Effect of Inhibin Immunization on Reproductive Hormones and Testicular Morphology of Dezhou Donkeys During the Non-Breeding Season
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Inhibin Immunogen Preparation
2.2. Experimental Design
2.3. Body Weights
2.4. Measurement of Body Weight, Blood, and Testes Tissue Collection
2.5. Antibody Titer
2.6. Measurement of Plasma Hormone Concentrations
2.7. Microscopy Performance
2.8. Criteria for Observing Apoptosis in Seminiferous Tubules
- Seminiferous epithelium lumen seemed quite empty, showing degeneration of spermatogonia, spermatocytes, and apoptotic bodies.
- The Seminiferous tubule’s basal membrane was observed empty. Pyknotic germ and Sertoli cells were also observed.
- Sertoli cells vacuolated, and most tubules had empty lumen depicting impaired spermatogenesis.
- Seminiferous tubules had irregularly shaped and degenerated germ cells.
2.9. Statistical Analysis
3. Results
3.1. Anti-Inhibin Antibody Titer
3.2. Body Weight of Animals
3.3. Plasma Hormone Concentrations
3.3.1. Follicle-Stimulating Hormone (FSH)
3.3.2. Luteinizing Hormone (LH)
3.3.3. Progesterone (P4)
3.3.4. Testosterone (T)
3.3.5. AntiMullerian Hormone
3.3.6. Activin A
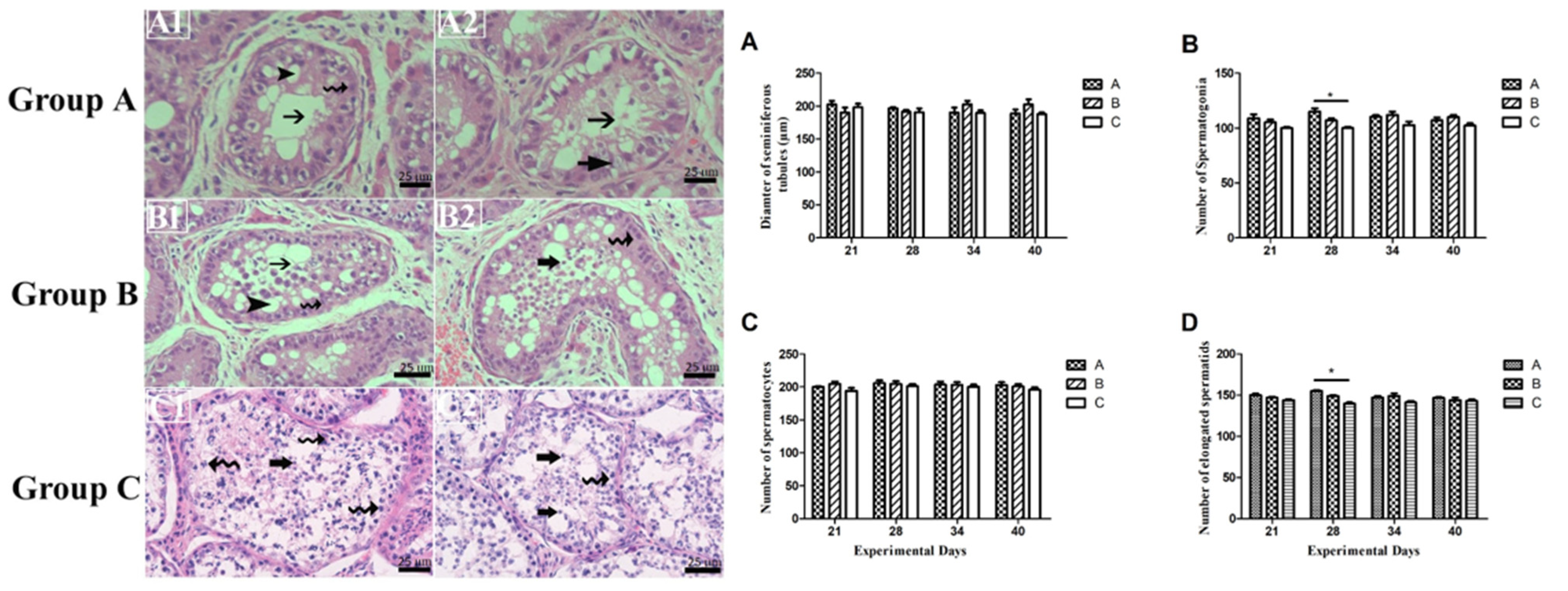
3.4. Germ Cell Count and Variations in Seminiferous Epithelium
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shi, T.; Hu, W.; Hou, H.; Zhao, Z.; Shang, M.; Zhang, L. Identification and comparative analysis of long non-coding RNA in the skeletal muscle of two dezhou donkey strains. Genes 2020, 11, 508. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Wang, J.; Li, Y.; Yang, C.; Zhang, R.; Wang, X.; Ju, Z.; Jiang, Q.; Huang, J.; Wang, C. Integrated analysis of mRNA and miRNA in testis and cauda epididymidis reveals candidate molecular markers associated with reproduction in Dezhou donkey. Livest. Sci. 2020, 234, 103885. [Google Scholar] [CrossRef]
- Zeng, L.; Dang, R.; Dong, H.; Li, F.; Chen, H.; Lei, C. Genetic diversity and relationships of Chinese donkeys using microsatellite markers. Arch. Anim. Breed. 2019, 62, 181–187. [Google Scholar] [CrossRef]
