2−ΔΔCT to calculate fold change.

Table 2.
Primers used for qRT-PCR analysis.
2.10. Statistical Analyses
Data were analyzed by one-way ANOVA using IBM SPSS 26.0 (SPSS Inc., Chicago, IL, USA), and the results of production performance were expressed as means ± SEM. The graphic depiction of the qRT-PCR results was performed using GraphPad Prism 9 (GraphPad Software Inc., San Diego, CA, USA). Significance between groups was assessed using Tukey’s multiple-range test; p < 0.05 indicated a significant difference.
3. Results
3.1. AEE Modulates the Growth Performance of HD Broilers
Broilers were reared in ND or HD environments with or without exogenous AEE in their basal diet and were assessed for changes in body weight and other parameters at intervals. Broilers in the HD group weighed significantly less (p < 0.05) than chickens in the ND environment at days 21, 28, 35, and 42 (Table 3). However, the body weight of chickens in the HD-AEE group at day 21 was significantly higher (p < 0.05) compared to the equivalent chickens without AEE. Broilers reared in HD-AEE conditions also showed a significant increase (p < 0.01) in average daily gain and average daily feed intake at days 22–28 relative to the HD group. Moreover, the addition of AEE to the basal diet of HD broilers significantly reduced (p < 0.05) the feed conversion ratio at days 22–28, 29–35, and 36–42 compared to the HD chickens. In contrast, the body weight, average daily gain, average daily feed intake, and feed conversion ratio of the broilers raised in ND and ND-AEE conditions did not differ significantly at any time. Thus, AEE specifically enhances the body weight, average daily gain, and average daily feed intake, and reduces the feed conversion ratio of broilers grown in HD environments. But the ND group still has the best feed conversion ratio compared to the ND-AEE group.

Table 3.
Production performance of broilers.
3.2. RNA-Sequencing Analysis
To better investigate the changes in production performance at 28 days of age, the hypothalami of 28-day-old broilers were comprehensively analyzed using high-throughput sequencing technology (Table 4). Hypothalamic sequencing results showed that an average of 54.75 million raw reads were obtained per sample. High-quality sequences accounted for >97.92% of the sequencing reads, and the percentage of bases with a base detection accuracy ≥99% ranged from 97.94 to 98.42%. In addition, the percentage of bases with a base detection accuracy ≥99.9% ranged from 94.43 to 95.73%. The total numbers of sequences from clean reads compared to the reference genome were 43,645,127 (95.50%)–56,006,660 (95.94%). Comparing the total number of sequences with only one position, the percentages were 33,698,525 (98.66%)–89,090,158 (98.46%). Finally, the total numbers of reads mapped to the gene region were compared, and the percentages were 40,102,134 (80.64%)–44,961,555 (83.07%).

Table 4.
Assessment of transcriptome quality.
3.3. Analysis of Differentially Expressed Genes
The volcano plots of the DEGs among the groups (ND vs. HD, ND vs. ND-AEE, HD vs. HD-AEE) are shown in Figure 1a–c. The expression of 215 genes differed in the transcriptomes of ND and HD groups: 114 and 101 genes were upregulated and downregulated, respectively, under HD conditions (Figure 1d). Furthermore, 153 DEGs were revealed in the hypothalami of HD and HD-AEE groups, with the upregulation of 41 genes and downregulation of 112 genes in the latter. Finally, the hypothalamic transcriptome of ND-AEE group showed 78 DEGs that were altered compared to the ND group: 34 and 44 genes were upregulated and downregulated.
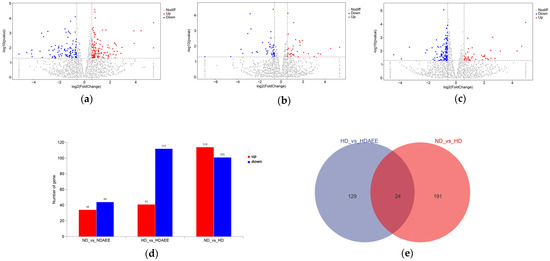
Figure 1.
DEGs in hypothalami of broiler chickens at 28 days of age. Volcano plots of DEGs for (a) ND and HD groups, (b) ND and ND-AEE groups, and (c) HD and HD-AEE groups. (d) Number of DEGs among groups. (e) Venn plots of DEGs for HD group compared to ND group and HD-AEE group compared to HD group. The horizontal dashed line is the significance level threshold.
According to the Venn plot results of the DEGs, 24 DEGs were common between the HD group compared to the ND group and the HD-AEE group compared to the HD group (Figure 1e). These DEGs principally involved the immune system (SERPINB1, GATA3), feeding (POMC, GAL, MC3R, NPY), neurological development (SLC9A9, EN2, BSX, ISL1, PAX2, PAX6), and growth hormone secretion (GHRH).
3.4. GO and KEGG Enrichment Analysis of Differentially Expressed Genes in Broiler Hypothalami
The DEGs that were identified in the preceding analysis were examined for GO and KEGG enrichment to better understand the biological functions of these genes. In the GO terms analysis, molecular functions and biological processes were primarily involved among groups (ND vs. HD, ND vs. ND-AEE, HD vs. HD-AEE) (Figure 2a–c). Compared with the ND group, the HD group exhibited 35 molecular functions and involvement in 218 biological processes. These molecular functions include neuropeptide receptor binding and neuropituitary hormone activity. The biological processes involved nervous system development, ingestive behavior, the modulation of hormone levels, the negative regulation of inflammation, and the modulation of glucocorticoid secretion. Compared to the HD group, the HD-AEE group exhibited 22 molecular functions and involvement in 91 biological processes. These molecular functions include hormone activity, receptor regulator activity, and neuropeptide hormone activity. The biological processes involve the development of the central nervous system, nutritional behavior, the regulation of hormone levels, and the regulation of glucocorticoid secretion.

