A Metric-Based, Meta-Analytic Appraisal of Environmental Enrichment Efficacy in Captive Primates
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Review and Data Consolidation
- Communication type (case report, research article or other);
- Institutional context (laboratory, rehabilitation centre, sanctuary, zoo or other);
- Primate species;
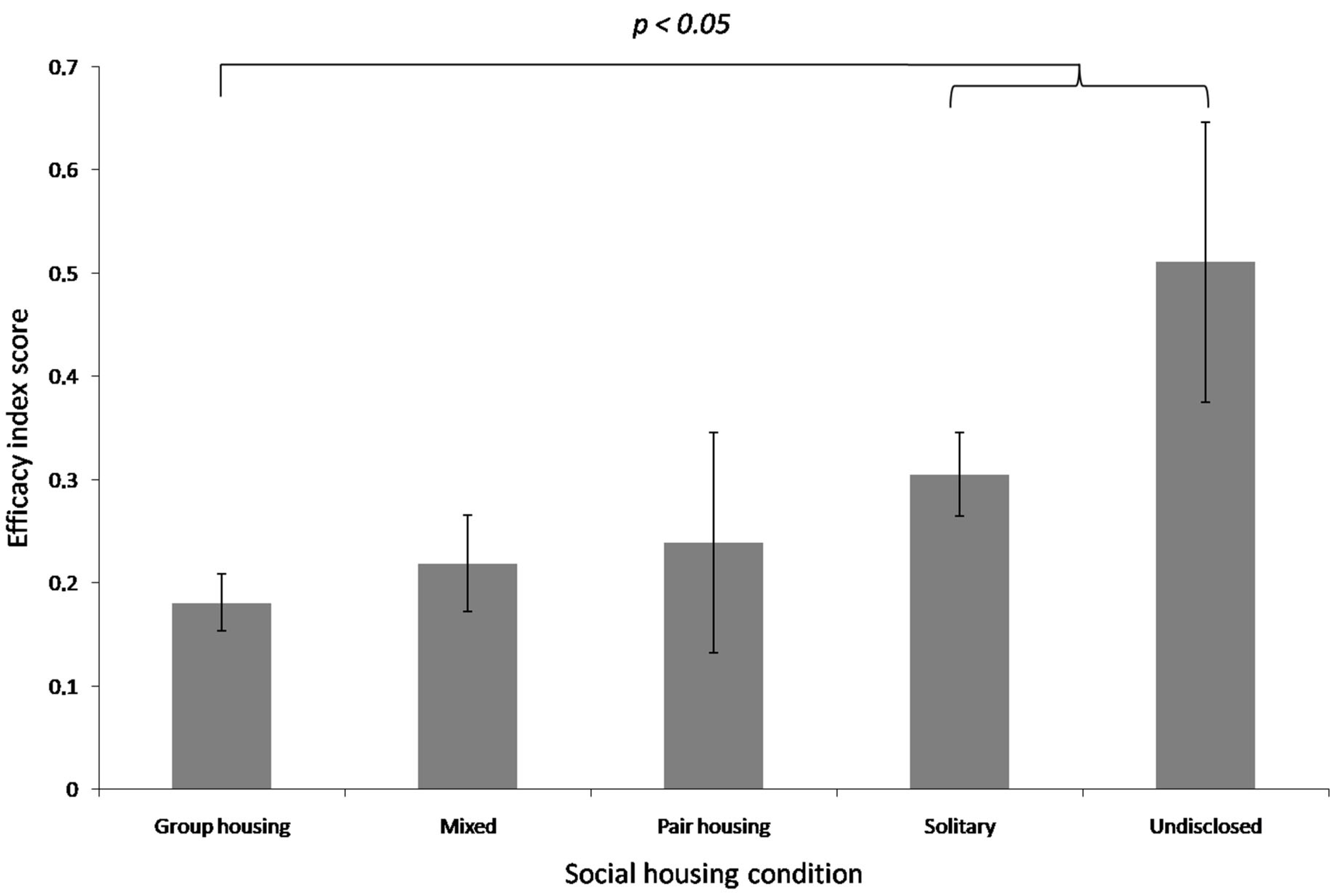
- Social conditions (group housing, pair housing, solitary, mixed housing);
- Housing type (indoor only, indoor–outdoor, outdoor only, standard housing (standard housing was considered as indoor-only cage rack systems, which typically employ enclosing steel mesh barriers, excretia trays below a steel mesh floor, a feeder hopper and a water supply) or other);
- Housing state (enriched, barren);
- Access to enrichment (0–3 h, 3–24 h, 1–3 days, >3 days);
- Enrichment type (see Table 1).
2.2. Data Analysis
3. Results
3.1. Summary of Findings in the Literature
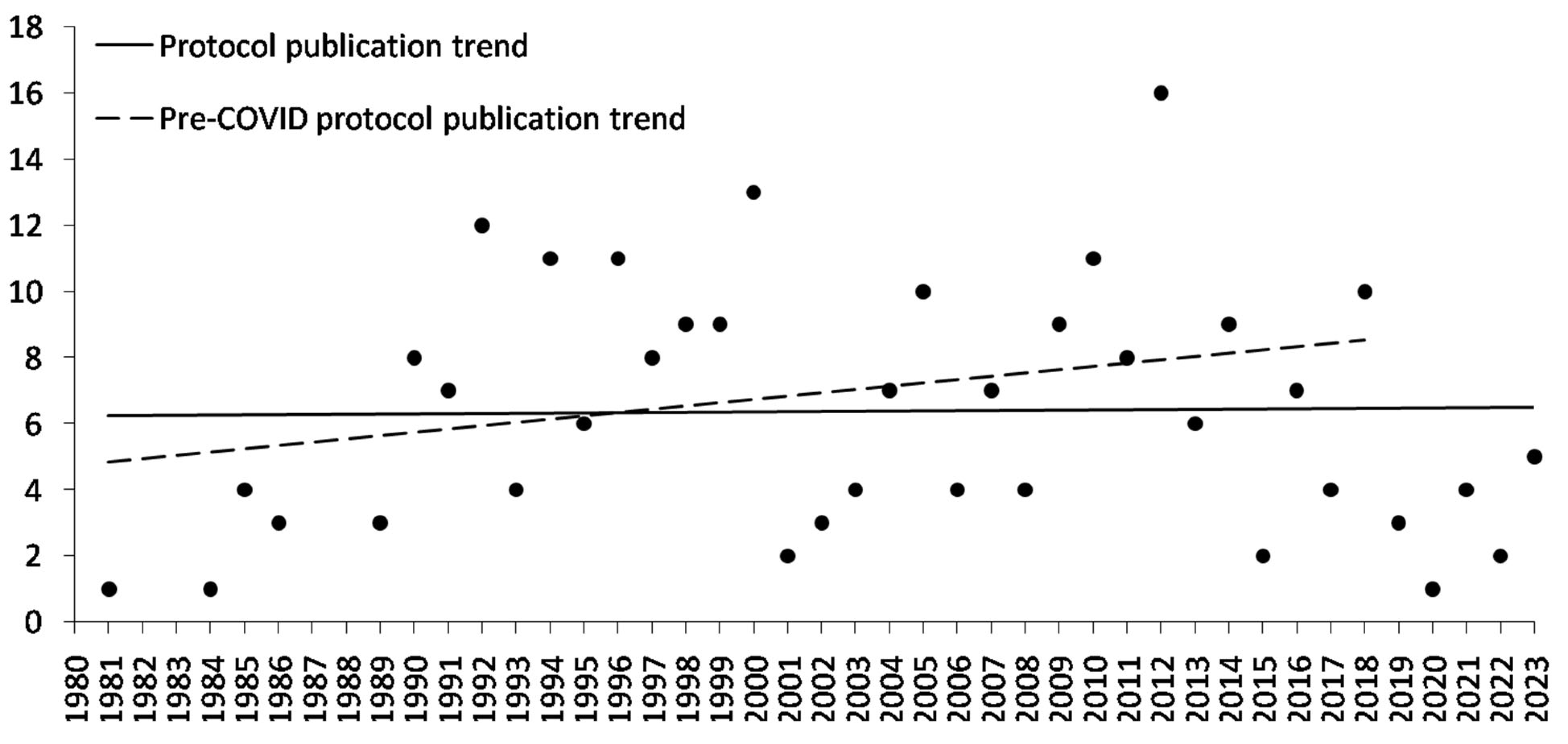
3.2. Errant Trends in Reporting
3.3. Statistical Models
4. Discussion
4.1. Contexts of Application and Reporting of Enrichment
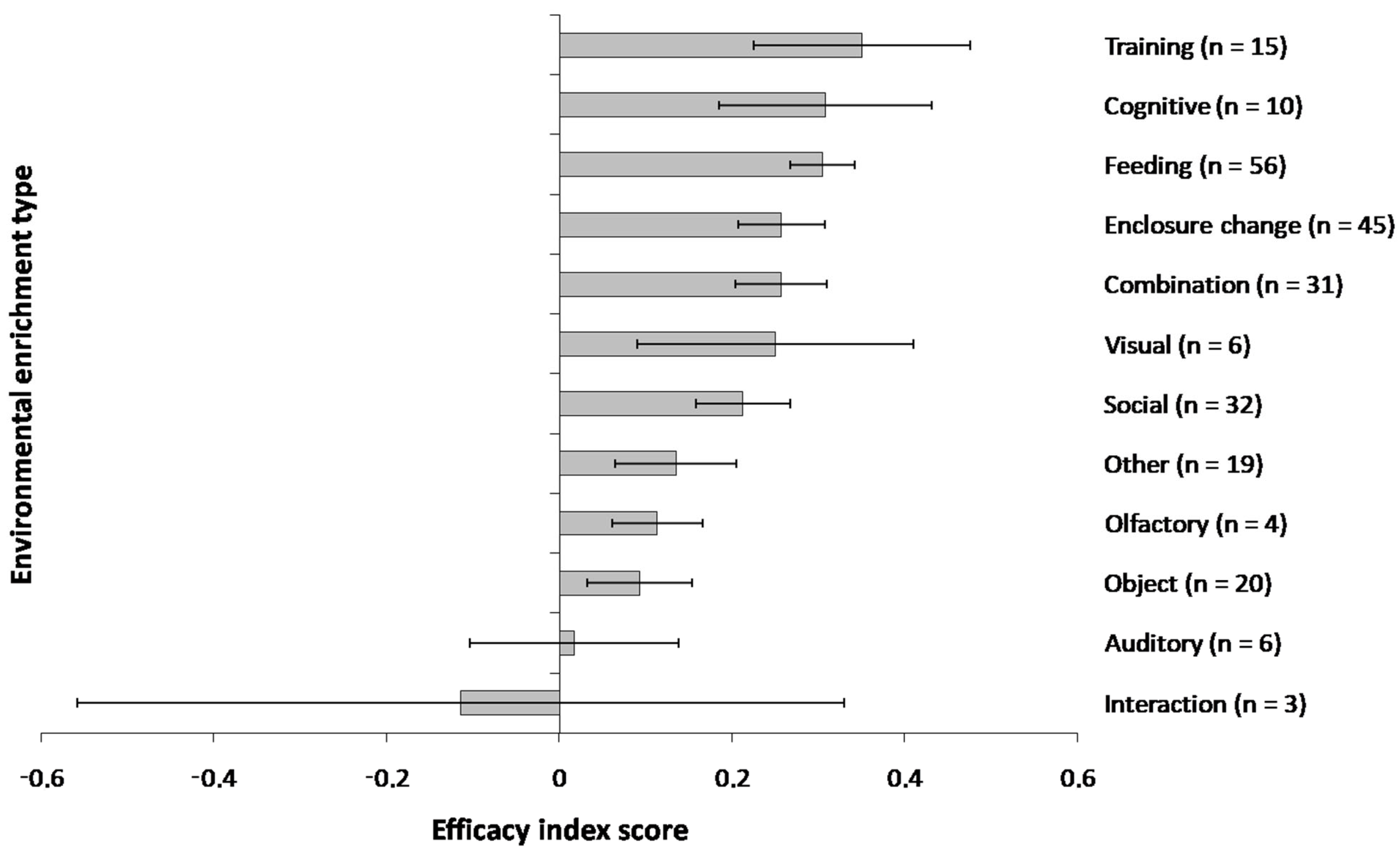
4.2. Enrichment Efficacy in Captive Primates
4.3. The Efficacy Index
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Rowell, T.E. A quantitative comparison of the behaviour of a wild and a caged baboon group. Anim. Behav. 1967, 15, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, E. The case for animal research in psychology. J. Soc. Issues 1993, 49, 121–131. [Google Scholar] [CrossRef]
- Archard, G.A.; Braithwaite, V.A. The importance of wild populations in studies of animal temperament. J. Zool. 2010, 281, 149–160. [Google Scholar] [CrossRef]
- Garner, J.P. Stereotypies and other abnormal repetitive behaviors: Potential impact on validity, reliability, and replicability of scientific outcomes. ILAR J. 2005, 46, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Tomasello, M.; Call, J. Methodological challenges in the study of primate cognition. Science 2011, 334, 1227–1228. [Google Scholar] [CrossRef]
- Buchanan-Smith, H.M. Environmental enrichment for primates in laboratories. Adv. Sci. Res. 2011, 5, 41–56. [Google Scholar] [CrossRef]
- Rothman, J.M.; Plumptre, A.J.; Dierenfeld, E.S.; Pell, A.N. Nutritional composition of the diet of the gorilla (Gorilla beringei): A comparison between two montane habitats. J. Trop. Ecol. 2007, 23, 673–682. [Google Scholar] [CrossRef]
- Symington, M. Fission-fusion social organization in Ateles and Pan. Int. J. Primatol. 1990, 11, 47–61. [Google Scholar] [CrossRef]
- Byrne, R.W.; Bates, L.A. Primate social cognition: Uniquely primate, uniquely social, or just unique? Neuron 2010, 65, 815–830. [Google Scholar] [CrossRef]
- Rogers, L.J. Cognition and animal welfare. WIREs Cogn. Sci. 2010, 1, 439–445. [Google Scholar] [CrossRef]
- Hosey, G.R. How does the zoo environment affect the behaviour of captive primates? Appl. Anim. Behav. Sci. 2005, 95, 107–129. [Google Scholar] [CrossRef]
- Morgan, K.N.; Tromborg, C.T. Sources of stress in captivity. Appl. Anim. Behav. Sci. 2007, 102, 262–302. [Google Scholar] [CrossRef]
- Fox, M.W. Animal freedom and well-being: Want or need? Appl. Anim. Ethol. 1984, 11, 205–209. [Google Scholar] [CrossRef]
- Hau, J.; Schapiro, S.J. The welfare of non-human primates. In The Welfare of Laboratory Animals; Kaliste, E., Ed.; Springer: Dordrecht, The Netherlands, 2007; pp. 291–314. [Google Scholar]
- Young, R.J. Environmental Enrichment for Captive Animals; Blackwell Publishing: Oxford, UK, 2003. [Google Scholar]
- Swaisgood, R.R.; Shepherdson, D.J. Scientific approaches to enrichment and stereotypies in zoo animals: What’s been done and where should we go next? Zoo Biol. 2005, 24, 499–518. [Google Scholar] [CrossRef]
- Morris, C.L.; Grandin, T.; Irlbeck, N.A. Companion Animals Symposium: Environmental enrichment for companion, exotic, and laboratory animals. J. Anim. Sci. 2011, 89, 4227–4238. [Google Scholar] [CrossRef]
- Hurme, K.; Gonzalez, K.; Halvorsen, M.; Foster, B.; Moore, D.; Chepko-Sade, B.D. Environmental enrichment for dendrobatid frogs. J. Appl. Anim. Welf. Sci. 2003, 6, 285–299. [Google Scholar] [CrossRef]
- Newberry, R.C. Environmental enrichment: Increasing the biological relevance of captive environments. Appl. Anim. Behav. Sci. 1995, 44, 229–243. [Google Scholar] [CrossRef]