- Lai, Z.; Wu, F.; Zhou, Z.; Li, M.; Gao, Y.; Yin, G.; Yu, J.; Lei, C.; Dang, R. Expression profiles and polymorphic identification of the ACSL1 gene and their association with body size traits in Dezhou donkeys. Arch. Anim. Breed. 2020, 63, 377–386. [Google Scholar] [CrossRef]
- Clauss, M.; Zerbe, P.; Bingaman Lackey, L.; Codron, D.; Müller, D.W. Basic considerations on seasonal breeding in mammals including their testing by comparing natural habitats and zoos. Mamm. Biol. 2021, 101, 373–386. [Google Scholar] [CrossRef]
- Tibary, A.; Sghiri, A.; Bakkoury, M.; Fite, C. Reproductive patterns in donkeys. In Proceedings of the 9th International Congress of the World Equine Veterinary Association, Marrakesh, Morocco, 22–26 January 2006; pp. 311–319. [Google Scholar]
- Aissanou, S.; Besseboua, O.; Abdelhanine, A. Some reproductive characteristics in common donkey male (Equus asinus)-A mini review. Turk. J. Vet. Res. 2022, 6, 77–84. [Google Scholar] [CrossRef]
- Akhtar, M.F.; Wei, Q.; Zhu, H.; Chen, Z.; Ahmad, E.; Zhendan, S.; Shi, F. The role of active immunization against inhibin α-subunit on testicular development, testosterone concentration and relevant genes expressions in testis, hypothalamus and pituitary glands in Yangzhou goose ganders. Theriogenology 2019, 128, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, T.K.; Mather, J.P. Inhibin, activin and the female reproductive axis. Annu. Rev. Physiol. 1995, 57, 219–244. [Google Scholar] [CrossRef]
- Liu, Y.; Mao, X.; Wei, Y.; Yu, J.; Li, H.; Chen, R.; Shi, Z. Studies on enhancing embryo quantity and quality by immunization against inhibin in repeatedly superovulated Holstein heifers and the associated endocrine mechanisms. Anim. Reprod. Sci. 2013, 142, 10–18. [Google Scholar] [CrossRef]
- Chen, F.; Lu, J.; Guo, R.; Mei, C.; Guo, B.; Li, W.; Tsigkou, A.; Shi, Z. Rectifying cow infertility under heat stress by immunization against inhibin and supplementation of progesterone. Domest. Anim. Endocrinol. 2022, 80, 106726. [Google Scholar] [CrossRef]