Figure 2.
GO terms for DEGs in the hypothalami of 28-day-old broilers. Histograms of GO terms in (a) ND and HD groups, (b) ND and ND-AEE groups, and (c) HD and HD-AEE groups. Blue bars represent molecular functions (MFs) and red bars represent biological processes (BPs).
The functions performed by the DEGs in the hypothalami of broilers in HD or ND environments with or without exogenous AEE were determined by KEGG enrichment analysis. The HD group was enriched in 45 signaling pathways compared to the ND group, including the adipocytokine, MAPK, Wnt, and Toll-like receptor signaling pathways. Similarly, the HD-AEE group was enriched in 35 signaling pathways compared to the HD group, including the adipocytokine, MAPK, and Wnt signaling pathways. Finally, 20 signaling pathways were commonly enriched among the groups (ND vs. HD, HD vs. HD-AEE) (Figure 3).

Figure 3.
KEGG enrichment analysis of DEGs in hypothalami of 28-day-old broilers. KEGG enrichment analysis histograms of (a) ND and HD groups, (b) ND and ND-AEE groups, and (c) HD and HD-AEE groups. Factor plots of KEGG enrichment analysis in (d) ND and HD groups, (e) ND and ND-AEE groups, and (f) HD and HD-AEE groups.
3.5. Identification of Candidate Differentially Expressed Genes and Validation of RNA-Seq Results
Based on the GO and KEGG pathway analyses, as well as the degree of significance of the DEGs, we identified several potential candidate genes that may be involved in appetite regulation and brain development (Table 5). Eight genes (NPY, AGRP, GAL, GHRH, POMC, BSX, SLC6A4 and PAX2) were selected to explore further the changes observed in the RNA-seq analysis. The qRT-PCR results of these genes exhibit similar regulatory trends to the RNA-seq results (Figure 4). Finally, protein–protein networks were further visualized, and links between potential candidate genes were established (Figure 5).

Table 5.
Candidate genes related to appetite regulation and brain development.

Figure 4.
qRT-PCR validation results of DEGs. Relative mRNA expression of (a) NPY, (b) AGRP, (c) GAL, (d) GHRH, (e) POMC, (f) BSX, (g) SLC6A4 and (h) PAX2. Values expressed as mean ± SEM (n = 6). Values with different letters are significantly different (p < 0.05).
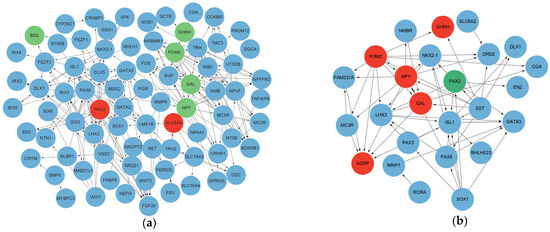
Figure 5.
Protein network interactions between DEGs. Protein network interactions between (a) ND and HD groups and (b) HD and HD-AEE groups. Red: gene upregulation; green: gene downregulation; blue: DEGs, but not candidate genes that are predictably involved in protein networks.
4. Discussion
4.1. AEE Modulates the Growth Performance of HD Broilers
An HD environment is a stressor that affects broiler performance [17], although results regarding the influence of stocking densities on performance are inconsistent. While HD stocking has been reported to dramatically reduce broiler performance [18,19,20,21], other data indicate that broiler performance is unaffected by stocking density [22,23]. Nevertheless, the results here support most previous studies, which highlight that HD stocking negatively impacts broiler performance. Broilers experience a decrease in average daily feed intake and average daily gain when stocking density exceeds 20 chickens/m2 [24]. A negative impact of stocking density on the feed conversion ratio has also been described [25]. Accordingly, the HD environment negatively affected average daily feed intake, feed conversion ratio, body weight, and average daily gain in this study. HD rearing had a greater impact on production performance during days 22–42 than during days 0–21.
We conducted an in-depth analysis of broiler production performance from days 22–42. Compared with the ND group, the HD group showed significant effects (p < 0.05) on average daily gain, average daily feed intake, and feed conversion ratio from days 22–28. Compared with the HD group, the HD-AEE group had a significant effect (p < 0.05) on the average daily feed intake, average daily gain, and feed conversion ratio of the HD broilers from days 22–28. AEE improved the production performance of the HD broilers, consistent with the findings of the previous study [5]. Interestingly, the effects of HD conditions on average daily feed intake in broilers at both days 22–28 and 29–35 were significantly lower (p < 0.05) than in ND chickens, whereas the addition of AEE increased (p < 0.05) feed intake in the HD group at days 22–28. Based on these observations, we investigated the hypothalamic transcriptome on day 28 to probe the gene expression changes involved in feeding regulation, energy metabolism, and brain development. A total of 78 DEGs (p < 0.05) were detected in the ND-AEE group compared to the ND group, and 153 DEGs (p < 0.05) were identified in the HD-AEE group compared to the HD group, which suggests a more potent effect of AEE on gene expression in the HD broiler hypothalami. As there was no discernible difference between the broiler production performances of the ND and ND-AEE groups (Table 3), we subsequently focussed on alterations in the hypothalamic transcriptome between ND and HD groups, as well as between HD and HD-AEE groups.