- Bloomsmith, M.A.; Brent, L.; Schapiro, S.J. Guidelines for developing and managing an environmental enrichment program for nonhuman primates. Lab. Anim. Sci. 1991, 41, 372–377. [Google Scholar]
- Brent, L.; Stone, A. Destructible toys as enrichment for captive chimpanzees. J. Appl. Anim. Welf. Sci. 1998, 1, 5–14. [Google Scholar] [CrossRef]
- Line, S.W.; Morgan, K.N.; Markowitz, H. Simple toys do not alter the behavior of aged rhesus monkeys. Zoo Biol. 1991, 10, 473–484. [Google Scholar] [CrossRef]
- Baker, K.C.; Crockett, C.M.; Lee, G.H.; Oettinger, B.C.; Schoof, V.A.M.; Thom, J.P. Pair housing for female Longtailed and Rhesus macaques in the laboratory: Behavior in protected contact versus full contact. J. Appl. Anim. Welf. Sci. 2012, 15, 126–143. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Brent, L.; Weaver, O. The physiological and behavioral effects of radio music on singly housed baboons. J. Med. Primatol. 1996, 25, 370–374. [Google Scholar] [CrossRef] [PubMed]
- Hinds, S.B.; Raimond, S.; Purcell, B.K. The effect of harp music on heart rate, mean blood pressure, respiratory rate, and body temperature in the African green monkey. J. Med. Primatol. 2007, 36, 95–100. [Google Scholar] [CrossRef]
- Carlstead, K.; Shepherdson, D. Effects of environmental enrichment on reproduction. Zoo Biol. 1994, 13, 447–458. [Google Scholar] [CrossRef]
- Markowitz, H.; Schmidt, M.J.; Moody, A. Behavioural engineering and animal health in the zoo. Int. Zoo Yearb. 1978, 18, 190–194. [Google Scholar] [CrossRef]
- Guerrero-Martin, S.M.; Rubin, L.H.; McGee, K.M.; Shirk, E.N.; Queen, S.E.; Li, M.; Bullock, B.; Carlson, B.W.; Adams, R.J.; Gama, L.; et al. Psychosocial stress alters the immune response and results in higher viral load during acute simian immunodeficiency virus infection in a pigtailed macaque model of human immunodeficiency virus. J. Infect. Dis. 2021, 224, 2113–2121. [Google Scholar] [CrossRef]
- Bethell, E.J.; Holmes, A.; Maclarnon, A.; Semple, S. Cognitive bias in a non-human primate: Husbandry procedures influence cognitive indicators of psychological well-being in captive rhesus macaques. Anim. Welf. 2012, 21, 185–195. [Google Scholar] [CrossRef]
- Palmer, S.; Oppler, S.H.; Graham, M.L. Behavioral management as a coping strategy for managing stressors in primates: The influence of temperament and species. Biology 2022, 11, 423. [Google Scholar] [CrossRef]
- Van de Weerd, H.A.; Aarsen, E.L.; Mulder, A.; Kruitwagen, C.L.J.J.; Hendriksen, C.F.M.; Baumans, V. Effects of environmental enrichment for mice: Variation in experimental results. J. Appl. Anim. Welf. Sci. 2002, 5, 87–109. [Google Scholar] [CrossRef]
- Watters, J.V. Toward a predictive theory for environmental enrichment. Zoo Biol. 2009, 28, 609–622. [Google Scholar] [CrossRef]
- Renner, M.J.; Feiner, A.J.; Orr, M.G.; Delaney, B.A. Environmental enrichment for New World primates: Introducing food-irrelevant objects and direct and secondary effects. J. Appl. Anim. Welf. Sci. 2000, 3, 23–32. [Google Scholar] [CrossRef]
- Spring, S.E.; Clifford, J.O.; Tomkol, D.L. Effect of environmental enrichment devices on behaviors of single- and group-housed squirrel monkeys (Saimiri sciureus). J. Am. Assoc. Lab. Anim. Sci. 1997, 36, 72–75. [Google Scholar]
- Van de Weerd, H.A.; Van Loo, P.L.P.; Van Zutphen, L.F.M.; Koolhaas, J.M.; Baumans, V. Nesting material as environmental enrichment has no adverse effects on behavior and physiology of laboratory mice. Physiol. Behav. 1997, 62, 1019–1028. [Google Scholar] [CrossRef] [PubMed]
- Fuller, G.; Sadowski, L.; Cassella, C.; Lukas, K.E. Examining deep litter as environmental enrichment for a family group of wolf’s guenons, Cercopithecus wolfi. Zoo Biol. 2010, 29, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Wells, D.L.; Coleman, D.; Challis, M.G. A note on the effect of auditory stimulation on the behaviour and welfare of zoo-housed gorillas. Appl. Anim. Behav. Sci. 2006, 100, 327–332. [Google Scholar] [CrossRef]
- Ogden, J.J.; Lindburg, D.G.; Maple, T.L. A preliminary study of the effects of ecologically relevant sounds on the behaviour of captive lowland gorillas. Appl. Anim. Behav. Sci. 1994, 39, 163–176. [Google Scholar] [CrossRef]