- Rehman, A.; Ahmad, E.; Sattar, A.; Riaz, A.; Khan, J.A.; Naseer, Z.; Akhtar, M.F.; Abbas, M.; Shi, Z. Long term effects of immunization against inhibin on fresh and post-thawed semen quality and sperm kinematics during low and peak breeding seasons in Beetal bucks. Small Rumin. Res. 2021, 201, 106442. [Google Scholar] [CrossRef]
- Rehman, A.; Ahmad, E.; Arshad, U.; Riaz, A.; Akhtar, M.S.; Ahmad, T.; Khan, J.A.; Mohsin, I.; Shi, Z.; Sattar, A. Effects of immunization against inhibin α-subunit on ovarian structures, pregnancy rate, embryonic and fetal losses, and prolificacy rate in goats where estrus was induced during the non-breeding season. Anim. Reprod. Sci. 2021, 224, 106654. [Google Scholar] [CrossRef]
- Ma, L.; Li, Z.; Ma, Z.; Ma, J.; Zhao, F. Immunization against inhibin promotes fertility in cattle: A meta-analysis and quality assessment. Front. Vet. Sci. 2021, 8, 687923. [Google Scholar] [CrossRef]
- Meng, J.; Feng, J.H.; Xiao, L.; Zhou, W.; Zhang, H.; Lan, X.; Wang, S. Active immunization with inhibin DNA vaccine promotes spermatogenesis and testicular development in rats. J. Appl. Anim. Res. 2024, 52, 2360408. [Google Scholar] [CrossRef]
- Lovell, T.M.; Knight, P.G.; Groome, N.P.; Gladwell, R.T. Measurement of dimeric inhibins and effects of active immunization against inhibin α-subunit on plasma hormones and testis morphology in the developing cockerel. Biol. Reprod. 2000, 63, 213–221. [Google Scholar] [CrossRef]
- Baqerkhani, M.; Soleimanzadeh, A.; Mohammadi, R. Effects of intratesticular injection of hypertonic mannitol and saline on the quality of donkey sperm, indicators of oxidative stress and testicular tissue pathology. BMC Vet. Res. 2024, 20, 99. [Google Scholar] [CrossRef]
- Akhtar, M.F.; Ahmad, E.; Ali, I.; Shafiq, M.; Chen, Z. The effect of inhibin immunization in seminiferous epithelium of Yangzhou goose ganders: A histological study. Animals 2021, 11, 2801. [Google Scholar] [CrossRef]
- Han, Y.; Jiang, T.; Liu, A.; Liu, L. Role and regulatory mechanism of inhibin in animal reproductive system. Theriogenology 2023, 202, 10–20. [Google Scholar] [CrossRef]