4.2. AEE Modulates Hypothalamic Feeding-Related Genes in HD Broilers
It is essential to modify animal food intake continuously to correspond with energy needs [26]. Appetite is the outcome of several pertinent regulatory factors that work in concert [12]. The control of feeding behavior by the central nervous system depends on signaling pathways that encompass key components, including core neuropeptide Y (NPY), agouti-related protein (AGRP), pro-opiomelanocortin (POMC), and cocaine- and amphetamine-regulated transcript (CART), that work in conjunction to regulate feeding behavior physiologically through an intricate interaction mechanism [27]. Feeding in poultry is encouraged particularly by the action of NPY in the hypothalamus. This neuropeptide is crucial for controlling feeding and preserving energy balance [28,29]. The expression of NPY mRNA is markedly increased in the hypothalamus of broiler chickens when there is a negative energy balance [30,31], and is accompanied by elevated neuronal activity [32]. NPY injected directly into brain ventricles greatly enhances and stimulates the feeding behavior of chickens [33]. The pro-feeding neuropeptide AGRP is secreted by the hypothalamus, and animals that express more AGRP consume more food while using less energy. A longer-lasting effect of increased food intake was observed when central injection of AGRP was administered instead of NPY [34]. NPY and AGRP neurons are co-expressed in the arcuate nucleus and, controlled by leptin and other factors, stimulate feeding by various modes. Melanocortin 3 receptor (MC3R) and melanocortin 4 receptor (MC4R) are blocked by AGRP [29]. The hypothalamic transcriptome analysis at day 28 here revealed that the HD group expressed lower levels (p < 0.05) of NPY than the ND group, whereas the HD-AEE group expressed higher levels (p < 0.05) of NPY and AGRP than the HD group. These changes in NPY and AGRP expression are congruent with variations in feed intake. Although there was no significant change, there was a tendency for the broilers in the HD environment to express hypothalamic AGRP at lower levels (Figure 4b). Consequently, AEE may promote broiler feeding by markedly raising NPY and AGRP expression in the HD broiler hypothalamus. In agreement with previous suggestions, brain-specific homeobox (BSX) expression in this study was consistent with NPY changes [35]. BSX may exert a regulatory role on NPY in broiler chickens. The neuropeptide NPY/AGRP controls feeding and body weight in mice when BSX is present [36]. Additionally, BSX is essential for modulating the expression of NPY and AGRP in mice [37] which influences feeding behavior and energy homeostasis. However, it is unknown why BSX exerts no effect on POMC expression in the hypothalamus [38]. The opposite effects of NPY/AGRP occur in neurons that express POMC/CART. POMC is a precursor polypeptide that is proteolytically processed to produce various hormones and neuropeptides, among which, the α-melanocyte-stimulating hormone (α-MSH) is an appetite suppressant. By binding to secondary neurons on MC3R and MC4R, α-MSH influences appetite and metabolism [39]. The POMC gene was downregulated in the hypothalamus of the HD group compared to the ND group, but was upregulated in the HD-AEE group relative to the HD group. As a result of the HD group’s reduced food intake, POMC changed in the opposite way from its food suppression effect. POMC has been shown to reduce appetite and cause weight loss [40]. The primary mechanism by which α-MSH regulates feeding is by activating bound MC4R [41]. Interestingly, the expression level of MC4R is upregulated (p < 0.05), contrary to the low expression of POMC, in low-feeding HD broilers, which may reflect competition for resources or environmental factors. Elevated MC4R expression also was noted following a period of food deprivation [42]. Appetite suppression also is linked to CART. Hypothalamic transcriptome data here revealed that, whereas POMC varied in both the ND and HD groups and in the HD and HD-AEE groups, CART expression was not significantly different, which may indicate that POMC and CART are not expressed exclusively in the same neurons.
Neuromedin U (NMU) was upregulated in the hypothalamus of 28-day-old broilers raised in the HD environment. This anorexigenic bioactive peptide is distributed widely in diverse tissues, expressed highly in the gut and brain, and plays a role in appetite regulation. Peripheral NMU also prevents ingestion by slowing stomach emptying, and may bind to NMU-R2 in the hypothalamus to suppress appetite [43]. Pharmacological studies have indicated that NMU may play a part in binge eating, and that NMU-R2 is a promising target for treating binge eating and changing eating patterns [44]. Furthermore, NMU may mediate the leptin regulation of hypothalamic–pituitary–adrenal (HPA) axis activity, thereby impacting the stress response [44]. Leptin also has a positive effect on NMU release [45]. However, only a few studies have confirmed the role of the NMU gene in appetite regulation in poultry. Notably, galanin and GMAP prepropeptide (GAL) changed in the transcriptomes, with GAL downregulated in the HD group compared to the ND group and upregulated in the HD-AEE group compared to the HD group. AEE may increase feed intake in HD broilers by controlling GAL, which is an appetitive regulator that exerts appetite-promoting effects [46]. Furthermore, GAL plays significant roles in feeding, energy regulation, and stress response, in addition to its involvement in neuroendocrine activities [47]. GAL and NPY are closely related neuroanatomically [48] and may interact to affect energy metabolism and feeding behavior [49]. Changes in the levels of the gene for growth hormone–releasing hormone (GHRH) in the hypothalamus transcriptomes of the HD and HD-AEE groups also may be significant. The endocrine system and hypothalamus are closely associated and regulate numerous physiological processes. Growth hormone (GH) secretion and release is stimulated by the production of GHRH by the hypothalamus. GHRH acts on the pituitary gland [50]. Although GHRH plays a critical role in the hypothalamus–pituitary–growth axis and the upregulation of GHRH in the HD-AEE group may have an impact on broiler growth, GH secretion is the consequence of several interrelated factors.