- Brooker, J.S. An investigation of the auditory perception of western lowland gorillas in an enrichment study. Zoo Biol. 2016, 35, 398–408. [Google Scholar] [CrossRef]
- Costa, R.; Sousa, C.; Llorente, M. Assessment of environmental enrichment for different primate species under low budget: A case study. J. Appl. Anim. Welf. Sci. 2018, 21, 185–199. [Google Scholar] [CrossRef]
- van Hoek, C.S.; Cate, C. Ten Abnormal behavior in caged birds kept as pets. J. Appl. Anim. Welf. Sci. 1998, 1, 51–64. [Google Scholar] [CrossRef]
- Ben-Ari, E.T. What’s new at the zoo? Bioscience 2001, 51, 172–177. [Google Scholar] [CrossRef]
- Rapaport, L.G. Optimal foraging theory predicts effects of environmental enrichment in a group of adult golden lion tamarins. Zoo Biol. 1998, 17, 231–244. [Google Scholar] [CrossRef]
- Almli, L.M.; Burghardt, G.M. Environmental enrichment alters the behavioral profile of ratsnakes (Elaphe). J. Appl. Anim. Welf. Sci. 2006, 9, 85–109. [Google Scholar] [CrossRef] [PubMed]
- Coleman, K.; Novak, M.A. Environmental enrichment in the 21st century. ILAR J. 2017, 58, 295–307. [Google Scholar] [CrossRef]
- Lutz, C.K.; Novak, M.A. Environmental enrichment for nonhuman primates: Theory and application. ILAR J. 2005, 46, 178–191. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Duncan, L.M.; Jones, M.A.; van Lierop, M.; Pillay, N. Chimpanzees use multiple strategies to limit aggression and stress during spatial density changes. Appl. Anim. Behav. Sci. 2013, 147, 159–171. [Google Scholar] [CrossRef]
- Olsson, I.A.S.; Dahlborn, K. Improving housing conditions for laboratory mice: A review of “environmental enrichment”. Lab. Anim. 2002, 36, 243–270. [Google Scholar] [CrossRef]
- Baker, K.C.; Seres, M.; Aureli, F.; de Waal, F.B.M. Injury risks among chimpanzees in three housing conditions. Am. J. Primatol. 2000, 51, 161–175. [Google Scholar] [CrossRef]
- Bloomsmith, M.A.; Alford, P.L.; Maple, T.L. Successful feeding enrichment for captive chimpanzees. Am. J. Primatol. 1988, 16, 155–164. [Google Scholar] [CrossRef]
- Clubb, R.; Mason, G.J. Natural behavioural biology as a risk factor in carnivore welfare: How analysing species differences could help zoos improve enclosures. Appl. Anim. Behav. Sci. 2007, 102, 303–328. [Google Scholar] [CrossRef]
- Queiroz, B.M.; Young, R.J. The different physical and behavioural characteristics of zoo mammals that influence their response to visitors. Animals 2018, 8, 139. [Google Scholar] [CrossRef] [PubMed]
- Head, J.S.; Boesch, C.; Robbins, M.M.; Rabanal, L.I.; Makaga, L.; Kühl, H.S. Effective sociodemographic population assessment of elusive species in ecology and conservation management. Ecol. Evol. 2013, 3, 2903–2916. [Google Scholar] [CrossRef] [PubMed]
- Stotrabhashyam, S.; Sharma, N.; Kumar, A.; Sinha, A. Winter foraging ecology of stump-tailed macaques Macaca arctoides in the Hollongapar Gibbon Sanctuary, Assam, India. J. Biosci. 2023, 48, 30. [Google Scholar] [CrossRef]
- Santhosh, K.; Kumara, H.N.; Velankar, A.D.; Sinha, A. Ranging behavior and resource use by lion-tailed macaques (Macaca silenus) in selectively logged forests. Int. J. Primatol. 2015, 36, 288–310. [Google Scholar] [CrossRef]
- Al-Razi, H.; Hasan, S.; Ahmed, T.; Muzaffar, S.B. Home Range, Activity Budgets and Habitat Use in the Bengal Slow Loris (Nycticebus bengalensis) in Bangladesh. In Evolution, Ecology and Conservation of Lorises and Pottos; Cambridge University Press: Cambridge, UK, 2020; pp. 193–203. [Google Scholar]
- Musyoki, C.M.; Strum, S.C. Spatial and temporal patterns of home range use by olive baboons (Papio anubis) in eastern Laikipia, Kenya. Afr. J. Ecol. 2016, 54, 349–356. [Google Scholar] [CrossRef]
- Sigg, H.; Stolba, A. Home range and daily march in a Hamadryas baboon troop. Folia Primatol. 1981, 36, 40–75. [Google Scholar] [CrossRef]
- Fischer, J.; Kopp, G.H.; Dal Pesco, F.; Goffe, A.; Hammerschmidt, K.; Kalbitzer, U.; Klapproth, M.; Maciej, P.; Ndao, I.; Patzelt, A.; et al. Charting the neglected West: The social system of Guinea baboons. Am. J. Phys. Anthropol. 2017, 162, 15–31. [Google Scholar] [CrossRef]
- Canale, G.R.; Kierulff, M.C.M.; Chivers, D.J. A critically endangered capuchin monkey (Sapajus xanthosternos) living in a highly fragmented hotspot. In Primates in Fragments: Complexity and Resilience; Marsh, L.K., Chapman, C.A., Eds.; Springer New York: New York, NY, USA, 2013; pp. 299–311. ISBN 978-1-4614-8839-2. [Google Scholar]