- Bernard, D.J.; Li, Y.; Toufaily, C.; Schang, G. Regulation of gonadotropins. In Oxford Research Encyclopedia of Neuroscience; Oxford University Press: New York, NY, USA, 2019. [Google Scholar]
- Guo, R.; Chen, F.; Mei, C.; Dai, Z.; Yan, L.; Shi, Z. Conception rate and reproductive hormone secretion in Holstein cows immunized against inhibin and subjected to the ovsynch protocol. Animals 2020, 10, 313. [Google Scholar] [CrossRef]
- Li, D.; Qin, G.; Wei, Y.; Lu, F.; Huang, Q.; Jiang, H.; Shi, D.; Shi, Z. Immunisation against inhibin enhances follicular development, oocyte maturation and superovulatory response in water buffaloes. Reprod. Fertil. Dev. 2011, 23, 788–797. [Google Scholar] [CrossRef]
- Anderson, R.; Groome, N.; Baird, D. Inhibin A and inhibin B in women with polycystic ovarian syndrome during treatment with FSH to induce mono-ovulation. Clin. Endocrinol. 1998, 48, 577–584. [Google Scholar] [CrossRef]
- Medan, M.; Akagi, S.; Kaneko, H.; Watanabe, G.; Tsonis, C.; Taya, K. Effects of re-immunization of heifers against inhibin on hormonal profiles and ovulation rate. Reproduction 2004, 128, 475–482. [Google Scholar] [CrossRef]
- Sasaki, K.; Medan, M.S.; Watanabe, G.; Sharawy, S.; Taya, K. Immunization of goats against inhibin increased follicular development and ovulation rate. J. Reprod. Dev. 2006, 52, 543–550. [Google Scholar] [CrossRef]
- Lazebny, O.; Kulikov, A.; Butovskaya, P.; Proshakov, P.; Fokin, A.; Butovskaya, M. Analysis of aggressive behavior in young Russian males using 250 SNP markers. Russ. J. Genet. 2020, 56, 1118–1128. [Google Scholar] [CrossRef]
- O’Donnell, L.; Whiley, P.A.; Loveland, K.L. Activin A and sertoli cells: Key to fetal testis steroidogenesis. Front. Endocrinol. 2022, 13, 898876. [Google Scholar] [CrossRef]
- Rodriguez, K.F.; Brown, P.R.; Amato, C.M.; Nicol, B.; Liu, C.-F.; Xu, X.; Yao, H.H.-C. Somatic cell fate maintenance in mouse fetal testes via autocrine/paracrine action of AMH and activin B. Nat. Commun. 2022, 13, 4130. [Google Scholar] [CrossRef]
- Shah, W.; Khan, R.; Shah, B.; Khan, A.; Dil, S.; Liu, W.; Wen, J.; Jiang, X. The molecular mechanism of sex hormones on Sertoli cell development and proliferation. Front. Endocrinol. 2021, 12, 648141. [Google Scholar] [CrossRef]