4.3. AEE Modulates Neurological Development-Related Genes in HD Broilers
Organism growth and nervous system development are interrelated processes. Serotonin transporter (SERT), which is distributed extensively throughout the central nervous system, gastrointestinal tract, and cardiovascular system, is encoded by the solute carrier family 6 member 4 (SLC6A4) gene [51]. By controlling 5-HT levels, SERT plays a crucial role in the central nervous system by promoting the re-uptake of the 5-hydroxytryptamine (5-HT) neurotransmitter in the synaptic gap [52]. Interestingly, 5-HT correlates strongly with mental health conditions, stress, and depression. Furthermore, 5-HT may contribute to bone formation [51] although the mechanism involved is unknown. Although it is not necessary for hypothalamus formation, BSX is crucial for the development of embryos and organs [38]. Paired box 2 (PAX2), a member of the paired box transcription factor family, primarily affects the kidney and central nervous system [53]. The deletion of the PAX2 gene impacts behavior and reduces synaptic plasticity in mice [54]. This trial validated it at the gene level, and it may later be extended to proteomics or epigenomics for a more comprehensive understanding.
4.4. GO and KEGG Enrichment Analysis of Differentially Expressed Genes in Broiler Hypothalami
For a more thorough comprehension of the role and function of DEGs, we can also pursue GO and KEGG enrichment analysis. The majority of the DEGs in the hypothalamus are associated with molecular function and biological processes. More specifically, activated entries related to feeding behavior, neuropeptide hormonal activity, neurohypophyseal hormone activity, nervous system development, and cellular developmental process were observed when the ND and HD groups were compared. Activated entries pertaining to feeding behavior, central nervous system development, neuropeptide hormone activity, and endocrine hormone secretion were identified in comparisons of the hypothalamic transcriptomes of the HD and HD-AEE groups. KEGG enrichment analysis identified 36 pathways in the HD and HD-AEE groups and 46 pathways in the ND and HD groups, respectively. Their identical pathways include adipocytokine signaling, MAPK signaling, Wnt signaling, cytokine–cytokine receptor interactions, caffeine metabolism, and tryptophan metabolism. Lipocalin and leptin are key proteins produced by adipocytes in the adipocytokine pathway. Leptin plays a crucial role in controlling energy intake and metabolic rate primarily through effects on the hypothalamus, where the protein regulates neuropeptide levels [55] via JAK kinase, nuclear transcription, and STAT3 phosphorylation [56]. Lipocalin triggers MAPK, which acts to reduce blood sugar [57] and promote glucose absorption and skeletal muscle lipid oxidation [58]. Therefore, the adipocytokine signaling pathway plays a crucial part in energy metabolism. The MAPK signaling pathway also is important for numerous cellular functions, including inflammation and cell migration, differentiation, and proliferation. Leptin signaling [59], lipocalin receptor signaling [57], growth hormone signaling [60], and inflammation mediation [61] are also linked to the MAPK signaling pathway. Moreover, cell survival, proliferation, and differentiation are regulated by Wnt signaling [62], which also influences body weight and food intake and is essential for neuroendocrine regulation in the hypothalamus [63]. Our analysis and discussion demonstrated that these signaling pathways affected the experiment, changing the HD group in comparison to the ND group and the HD-AEE group in comparison to the HD group. AEE may intercalate in these pathways to enhance HD broiler growth.
5. Conclusions
The results of the experiment showed that broiler growth performance is negatively impacted by HD, but this effect is mitigated by supplementing their basic diet with AEE. The addition of AEE to the basal diet significantly mitigated the HD-induced decrease in the average daily feed intake of broilers during the period of 22–28 days. On day 28, it upregulated the expression levels of the feeding genes NPY, AGRP, and GAL in the hypothalamus of HD broilers. The modulation of expression of these genes may be the primary mechanism by which AEE encourages feeding, regulates energy metabolism, and supports HD broiler growth. Thus, the current study provides new insights into hypothalamic transcription patterns that are associated with the ameliorative effects of AEE in HD broilers and will serve as a reference for the future feeding management of poultry. In the future, we will continue to focus on the effects of HD environments on production performance and further test these research results at different levels through proteomics or epigenomics.
Author Contributions
Investigation, X.Z. and Y.Z.; methodology, X.Z., C.G., Z.W. and P.M.; software, X.Z. and Z.W.; validation, X.Z., C.G., X.X. and X.M.; formal analysis, X.Z., Y.Z., W.Z. and D.B.; resources, Y.M., D.B., B.Z., Y.Y. and J.L.; data curation, X.Z., C.G., Z.W. and P.M.; writing—original draft preparation, X.Z. and Y.Z.; reviewing and editing, D.B., K.I., B.Z. and Y.M.; visualization, X.Z. and Y.Z.; supervision, Y.Z. and Y.M.; project administration, Y.M.; funding acquisition, Y.M. All authors have read and agreed to the published version of the manuscript.
Funding
The research was supported partly by the National Key Research and Development Program of China (Grant Number 2022YFE0111100), the Key Research and Development Program of Henan Province (Grant Number 241111113800), the Program for International S&T Cooperation Projects of Henan (Grant Number 232102521012), the Key Scientific Research Foundation of the Higher Education Institutions of Henan Province (Grant Number 22A230001), the Key Research and Development and Promotion of Special (Science and Technology) Project of Henan Province (Grant Number 242102110018), the Trendy Industry Projects of Longmen Laboratory (Grant Number LMFKCY2023002), and the Frontier Exploration Projects of Longmen Laboratory (Grant Number LMQYTSKT037).
Institutional Review Board Statement
The animal study protocol was approved by the Animal Care and Use Committee of Henan University of Science and Technology (DWFL36891-2023), and the management and experimental procedures complied with the regulations of the Institutional Animal Care and Use Committee.
Informed Consent Statement
Not applicable.
Data Availability Statement
The corresponding author can provide access to the collected and analyzed data sets from this study upon request.