- Morgan, B.J.; Abwe, E.E.; Dixson, A.F.; Astaras, C. The distribution, status, and conservation outlook of the drill (Mandrillus leucophaeus) in Cameroon. Int. J. Primatol. 2013, 34, 281–302. [Google Scholar] [CrossRef]
- Astaras, C.; Mühlenberg, M.; Waltert, M. Note on drill (Mandrillus leucophaeus) ecology and conservation status in Korup National Park, Southwest Cameroon. Am. J. Primatol. 2008, 70, 306–310. [Google Scholar] [CrossRef]
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2020. [Google Scholar]
- Fox, J.; Weisberg, S. An {R} Companion to Applied Regression, 2nd ed.; Sage Publications Inc.: Thousand Oaks, CA, USA, 2011. [Google Scholar]
- Bennett, A.J.; Perkins, C.M.; Harty, N.M.; Niu, M.; Buelo, A.K.; Luck, M.L.; Pierre, P.J. Assessment of foraging devices as a model for decision-making in nonhuman primate environmental enrichment. J. Am. Assoc. Lab. Anim. Sci. 2014, 53, 452–463. [Google Scholar] [PubMed]
- Shapiro, M.E.; Shapiro, H.G.; Ehmke, E.E. Behavioral responses of three lemur species to different food enrichment devices. Zoo Biol. 2018, 37, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Kalcher-Sommersguter, E.; Franz-Schaider, C.; Preuschoft, S.; Crailsheim, K. Long-term evaluation of abnormal behavior in adult ex-laboratory chimpanzees (Pan troglodytes) following re-socialization. Behav. Sci. 2013, 3, 99. [Google Scholar] [CrossRef] [PubMed]
- Pruetz, J.D.; Bloomsmith, M.A. Comparing two manipulable objects as enrichment for captive chimpanzees. Anim. Welf. 1992, 1, 127–137. [Google Scholar] [CrossRef]
- Eaton, G.G.; Kelley, S.T.; Axthelm, M.K.; Iliff-Sizemore, S.A.; Shiigi, S.M. Psychological well-being in paired adult female rhesus (Macaca mulatta). Am. J. Primatol. 1994, 33, 89–99. [Google Scholar] [CrossRef]
- Anderson, M.R. Reaching new heights: The effect of an environmentally enhanced outdoor enclosure on gibbons in a zoo setting. J. Appl. Anim. Welf. Sci. 2014, 17, 216–227. [Google Scholar] [CrossRef]
- Cannon, T.H.; Heistermann, M.; Hankison, S.J.; Hockings, K.J.; McLennan, M.R. Tailored enrichment strategies and stereotypic behavior in captive individually housed macaques (Macaca spp.). J. Appl. Anim. Welf. Sci. 2016, 19, 171–182. [Google Scholar] [CrossRef]
- Mallavarapu, S.; Bloomsmith, M.A.; Kuhar, C.W.; Maple, T.L. Using multiple joystick systems in computerised enrichment for captive orangutans. Anim. Welf. 2013, 22, 401–409. [Google Scholar] [CrossRef][Green Version]
- Maloney, M.A.; Meiers, S.T.; White, J.; Romano, M.A. Effects of three food enrichment items on the behavior of black lemurs (Eulemur macaco macaco) and ringtail lemurs (Lemur catta) at the Henson Robinson Zoo, Springfield, Illinois. J. Appl. Anim. Welf. Sci. 2006, 9, 111–127. [Google Scholar] [CrossRef]
- Baker, K.C.; Springer, D.A. Frequency of feeding enrichment and response of laboratory nonhuman primates to unfamiliar people. J. Am. Assoc. Lab. Anim. Sci. 2006, 45, 69–73. [Google Scholar]
- Chatpongcharoen, P.; Campera, M.; Laithong, P.; Gibson, N.L.; Nekaris, K.A.I. Naturalising diet to reduce stereotypic behaviours in slow lorises rescued from wildlife trade. Appl. Anim. Behav. Sci. 2021, 242, 105413. [Google Scholar] [CrossRef]
- Sanders, K.; Fernandez, E.J. Behavioral Implications of Enrichment for Golden Lion Tamarins: A Tool for Ex Situ Conservation. J. Appl. Anim. Welf. Sci. 2022, 25, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Bakker, J.; Ouwerling, B.; Heidt, P.J.; Kondova, I.; Langermans, J.A.M. Advantages and risks of husbandry and housing changes to improve animal wellbeing in a breeding colony of common marmosets (Callithrix jacchus). J. Am. Assoc. Lab. Anim. Sci. 2015, 54, 273–279. [Google Scholar] [PubMed]
- Kondo, S.Y.; Yudko, E.B.; Magee, L.K. A novel approach for documentation and evaluation of activity patterns in owl monkeys during development of environmental enrichment programs. J. Am. Assoc. Lab. Anim. Sci. 2003, 42, 17–21. [Google Scholar]
- Kristie, C.; Tim, S.; Lance, J.M. Impact of different forms of environmental enrichment on foraging and activity levels in gorillas (Gorilla gorilla gorilla). Anim. Behav. Cogn. 2015, 2, 233–240. [Google Scholar] [CrossRef]