- Arato, I.; Grande, G.; Barrachina, F.; Bellucci, C.; Lilli, C.; Jodar, M.; Aglietti, M.C.; Mancini, F.; Vincenzoni, F.; Pontecorvi, A. “In vitro” Effect of Different Follicle—Stimulating Hormone Preparations on Sertoli Cells: Toward a Personalized Treatment for Male Infertility. Front. Endocrinol. 2020, 11, 401. [Google Scholar] [CrossRef]
- Santi, D.; Crépieux, P.; Reiter, E.; Spaggiari, G.; Brigante, G.; Casarini, L.; Rochira, V.; Simoni, M. Follicle-stimulating hormone (FSH) action on spermatogenesis: A focus on physiological and therapeutic roles. J. Clin. Med. 2020, 9, 1014. [Google Scholar] [CrossRef]
- Wang, J.-M.; Li, Z.-F.; Yang, W.-X.; Tan, F.-Q. Follicle-stimulating hormone signaling in Sertoli cells: A licence to the early stages of spermatogenesis. Reprod. Biol. Endocrinol. 2022, 20, 97. [Google Scholar] [CrossRef]
- Ni, F.-D.; Hao, S.-L.; Yang, W.-X. Molecular insights into hormone regulation via signaling pathways in Sertoli cells: With discussion on infertility and testicular tumor. Gene 2020, 753, 144812. [Google Scholar] [CrossRef]
- Olsen, O.E.; Hella, H.; Elsaadi, S.; Jacobi, C.; Martinez-Hackert, E.; Holien, T. Activins as dual specificity TGF-β family molecules: SMAD-activation via activin-and BMP-type 1 receptors. Biomolecules 2020, 10, 519. [Google Scholar] [CrossRef]
- Jiang, L.; Liu, B.; Qi, Y.; Zhu, L.; Cui, X.; Liu, Z. Antagonistic effects of activin A and TNF-α on the activation of L929 fibroblast cells via Smad3-independent signaling. Sci. Rep. 2020, 10, 20623. [Google Scholar] [CrossRef]
- Zeng, X.; Turkstra, J.; Tsigos, A.; Meloen, R.; Liu, X.; Chen, F.; Schaaper, W.; Guo, D.; van de Wiel, D. Effects of active immunization against GnRH on serum LH, inhibin A, sexual development and growth rate in Chinese female pigs. Theriogenology 2002, 58, 1315–1326. [Google Scholar] [CrossRef] [PubMed]
- Avital-Cohen, N.; Heiblum, R.; Argov, N.; Rosenstrauch, A.; Chaiseha, Y.; Mobarkey, N.; Rozenboim, I. The effect of active immunization against vasoactive intestinal peptide and inhibin on reproductive performance of young White Leghorn roosters. Poult. Sci. 2011, 90, 2321–2331. [Google Scholar] [CrossRef]