Acknowledgments
The authors are grateful to the College of Animal Science and Technology, Henan University of Science and Technology for the use of experimental facilities, and greatly acknowledge the Longmen Laboratory, International Joint Lab for Animal Welfare and Health Breeding of Henan Province, and Expat Scientist Studio for Animal Stress and Health Breeding of Henan Province for their helpful academic advice during this study.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| HD | high stocking density |
| ND | normal stocking density |
| AEE | aspirin eugenol esters |
| DEGs | differential expression genes |
| MC3R | melanocortin 3 receptor |
| MC4R | melanocortin 4 receptor |
| PAX6 | paired box 6 |
| α-MSH | alpha-melanocyte-stimulating hormone |
| NMU | neuromedin U |
| GH | growth hormone |
| SERT | serotonin transporter |
| 5-HT | 5-hydroxytryptamine |
| SERPINB1 | serpin family B member 1 |
| GATA3 | gata binding protein 3 |
| SLC9A9 | solute carrier family 9 member A9 |
| EN2 | engrailed homeobox 2 |
| ISL1 | isl lim homeobox 1 |
| PAX2 | paired box 2 |
| SLC6A4 | solute carrier family 6 member 4 |
| BSX | Brain-specific homeobox |
| GHRH | growth hormone–releasing hormone |
| GAL | galanin and GAMP prepropeptide |
| POMC | proopiomelanocortin |
| AGRP | agouti-related peptide |
| NPY | neuropeptide Y |
| CART | cocaine- and amphetamine-regulated transcript |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| GO | Gene Ontology |
References
- Kwon, B.Y.; Park, J.; Kim, D.H.; Lee, K.W. Assessment of Welfare Problems in Broilers: Focus on Musculoskeletal Problems Associated with Their Rapid Growth. Animals 2024, 14, 1116. [Google Scholar] [CrossRef]
- Li, X.M.; Zhang, M.H.; Liu, S.M.; Feng, J.H.; Ma, D.D.; Liu, Q.X.; Zhou, Y.; Wang, X.J.; Xing, S. Effects of Stocking Density on Growth Performance, Growth Regulatory Factors, and Endocrine Hormones in Broilers under Appropriate Environments. Poult. Sci. 2019, 98, 6611–6617. [Google Scholar] [CrossRef]
- Riber, A.B.; van de Weerd, H.A.; de Jong, I.C.; Steenfeldt, S. Review of Environmental Enrichment for Broiler Chickens. Poult. Sci. 2018, 97, 378–396. [Google Scholar] [CrossRef]
- Obeidat, M.D.; Alkhateeb, M.E.M.; Jawasreh, K.I.; Riley, D.G.; Al Sukhni, I.A. Herbal Extract Dietary Supplementation Effect on Growth Performance and Meat Quality in Broiler Raised under Two Stocking Densities. Sci. Rep. 2024, 14, 1863. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, Y.; Bai, D.; Zhong, J.; Hu, X.; Zhang, R.; Zhen, W.; Ito, K.; Zhang, B.; Yang, Y.; et al. Effect of Dietary Aspirin Eugenol Ester on the Growth Performance, Antioxidant Capacity, Intestinal Inflammation, and Cecal Microbiota of Broilers under High Stocking Density. Poult. Sci. 2024, 103, 103825. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Bai, D.; Li, Y.; He, X.; Ito, K.; Liu, K.; Tan, H.; Zhen, W.; Zhang, B.; et al. Dietary Supplementation with Chlorogenic Acid Enhances Antioxidant Capacity, Which Promotes Growth, Jejunum Barrier Function, and Cecum Microbiota in Broilers under High Stocking Density Stress. Animals 2023, 13, 303. [Google Scholar] [CrossRef]
- Thomas, D.G.; Ravindran, V.; Thomas, D.V.; Camden, B.J.; Cottam, Y.H.; Morel, P.C.H.; Cook, C.J. Influence of Stocking Density on the Performance, Carcass Characteristics and Selected Welfare Indicators of Broiler Chickens. N. Z. Vet. J. 2004, 52, 76–81. [Google Scholar] [CrossRef]
- Taira, K.; Nagai, T.; Obi, T.; Takase, K. Effect of Litter Moisture on the Development of Footpad Dermatitis in Broiler Chickens. J. Vet. Med. Sci. 2014, 76, 583–586. [Google Scholar] [CrossRef]
- Sun, Z.W.; Yan, L.; G, Y.Y.; Zhao, J.P.; Lin, H.; Guo, Y.M. Increasing Dietary Vitamin D3 Improves the Walking Ability and Welfare Status of Broiler Chickens Reared at High Stocking Densities. Poult. Sci. 2013, 92, 3071–3307. [Google Scholar] [CrossRef]
- Dai, D.; Qi, G.; Wang, J.; Zhang, H.; Qiu, K.; Han, Y.; Wu, Y.; Wu, S. Dietary Organic Acids Ameliorate High Stocking Density Stress-Induced Intestinal Inflammation through the Restoration of Intestinal Microbiota in Broilers. J. Anim. Sci. Biotechnol. 2022, 13, 124. [Google Scholar] [CrossRef]