- Ogura, T. Use of video system and its effects on abnormal behaviour in captive Japanese macaques (Macaca fuscata). Appl. Anim. Behav. Sci. 2012, 141, 173–183. [Google Scholar] [CrossRef]
- Baker, K.C. Straw and forage material ameliorate abnormal behaviors in adult chimpanzees. Zoo Biol. 1997, 16, 225–236. [Google Scholar] [CrossRef]
- Schapiro, S.J.; Porter, L.M.; Suarez, S.A.; Bloomsmith, M.A. The behavior of singly-caged, yearling rhesus monkeys is affected by the environment outside of the cage. Appl. Anim. Behav. Sci. 1995, 45, 151–163. [Google Scholar] [CrossRef]
- American Psychological Association. APA Style Numbers and Statistics Guide; American Psychological Association: Washington, DC, USA, 2024. [Google Scholar]
- Bayne, K.A.L.; Dexter, S.; Mainzer, H.; McCully, C.; Campbell, G.; Yamada, F. The use of artificial turf as a foraging substrate for individually housed rhesus monkeys (Macaca mulatta). Anim. Welf. 1992, 1, 39–54. [Google Scholar] [CrossRef]
- Carrasco, L.; Colell, M.; Calvo, M.; Abelló, M.T.; Velasco, M.; Posada, S. Benefits of training/playing therapy in a group of captive lowland gorillas (Gorilla gorilla gorilla). Anim. Welf. 2009, 18, 9–19. [Google Scholar] [CrossRef]
- Bayne, K.A.L.; Würbel, H. The impact of environmental enrichment on the outcome variability and scientific validity of laboratory animal studies. Sci. Tech. Rev. Off. Int. Epizoot. 2014, 33, 273–280. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes. Off. J. Eur. Union 2010, L 276, 33–79. [Google Scholar]
- Cameron, D.; Clemence, M.; Xypolia, K. Public Attitudes to Animal Research in 2018; ISPOS: London, UK, 2018. [Google Scholar]
- Celli, M.L.; Tomonaga, M.; Udono, T.; Teramoto, M.; Nagano, K. Tool use task as environmental enrichment for captive chimpanzees. Appl. Anim. Behav. Sci. 2003, 81, 171–182. [Google Scholar] [CrossRef]
- Coleman, G.J. Public perceptions of animal welfare: An international perspective. In Proceedings of the AAWS08 International Animal Welfare Conference, Gold Coast, Australia, 31 August–3 September 2008. [Google Scholar]
- Carlsson, H.-E.; Schapiro, S.J.; Farah, I.; Hau, J. Use of primates in research: A global overview. Am. J. Primatol. 2004, 63, 225–237. [Google Scholar] [CrossRef]
- Feister, A.J.; DiPietrantonio, A.; Yuenger, J.; Ireland, K.; Rao, A. Nonhuman Primate Evaluation and Analysis Part 1: Analysis of Future Demand and Supply; National Institutes of Health: Bethesda, MD, USA, 2018. [Google Scholar]
- Knight, A. The beginning of the end for chimpanzee experiments? Philos. Ethics Humanit. Med. 2008, 3, 16. [Google Scholar] [CrossRef]
- Moss, A.; Esson, M. Visitor interest in zoo animals and the implications for collection planning and zoo education programmes. Zoo Biol. 2010, 29, 715–731. [Google Scholar] [CrossRef]
- Hopper, L.M. Primatology in zoos: Studying behavior, cognition, and welfare. Am. J. Primatol. 2022, 84, e23385. [Google Scholar] [CrossRef]
- Colléony, A.; Clayton, S.; Couvet, D.; Saint Jalme, M.; Prévot, A.-C. Human preferences for species conservation: Animal charisma trumps endangered status. Biol. Conserv. 2017, 206, 263–269. [Google Scholar] [CrossRef]
- Ward, P.I.; Mosberger, N.; Kistler, C.; Fischer, O. The relationship between popularity and body size in zoo animals. Conserv. Biol. 1998, 12, 1408–1411. [Google Scholar] [CrossRef]
- McPhee, M.E.; Foster, J.S.; Sevenich, M.; Saunders, C.D. Public perceptions of behavioral enrichment: Assumptions gone awry. Zoo Biol. 1998, 17, 525–534. [Google Scholar] [CrossRef]
- Melfi, V.A. The appliance of science to zoo-housed primates. Appl. Anim. Behav. Sci. 2005, 90, 97–106. [Google Scholar] [CrossRef]
- Mason, G.J. Species differences in responses to captivity: Stress, welfare and the comparative method. Trends Ecol. Evol. 2010, 25, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Pomerantz, O.; Meiri, S.; Terkel, J. Socio-ecological factors correlate with levels of stereotypic behavior in zoo-housed primates. Behav. Processes 2013, 98, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Sakuraba, Y.; Tomonaga, M.; Hayashi, M. A new method of walking rehabilitation using cognitive tasks in an adult chimpanzee (Pan troglodytes) with a disability: A case study. Primates 2016, 57, 403–412. [Google Scholar] [CrossRef]