- Samir, H.; El Sayed, M.A.; Nagaoka, K.; Sasaki, K.; El-Maaty, A.M.A.; Karen, A.; Abou-Ahmed, M.M.; Watanabe, G. Passive immunization against inhibin increases testicular blood flow in male goats. Theriogenology 2020, 147, 85–91. [Google Scholar] [CrossRef]
- Oduwole, O.O.; Huhtaniemi, I.T.; Misrahi, M. The roles of luteinizing hormone, follicle-stimulating hormone and testosterone in spermatogenesis and folliculogenesis revisited. Int. J. Mol. Sci. 2021, 22, 12735. [Google Scholar] [CrossRef]
- Ali, A.; Derar, D.R.; Zeitoun, M.M.; Al-Sobayil, F. Impotentia generandi in male dromedary camels: FSH, LH and testosterone profiles and their association with clinical findings and semen analysis data. Theriogenology 2018, 120, 98–104. [Google Scholar] [CrossRef]
- Swelum, A.A.-A.; Saadeldin, I.M.; Zaher, H.A.; Alsharifi, S.A.; Alowaimer, A.N. Effect of sexual excitation on testosterone and nitric oxide levels of water buffalo bulls (Bubalus bubalis) with different categories of sexual behavior and their correlation with each other. Anim. Reprod. Sci. 2017, 181, 151–158. [Google Scholar] [CrossRef]
- ur Rehman, Z.; Worku, T.; Davis, J.S.; Talpur, H.S.; Bhattarai, D.; Kadariya, I.; Hua, G.; Cao, J.; Dad, R.; Hussain, T. Role and mechanism of AMH in the regulation of Sertoli cells in mice. J. Steroid Biochem. Mol. Biol. 2017, 174, 133–140. [Google Scholar] [CrossRef]
- Matuszczak, E.; Hermanowicz, A.; Komarowska, M.; Debek, W. Serum AMH in physiology and pathology of male gonads. Int. J. Endocrinol. 2013, 2013, 128907. [Google Scholar] [CrossRef] [PubMed]
- Rey, R.A.; Grinspon, R.P. Normal male sexual differentiation and aetiology of disorders of sex development. Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 221–238. [Google Scholar] [CrossRef]
- Hui, H.-B.; Xiao, L.; Sun, W.; Zhou, Y.-J.; Zhang, H.-Y.; Ge, C.-T. Sox9 is indispensable for testis differentiation in the red-eared slider turtle, a reptile with temperature-dependent sex determination. Zool. Res. 2021, 42, 721. [Google Scholar] [CrossRef]