- He, X.; Lu, Z.; Ma, B.; Zhang, L.; Li, J.; Jiang, Y.; Zhou, G.; Gao, F. Chronic Heat Stress Alters Hypothalamus Integrity, the Serum Indexes and Attenuates Expressions of Hypothalamic Appetite Genes in Broilers. J. Therm. Biol. 2019, 81, 110–117. [Google Scholar] [CrossRef]
- Jones, B.J.; Bloom, S.R. The New Era of Drug Therapy for Obesity: The Evidence and the Expectations. Drugs 2015, 75, 935–945. [Google Scholar] [CrossRef]
- Boswell, T.; Dunn, I.C. Regulation of Agouti-Related Protein and Pro-Opiomelanocortin Gene Expression in the Avian Arcuate Nucleus. Front. Endocrinol. 2017, 8, 75. [Google Scholar] [CrossRef]
- Li, J.; Yu, Y.; Wang, Q.; Zhang, J.; Yang, Y.; Li, B.; Zhou, X.; Niu, J.; Wei, X.; Liu, X.; et al. Synthesis of Aspirin Eugenol Ester and Its Biological Activity. Med. Chem. Res. 2012, 21, 995–999. [Google Scholar] [CrossRef]
- Huang, M.Z.; Zhang, Z.D.; Yang, Y.J.; Liu, X.W.; Qin, Z.; Li, J.Y. Aspirin Eugenol Ester Protects Vascular Endothelium from Oxidative Injury by the Apoptosis Signal Regulating Kinase-1 Pathway. Front. Pharmacol. 2020, 11, 588755. [Google Scholar] [CrossRef]
- Ma, N.; Liu, X.W.; Yang, Y.J.; Shen, D.S.; Zhao, X.L.; Mohamed, I.; Kong, X.J.; Li, J.-Y. Evaluation on Antithrombotic Effect of Aspirin Eugenol Ester from the View of Platelet Aggregation, Hemorheology, TXB2/6-Keto-PGF1α and Blood Biochemistry in Rat Model. BMC Vet. Res. 2016, 12, 108. [Google Scholar] [CrossRef]
- Kridtayopas, C.; Rakangtong, C.; Bunchasak, C.; Loongyai, W. Effect of Prebiotic and Synbiotic Supplementation in Diet on Growth Performance, Small Intestinal Morphology, Stress, and Bacterial Population under High Stocking Density Condition of Broiler Chickens. Poult. Sci. 2019, 98, 4595–4605. [Google Scholar] [CrossRef]
- Guardia, S.; Konsak, B.; Combes, S.; Levenez, F.; Cauquil, L.; Guillot, J.-F.; Moreau-Vauzelle, C.; Lessire, M.; Juin, H.; Gabriel, I. Effects of Stocking Density on the Growth Performance and Digestive Microbiota of Broiler Chickens. Poult. Sci. 2011, 90, 1878–1889. [Google Scholar] [CrossRef]
- Simitzis, P.E.; Kalogeraki, E.; Goliomytis, M.; Charismiadou, M.A.; Triantaphyllopoulos, K.; Ayoutanti, A.; Niforou, K.; Hager-Theodorides, A.L.; Deligeorgis, S.G. Impact of Stocking Density on Broiler Growth Performance, Meat Characteristics, Behavioural Components and Indicators of Physiological and Oxidative Stress. Br. Poult. Sci. 2012, 53, 721–730. [Google Scholar] [CrossRef]
- Chegini, S.; Kiani, A.; Rokni, H. Alleviation of Thermal and Overcrowding Stress in Finishing Broilers by Dietary Propolis Supplementation. Ital. J. Anim. Sci. 2018, 17, 377–385. [Google Scholar] [CrossRef]
- Rambau, M.D.; Mudau, M.L.; Makhanya, S.D.; Benyi, K. Effects of Stocking Density and Daily Feed Withdrawal Periods on the Performance of Broiler Chickens in a Semi-Arid Environment. Trop. Anim. Health Prod. 2016, 48, 1547–1554. [Google Scholar] [CrossRef]
- Buijs, S.; Keeling, L.; Rettenbacher, S.; Van Poucke, E.; Tuyttens, F.A.M. Stocking Density Effects on Broiler Welfare: Identifying Sensitive Ranges for Different Indicators. Poult. Sci. 2009, 88, 1536–1543. [Google Scholar] [CrossRef]
- Mahrose, K.M.; El-Hack, M.E.A.; Amer, S.A. Influences of Dietary Crude Protein and Stocking Density on Growth Performance and Body Measurements of Ostrich Chicks. An. Acad. Bras. Cienc. 2019, 91, e20180479. [Google Scholar] [CrossRef]
- Jobe, M.C.; Ncobela, C.N.; Kunene, N.W.; Opoku, A.R. Effects of Cassia abbreviata Extract and Stocking Density on Growth Performance, Oxidative Stress and Liver Function of Indigenous Chickens. Trop. Anim. Health Prod. 2019, 51, 2567–2574. [Google Scholar] [CrossRef]
- Cengiz, Ö.; Köksal, B.H.; Tatlı, O.; Sevim, Ö.; Ahsan, U.; Üner, A.G.; Ulutaş, P.A.; Beyaz, D.; Büyükyörük, S.; Yakan, A.; et al. Effect of Dietary Probiotic and High Stocking Density on the Performance, Carcass Yield, Gut Microflora, and Stress Indicators of Broilers. Poult. Sci. 2015, 94, 2395–2403. [Google Scholar] [CrossRef]
- Morton, G.J.; Cummings, D.E.; Baskin, D.G.; Barsh, G.S.; Schwartz, M.W. Central Nervous System Control of Food Intake and Body Weight. Nature 2006, 443, 289–295. [Google Scholar] [CrossRef]
- Wen, S.; Wang, C.; Gong, M.; Zhou, L. An Overview of Energy and Metabolic Regulation. Sci. China Life Sci. 2019, 62, 771–790. [Google Scholar] [CrossRef]