- Ongman, L.; Colin, C.; Raballand, E.; Humle, T. The “super chimpanzee”: The ecological dimensions of rehabilitation of orphan chimpanzees in Guinea, West Africa. Animals 2013, 3, 109. [Google Scholar] [CrossRef]
- Lonsdorf, E.V.; Ross, S.R.; Linick, S.A.; Milstein, M.S.; Melber, T.N. An experimental, comparative investigation of tool use in chimpanzees and gorillas. Anim. Behav. 2009, 77, 1119–1126. [Google Scholar] [CrossRef]
- Visalberghi, E.; Vitale, A.F. Coated nuts as an enrichment device to elicit tool use in tufted capuchins (Cebus apella). Zoo Biol. 1990, 9, 65–71. [Google Scholar] [CrossRef]
- Morimura, N.; Idani, G.; Matsuzawa, T. The first chimpanzee sanctuary in Japan: An attempt to care for the “surplus” of biomedical research. Am. J. Primatol. 2011, 73, 226–232. [Google Scholar] [CrossRef]
- Ross, S.R.; Leinwand, J.G. A review of research in primate sanctuaries. Biol. Lett. 2020, 16, 20200033. [Google Scholar] [CrossRef]
- Nunes, E.V.; Pavlicova, M.; Hu, M.-C.; Campbell, A.N.; Miele, G.; Hien, D.; Klein, D.F. Baseline matters: The importance of covariation for baseline severity in the analysis of clinical trials. Am. J. Drug Alcohol Abuse 2011, 37, 446–452. [Google Scholar] [CrossRef]
- Hylander, B.L.; Repasky, E.A.; Sexton, S. Using mice to model human disease: Understanding the roles of baseline housing-induced and experimentally imposed stresses in animal welfare and experimental reproducibility. Animals 2022, 12, 371. [Google Scholar] [CrossRef] [PubMed]
- Fraser, D. Assessing animal welfare: Different philosophies, different scientific approaches. Zoo Biol. 2009, 28, 507–518. [Google Scholar] [CrossRef] [PubMed]
- Carenzi, C.; Verga, M. Animal welfare: Review of the scientific concept and definition. Ital. J. Anim. Sci. 2009, 8, 21–30. [Google Scholar] [CrossRef]
- Hahn, N.E.; Lau, D.; Eckert, K.; Markowitz, H. Environmental enrichment-related injury in a macaque (Macaca fascicularis): Intestinal linear foreign body. Comp. Med. 2000, 50, 556–558. [Google Scholar]
- Mätz-Rensing, K.; Floto, A.; Kaup, F.-J.J. Intraperitoneal foreign body disease in a baboon (Papio hamadryas). J. Med. Primatol. 2004, 33, 113–116. [Google Scholar] [CrossRef]
- Seier, J.V.; Dhansay, M.A.; Davids, A. Risks associated with environmental enrichment: Intestinal obstruction caused by foraging substrate. J. Med. Primatol. 2005, 34, 154–155. [Google Scholar] [CrossRef]
- Mook, D.M. Gastric trichobezoars in a rhesus macaque (Macaca mulatta). Comp. Med. 2002, 52, 560–562. [Google Scholar]
- Pika, S.; Tomasello, M. ‘Separating the wheat from the chaff’: A novel food processing technique in captive gorillas (Gorilla g. gorilla). Primates 2001, 42, 167–170. [Google Scholar] [CrossRef]
- Jones, M.; Pillay, N. Foraging in captive hamadryas baboons: Implications for enrichment. Appl. Anim. Behav. Sci. 2004, 88, 101–110. [Google Scholar] [CrossRef]
- Kessel, A.; Brent, L. The rehabilitation of captive baboons. J. Med. Primatol. 2001, 30, 71–80. [Google Scholar] [CrossRef]
- Francis, D.D.; Diorio, J.; Plotsky, P.M.; Meaney, M.J. Environmental enrichment reverses the effects of maternal separation on stress reactivity. J. Neurosci. 2002, 22, 7840–7843. [Google Scholar] [CrossRef] [PubMed]
- Rampon, C.; Jiang, C.H.; Dong, H.; Tang, Y.-P.; Lockhart, D.J.; Schultz, P.G.; Tsien, J.Z.; Hu, Y. Effects of environmental enrichment on gene expression in the brain. Proc. Natl. Acad. Sci. USA 2000, 97, 12880. [Google Scholar] [CrossRef]
- Young, D.; Lawlor, P.A.; Leone, P.; Dragunow, M.; During, M.J. Environmental enrichment inhibits spontaneous apoptosis, prevents seizures and is neuroprotective. Nat. Med. 1999, 5, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Blackman, N. Corporate social responsibility & animal welfare. Aust. Vet. J. 2005, 83, 250. [Google Scholar] [CrossRef]
- Foltin, R.W. “Tasting and wasting” behavior in non-human primates: Aberrant behavior or normal behavior in “times of plenty”. Physiol. Behav. 2006, 89, 587–597. [Google Scholar] [CrossRef]
- Melfi, V.A.; McCormick, W.; Gibbs, A. A preliminary assessment of how zoo visitors evaluate animal welfare according to enclosure style and the expression of behavior. Anthrozoos 2004, 17, 98–108. [Google Scholar] [CrossRef]
- Silk, J.B.; Kappeler, P.M. Sociality in primates. In Comparative Social Evolution; Rubenstein, D.R., Abbot, P., Eds.; Cambridge University Press: Cambridge, UK, 2017; pp. 253–283. ISBN 9781107043398. [Google Scholar]