- Shima, Y.; Miyabayashi, K.; Haraguchi, S.; Arakawa, T.; Otake, H.; Baba, T.; Matsuzaki, S.; Shishido, Y.; Akiyama, H.; Tachibana, T. Contribution of Leydig and Sertoli cells to testosterone production in mouse fetal testes. Mol. Endocrinol. 2013, 27, 63–73. [Google Scholar] [CrossRef]
- Zhou, R.; Wu, J.; Liu, B.; Jiang, Y.; Chen, W.; Li, J.; He, Q.; He, Z. The roles and mechanisms of Leydig cells and myoid cells in regulating spermatogenesis. Cell. Mol. Life Sci. 2019, 76, 2681–2695. [Google Scholar] [CrossRef]
- Banerjee, S.; Chaturvedi, C.M. Apoptotic mechanism behind the testicular atrophy in photorefractory and scotosensitive quail: Involvement of GnIH induced p-53 dependent Bax-Caspase-3 mediated pathway. J. Photochem. Photobiol. B Biol. 2017, 176, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, R.; Burgos, M.; Barrionuevo, F.J. Circannual testis changes in seasonally breeding mammals. Sex. Dev. 2015, 9, 205–215. [Google Scholar] [CrossRef]
- Beltrán-Frutos, E.; Seco-Rovira, V.; Martínez-Hernández, J.; Ferrer, C.; Serrano-Sánchez, M.I.; Pastor, L.M. Cellular modifications in spermatogenesis during seasonal testicular regression: An update review in mammals. Animals 2022, 12, 1605. [Google Scholar] [CrossRef]
 , Group B
, Group B  and control Group C
and control Group C  of Dezhou donkeys at 21, 28, 34, and 40 days of the experiment. Vertical bars represent the standard error of the mean (SEM). The values with ** indicate the difference (p < 0.01) whereas the values with *** indicate the difference (p < 0.001) between groups A, B, and C. Arrows indicate primary and booster Inhibin (INH) immunization at 1st and 23rd day of the experiment. Each group (n = 10).
of Dezhou donkeys at 21, 28, 34, and 40 days of the experiment. Vertical bars represent the standard error of the mean (SEM). The values with ** indicate the difference (p < 0.01) whereas the values with *** indicate the difference (p < 0.001) between groups A, B, and C. Arrows indicate primary and booster Inhibin (INH) immunization at 1st and 23rd day of the experiment. Each group (n = 10).