- Furuse, M. Central Regulation of Food Intake in the Neonatal Chick. Anim. Sci. J. 2002, 73, 83–94. [Google Scholar] [CrossRef]
- Kuenzel, W.J.; Beck, M.M.; Teruyama, R. Neural Sites and Pathways Regulating Food Intake in Birds: A Comparative Analysis to Mammalian Systems. J. Exp. Zool. 1999, 283, 348–364. [Google Scholar] [CrossRef]
- Boswell, T.; Dunn, I.C.; Corr, S.A. Neuropeptide Y Gene Expression in the Brain Is Stimulated by Fasting and Food Restriction in Chickens. Br. Poult. Sci. 1999, 40 (Suppl. S1), S42–S43. [Google Scholar] [CrossRef]
- Boswell, T.; Dunn, I.C.; Corr, S.A. Hypothalamic Neuropeptide Y mRNA Is Increased after Feed Restriction in Growing Broilers. Poult. Sci. 1999, 78, 1203–1207. [Google Scholar] [CrossRef]
- Boswell, T.; Li, Q.; Takeuchi, S. Neurons Expressing Neuropeptide Y mRNA in the Infundibular Hypothalamus of Japanese Quail Are Activated by Fasting and Co-Express Agouti-Related Protein mRNA. Brain Res. Mol. Brain Res. 2002, 100, 31–42. [Google Scholar] [CrossRef]
- Furuse, M.; Matsumoto, M.; Mori, R.; Sugahara, K.; Kano, K.; Hasegawa, S. Influence of Fasting and Neuropeptide Y on the Suppressive Food Intake Induced by Intracerebroventricular Injection of Glucagon-like Peptide-1 in the Neonatal Chick. Brain Res. 1997, 764, 289–292. [Google Scholar] [CrossRef]
- Small, C.J.; Kim, M.S.; Stanley, S.A.; Mitchell, J.R.; Murphy, K.; Morgan, D.G.; Ghatei, M.A.; Bloom, S.R. Effects of Chronic Central Nervous System Administration of Agouti-Related Protein in Pair-Fed Animals. Diabetes 2001, 50, 248–254. [Google Scholar] [CrossRef]
- Piórkowska, K.; Żukowski, K.; Połtowicz, K.; Nowak, J.; Ropka-Molik, K.; Derebecka, N.; Wesoły, J.; Wojtysiak, D. Identification of Candidate Genes and Regulatory Factors Related to Growth Rate through Hypothalamus Transcriptome Analyses in Broiler Chickens. BMC Genom. 2020, 21, 509. [Google Scholar] [CrossRef]
- Sakkou, M.; Wiedmer, P.; Anlag, K.; Hamm, A.; Seuntjens, E.; Ettwiller, L.; Tschöp, M.H.; Treier, M. A Role for Brain-Specific Homeobox Factor Bsx in the Control of Hyperphagia and Locomotory Behavior. Cell Metab. 2007, 5, 450–463. [Google Scholar] [CrossRef]
- Nogueiras, R.; López, M.; Lage, R.; Perez-Tilve, D.; Pfluger, P.; Mendieta-Zerón, H.; Sakkou, M.; Wiedmer, P.; Benoit, S.C.; Datta, R.; et al. Bsx, a Novel Hypothalamic Factor Linking Feeding with Locomotor Activity, Is Regulated by Energy Availability. Endocrinology 2008, 149, 3009–3015. [Google Scholar] [CrossRef]
- Kaji, T.; Nonogaki, K. Role of Homeobox Genes in the Hypothalamic Development and Energy Balance. Front. Biosci. 2013, 18, 740–747. [Google Scholar]
- Huszar, D.; Lynch, C.A.; Fairchild-Huntress, V.; Dunmore, J.H.; Fang, Q.; Berkemeier, L.R.; Gu, W.; Kesterson, R.A.; Boston, B.A.; Cone, R.D.; et al. Targeted Disruption of the Melanocortin-4 Receptor Results in Obesity in Mice. Cell 1997, 88, 131–141. [Google Scholar] [CrossRef]
- Honda, K.; Saneyasu, T.; Hasegawa, S.; Kamisoyama, H. A Comparative Study of the Central Effects of Melanocortin Peptides on Food Intake in Broiler and Layer Chicks. Peptides 2012, 37, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, S.; Takahashi, S. Melanocortin Receptor Genes in the Chicken—Tissue Distributions. Gen. Comp. Endocrinol. 1998, 112, 220–231. [Google Scholar] [CrossRef]
- Higgins, S.E.; Ellestad, L.E.; Trakooljul, N.; McCarthy, F.; Saliba, J.; Cogburn, L.A.; Porter, T.E. Transcriptional and Pathway Analysis in the Hypothalamus of Newly Hatched Chicks during Fasting and Delayed Feeding. BMC Genom. 2010, 11, 162. [Google Scholar] [CrossRef]
- Graham, E.S.; Turnbull, Y.; Fotheringham, P.; Nilaweera, K.; Mercer, J.G.; Morgan, P.J.; Barrett, P. Neuromedin U and Neuromedin U Receptor-2 Expression in the Mouse and Rat Hypothalamus: Effects of Nutritional Status. J. Neurochem. 2003, 87, 1165–1173. [Google Scholar] [CrossRef]
- Botticelli, L.; Micioni Di Bonaventura, E.; Del Bello, F.; Giorgioni, G.; Piergentili, A.; Quaglia, W.; Bonifazi, A.; Cifani, C.; Micioni Di Bonaventura, M.V. The Neuromedin U System: Pharmacological Implications for the Treatment of Obesity and Binge Eating Behavior. Pharmacol. Res. 2023, 195, 106875. [Google Scholar] [CrossRef]