- van de Weerd, H.A.; Day, J.E.L. A review of environmental enrichment for pigs housed in intensive housing systems. Appl. Anim. Behav. Sci. 2009, 116, 1–20. [Google Scholar] [CrossRef]
- Wells, D.L. A review of environmental enrichment for kennelled dogs, Canis familiaris. Appl. Anim. Behav. Sci. 2004, 85, 307–317. [Google Scholar] [CrossRef]
- Bearder, S.K. Lorises, bushbabies, and tarsiers: Diverse societies in solitary foragers. In Primate Societies; Smuts, B.B., Cheney, D.L., Seyfarth, R.M., Wrangham, R.W., Struhsaker, T.T., Eds.; The University of Chicago Press: Chicago, IL, USA; London, UK, 1987; pp. 11–24. [Google Scholar]
- Forss, S.I.F.; Koski, S.E.; van Schaik, C.P. Explaining the Paradox of Neophobic Explorers: The Social Information Hypothesis. Int. J. Primatol. 2017, 38, 799–822. [Google Scholar] [CrossRef]
- Schaffer, A.; Caicoya, A.L.; Colell, M.; Holland, R.; von Fersen, L.; Widdig, A.; Amici, F. Neophobia in 10 ungulate species—A comparative approach. Behav. Ecol. Sociobiol. 2021, 75, 102. [Google Scholar] [CrossRef]
- Schapiro, S.J.; Bloomsmith, M.A.; Laule, G.E. Positive reinforcement training as a technique to alter nonhuman primate behavior: Quantitative assessments of effectiveness. J. Appl. Anim. Welf. Sci. 2003, 6, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Bayne, K.A.L. Environmental enrichment of nonhuman primates, dogs and rabbits used in toxicology studies. Toxicol. Pathol. 2003, 31, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Lambeth, S.P.; Hau, J.; Perlman, J.E.; Martino, M.; Schapiro, S.J. Positive reinforcement training affects hematologic and serum chemistry values in captive chimpanzees (Pan troglodytes). Am. J. Primatol. 2006, 68, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Melfi, V.A. Is training zoo animals enriching? Appl. Anim. Behav. Sci. 2013, 147, 299–305. [Google Scholar] [CrossRef]
- Videan, E.N.; Fritz, J.; Schwandt, M.L.; Smith, H.F.; Howell, S. Controllability in environmental enrichment for captive chimpanzees (Pan troglodytes). J. Appl. Anim. Welf. Sci. 2005, 8, 117–130. [Google Scholar] [CrossRef]
- Heymann, E.W. The neglected sense–olfaction in primate behavior, ecology, and evolution. Am. J. Primatol. 2006, 68, 519–524. [Google Scholar] [CrossRef]
- Wells, D.L.; Hepper, P.G.; Coleman, D.; Challis, M.G. A note on the effect of olfactory stimulation on the behaviour and welfare of zoo-housed gorillas. Appl. Anim. Behav. Sci. 2007, 106, 155–160. [Google Scholar] [CrossRef]
- Clark, F.E.; King, A.J. A critical review of zoo-based olfactory enrichment. In Chemical Signals in Vertebrates 11; Hurst, J.L., Beynon, R.J., Roberts, S.C., Wyatt, T.D., Eds.; Springer: New York, NY, USA, 2008; pp. 391–398. [Google Scholar]
- Wells, D.L.; Egli, J.M. The influence of olfactory enrichment on the behaviour of captive black-footed cats, Felis nigripes. Appl. Anim. Behav. Sci. 2004, 85, 107–119. [Google Scholar] [CrossRef]
- Ellis, S.L.H.; Wells, D.L. The influence of olfactory stimulation on the behaviour of cats housed in a rescue shelter. Appl. Anim. Behav. Sci. 2010, 123, 56–62. [Google Scholar] [CrossRef]
- Wells, D.L. Sensory stimulation as environmental enrichment for captive animals: A review. Appl. Anim. Behav. Sci. 2009, 118, 1–11. [Google Scholar] [CrossRef]
- Case, L.; Yanagi, A.; Loeser, E.; Fultz, A. Human-animal relationships: The use of species-typical food calls and chimpanzee (Pan troglodytes) names: Welfare-oriented tools to manage sanctuary chimpanzees. Anim. Behav. Cogn. 2015, 2, 254–266. [Google Scholar] [CrossRef]
- Ward, S.J.; Melfi, V.A. Keeper-animal interactions: Differences between the behaviour of zoo animals affect stockmanship. PLoS ONE 2015, 10, e0140237. [Google Scholar] [CrossRef] [PubMed]
- Nimon, A.J.; Dalziel, F.R. Cross-species interaction and communication: A study method applied to captive siamang (Hylobates syndactylus) and long-billed corella (Cacatua tenuirostris) contacts with humans. Appl. Anim. Behav. Sci. 1992, 33, 261–272. [Google Scholar] [CrossRef]
- Baker, K.C. Enrichment and primate centers: Closing the gap between research and practice. J. Appl. Anim. Welf. Sci. 2007, 10, 49–54. [Google Scholar] [CrossRef][Green Version]