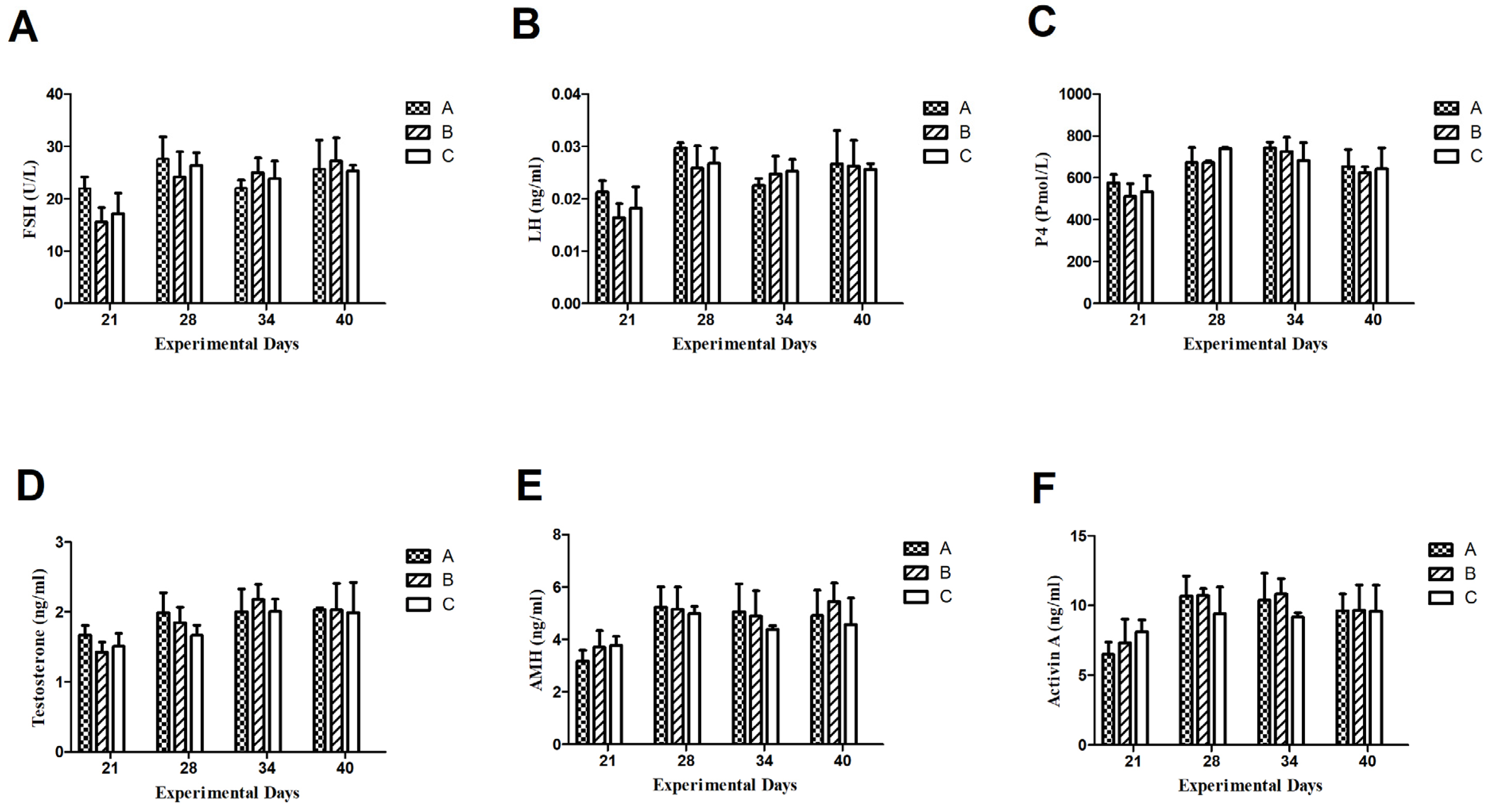
 , Group B
, Group B  and control Group C
and control Group C  of Dezhou donkeys at 21, 28, 34, and 40 days of the experiment. Vertical bars represent the standard error of the mean (SEM). The values with ** indicate the difference (p < 0.01) whereas the values with *** indicate the difference (p < 0.001) between groups A, B, and C. Arrows indicate primary and booster Inhibin (INH) immunization at 1st and 23rd day of the experiment. Each group (n = 10).
of Dezhou donkeys at 21, 28, 34, and 40 days of the experiment. Vertical bars represent the standard error of the mean (SEM). The values with ** indicate the difference (p < 0.01) whereas the values with *** indicate the difference (p < 0.001) between groups A, B, and C. Arrows indicate primary and booster Inhibin (INH) immunization at 1st and 23rd day of the experiment. Each group (n = 10).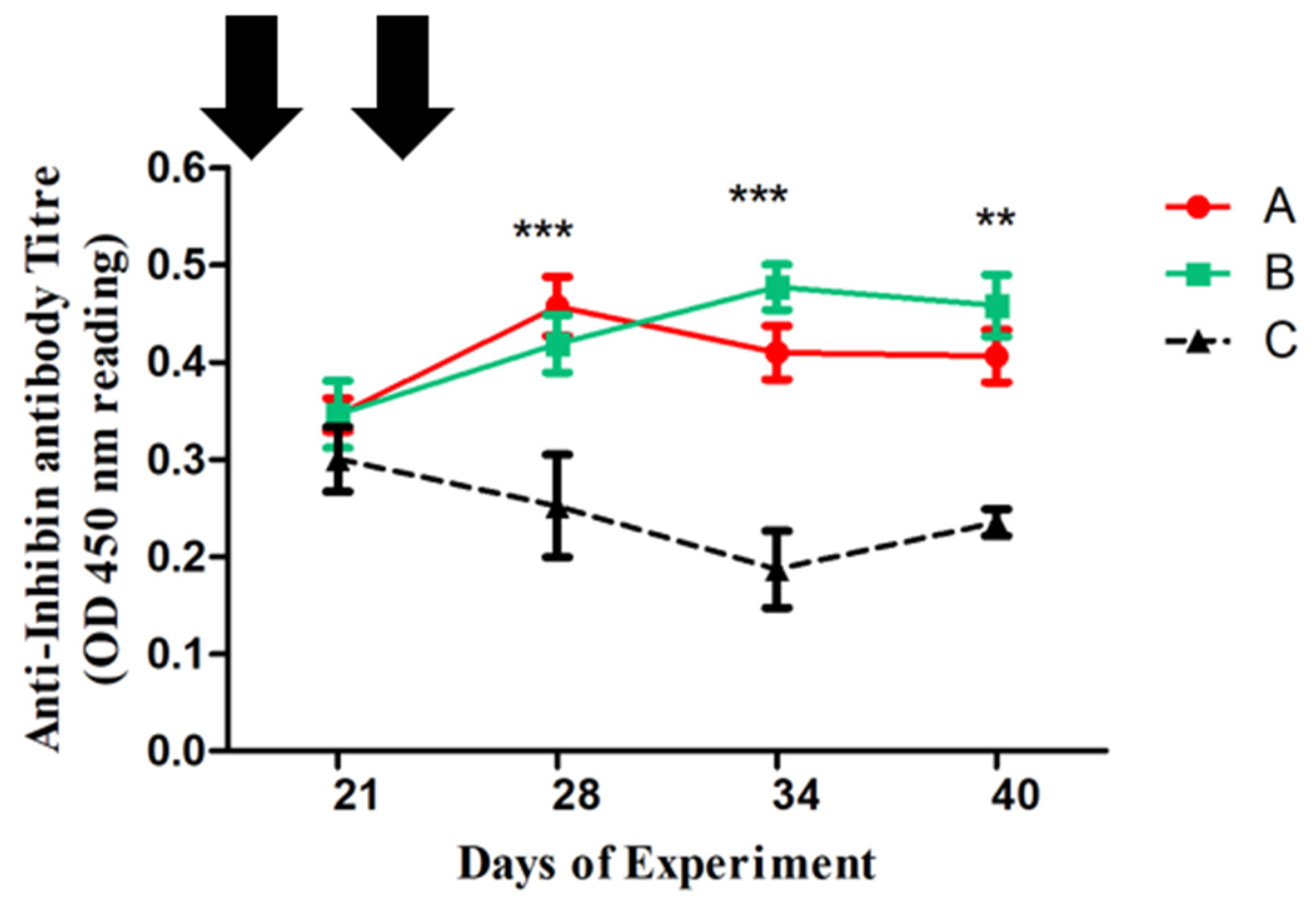

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akhtar, M.F.; Umar, M.; Chai, W.; Li, L.; Ahmad, E.; Wang, C. Effect of Inhibin Immunization on Reproductive Hormones and Testicular Morphology of Dezhou Donkeys During the Non-Breeding Season. Animals 2025, 15, 813. https://doi.org/10.3390/ani15060813
Akhtar MF, Umar M, Chai W, Li L, Ahmad E, Wang C. Effect of Inhibin Immunization on Reproductive Hormones and Testicular Morphology of Dezhou Donkeys During the Non-Breeding Season. Animals. 2025; 15(6):813. https://doi.org/10.3390/ani15060813
Chicago/Turabian StyleAkhtar, Muhammad Faheem, Muhammad Umar, Wenqiong Chai, Liangliang Li, Ejaz Ahmad, and Changfa Wang. 2025. "Effect of Inhibin Immunization on Reproductive Hormones and Testicular Morphology of Dezhou Donkeys During the Non-Breeding Season" Animals 15, no. 6: 813. https://doi.org/10.3390/ani15060813
APA StyleAkhtar, M. F., Umar, M., Chai, W., Li, L., Ahmad, E., & Wang, C. (2025). Effect of Inhibin Immunization on Reproductive Hormones and Testicular Morphology of Dezhou Donkeys During the Non-Breeding Season. Animals, 15(6), 813. https://doi.org/10.3390/ani15060813