- Wren, A.M.; Small, C.J.; Abbott, C.R.; Jethwa, P.H.; Kennedy, A.R.; Murphy, K.G.; Stanley, S.A.; Zollner, A.N.; Ghatei, M.A.; Bloom, S.R. Hypothalamic Actions of Neuromedin U. Endocrinology 2002, 143, 4227–4234. [Google Scholar] [CrossRef][Green Version]
- Mills, E.G.; Izzi-Engbeaya, C.; Abbara, A.; Comninos, A.N.; Dhillo, W.S. Functions of Galanin, Spexin and Kisspeptin in Metabolism, Mood and Behaviour. Nat. Rev. Endocrinol. 2021, 17, 97–113. [Google Scholar] [CrossRef]
- Mohd Zahir, I.; Ogawa, S.; Dominic, N.A.; Soga, T.; Parhar, I.S. Spexin and Galanin in Metabolic Functions and Social Behaviors with a Focus on Non-Mammalian Vertebrates. Front. Endocrinol. 2022, 13, 882772. [Google Scholar] [CrossRef]
- Parrado, C.; Díaz-Cabiale, Z.; García-Coronel, M.; Agnati, L.F.; Coveñas, R.; Fuxe, K.; Narváez, J.A. Region Specific Galanin Receptor/Neuropeptide Y Y1 Receptor Interactions in the Tel- and Diencephalon of the Rat. Relevance for Food Consumption. Neuropharmacology 2007, 52, 684–692. [Google Scholar] [CrossRef]
- Marcos, P.; Coveñas, R. Neuropeptidergic Control of Feeding: Focus on the Galanin Family of Peptides. Int. J. Mol. Sci. 2021, 22, 2544. [Google Scholar] [CrossRef]
- Krulich, L.; Dhariwal, A.P.; McCann, S.M. Stimulatory and Inhibitory Effects of Purified Hypothalamic Extracts on Growth Hormone Release from Rat Pituitary in Vitro. Endocrinology 1968, 83, 783–790. [Google Scholar] [CrossRef]
- Warden, S.J.; Haney, E.M. Skeletal Effects of Serotonin (5-Hydroxytryptamine) Transporter Inhibition: Evidence from In Vitro and Animal-Based Studies. J. Musculoskelet. Neuronal Interact. 2008, 8, 121–132. [Google Scholar]
- Chen, F.X.; Chen, X.S.; Guo, J.-C.; Zheng, B.-A.; Guo, M. Serotonin Transporter-Linked Polymorphic Region Genotypes in Relation to Stress Conditions among Patients with Papillary Thyroid Carcinoma. Int. J. Clin. Exp. Pathol. 2019, 12, 968–977. [Google Scholar]
- Wang, Y.; Wang, Y.; Tang, J.; Li, R.; Jia, Y.; Yang, H.; Wei, H. Impaired Neural Circuitry of Hippocampus in Pax2 Nervous System-Specific Knockout Mice Leads to Restricted Repetitive Behaviors. CNS Neurosci. Ther. 2024, 30, e14482. [Google Scholar] [CrossRef]
- Li, R.; Tang, J.; Wang, Y.; Wang, Y.; Yang, H.; Wei, H. Metabolomics and Transcriptomics Analysis of Prefrontal Cortex in the Pax2 Neuron-Specific Deletion Mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 2024, 128, 110858. [Google Scholar] [CrossRef]
- Münzberg, H.; Morrison, C.D. Structure, Production and Signaling of Leptin. Metabolism 2015, 64, 13–23. [Google Scholar] [CrossRef]
- Myers, M.G.; Cowley, M.A.; Münzberg, H. Mechanisms of Leptin Action and Leptin Resistance. Annu. Rev. Physiol. 2008, 70, 537–556. [Google Scholar] [CrossRef]
- Yamauchi, T.; Kamon, J.; Minokoshi, Y.; Ito, Y.; Waki, H.; Uchida, S.; Yamashita, S.; Noda, M.; Kita, S.; Ueki, K.; et al. Adiponectin Stimulates Glucose Utilization and Fatty-Acid Oxidation by Activating AMP-Activated Protein Kinase. Nat. Med. 2002, 8, 1288–1295. [Google Scholar] [CrossRef]
- Mao, X.; Kikani, C.K.; Riojas, R.A.; Langlais, P.; Wang, L.; Ramos, F.J.; Fang, Q.; Christ-Roberts, C.Y.; Hong, J.Y.; Kim, R.-Y.; et al. APPL1 Binds to Adiponectin Receptors and Mediates Adiponectin Signalling and Function. Nat. Cell Biol. 2006, 8, 516–523. [Google Scholar] [CrossRef]
- Li, X.-M.; Yan, H.-J.; Guo, Y.-S.; Wang, D. The Role of Leptin in Central Nervous System Diseases. Neuroreport 2016, 27, 350–355. [Google Scholar] [CrossRef]
- Qiu, H.; Yang, J.-K.; Chen, C. Influence of Insulin on Growth Hormone Secretion, Level and Growth Hormone Signalling. Sheng Li Xue Bao 2017, 69, 541–556. [Google Scholar]
- Sheldon, I.M.; Cronin, J.G.; Healey, G.D.; Gabler, C.; Heuwieser, W.; Streyl, D.; Bromfield, J.J.; Miyamoto, A.; Fergani, C.; Dobson, H. Innate Immunity and Inflammation of the Bovine Female Reproductive Tract in Health and Disease. Reproduction 2014, 148, R41–R51. [Google Scholar] [CrossRef]
- Helfer, G.; Tups, A. Hypothalamic Wnt Signalling and Its Role in Energy Balance Regulation. J. Neuroendocrinol. 2016, 28, 12368. [Google Scholar] [CrossRef] [PubMed]
- Timper, K.; Brüning, J.C. Hypothalamic Circuits Regulating Appetite and Energy Homeostasis: Pathways to Obesity. Dis. Model. Mech. 2017, 10, 679–689. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).