- Eberle, M.; Kappeler, P.M. Family insurance: Kin selection and cooperative breeding in a solitary primate (Microcebus murinus). Behav. Ecol. Sociobiol. 2006, 60, 582–588. [Google Scholar] [CrossRef]
- Gilbert, M.H.; Baker, K.C. Social buffering in adult male rhesus macaques (Macaca mulatta): Effects of stressful events in single vs. pair housing. J. Med. Primatol. 2011, 40, 71–78. [Google Scholar] [CrossRef]
- Sanchez, M.M.; McCormack, K.M.; Howell, B.R. Social buffering of stress responses in nonhuman primates: Maternal regulation of the development of emotional regulatory brain circuits. Soc. Neurosci. 2015, 10, 512–526. [Google Scholar] [CrossRef]
- Mlinarić, A.; Horvat, M.; Šupak Smolčić, V. Dealing with the positive publication bias: Why you should really publish your negative results. Biochem. medica 2017, 27, 30201. [Google Scholar] [CrossRef]
- van der Schot, A.A.; Phillips, C. Publication bias in animal welfare scientific literature. J. Agric. Environ. Ethics 2013, 26, 945–958. [Google Scholar] [CrossRef]
- Dalton, J.E.; Bolen, S.D.; Mascha, E.J. Publication bias: The elephant in the review. Anesth. Analg. 2016, 123, 812–813. [Google Scholar] [CrossRef]
- Boogaard, B.K.; Oosting, S.J.; Bock, B.B. Elements of societal perception of farm animal welfare: A quantitative study in The Netherlands. Livest. Sci. 2006, 104, 13–22. [Google Scholar] [CrossRef]
- Jaakkola, K.; Bruck, J.N.; Connor, R.C.; Montgomery, S.H.; King, S.L. Bias and misrepresentation of science undermines productive discourse on animal welfare policy: A case study. Animals 2020, 10, 1118. [Google Scholar] [CrossRef] [PubMed]
- Bloomsmith, M.A.; Pazol, K.A.; Alford, P.L. Juvenile and adolescent chimpanzee behavioral development in complex groups. Appl. Anim. Behav. Sci. 1994, 39, 73–87. [Google Scholar] [CrossRef]
- Bielefeldt-Ohmann, H.; Bellanca, R.U.; Crockett, C.M.; Curnow, E.; Eiffert, K.; Gillen, M.; Glanister, D.; Hayes, E.; Kelley, S.; Minoshima, S.; et al. Subacute necrotizing encephalopathy in a pig-tailed macaque (Macaca nemestrina) that resembles mitochondrial encephalopathy in humans. Comp. Med. 2004, 54, 422–433. [Google Scholar]
- Hutsell, B.A.; Banks, M.L. Effects of environmental and pharmacological manipulations on a novel delayed nonmatching-to-sample ‘working memory’ procedure in unrestrained rhesus monkeys. J. Neurosci. Methods 2015, 251, 62–71. [Google Scholar] [CrossRef]
- Schapiro, S.J.; Bloomsmith, M.A.; Suarez, S.A.; Porter, L.M. A comparison of the effects of simple versus complex environmental enrichment on the behaviour of group-housed, subadult rhesus macaques. Anim. Welf. 1997, 6, 17–28. [Google Scholar] [CrossRef]
- Tsuchida, J.; Izumi, A. The effects of age and sex on interest toward movies of conspecifics in Japanese macaques (Macaca fuscata). J. Am. Assoc. Lab. Anim. Sci. 2009, 48, 286–291. [Google Scholar]
- Whitham, J.C.; Wielebnowski, N. Animal-based welfare monitoring: Using keeper ratings as an assessment tool. Zoo Biol. 2009, 28, 545–560. [Google Scholar] [CrossRef]
- FitzGerald, C.; Hurst, S. Implicit bias in healthcare professionals: A systematic review. BMC Med. Ethics 2017, 18, 19. [Google Scholar] [CrossRef]
- Feely, M.; Bosk, E.A. That which is essential has been made invisible: The need to bring a structural risk perspective to reduce racial disproportionality in child welfare. Race Soc. Probl. 2021, 13, 49–62. [Google Scholar] [CrossRef]
- Ly, L.H.; Brown, K.; Yau, E.; Kenworthy, B.; Segurson, S.; Protopopova, A. A mixed-methods exploration of opportunities for barriers and bias during off site animal adoption events. J. Shelter Med. Community Anim. Health 2024, 3, 1–18. [Google Scholar] [CrossRef]
- Broom, D.M. Animal welfare: Concepts and measurement. J. Anim. Sci. 1991, 69, 4167–4175. [Google Scholar] [CrossRef] [PubMed]
- Mellor, D.J.; Beausoleil, N.J.; Littlewood, K.E.; McLean, A.N.; McGreevy, P.D.; Jones, B.; Wilkins, C. The 2020 Five Domains Model: Including Human–Animal Interactions in Assessments of Animal Welfare. Animals 2020, 10, 1870. [Google Scholar] [CrossRef]
- Fuller, G.; Allard, S. Preliminary data showing potential for salivary C-reactive protein as an indicator of welfare in western lowland gorillas (Gorilla gorilla gorilla). J. Vet. Behav. 2018, 28, 58–62. [Google Scholar] [CrossRef]
- Howell, C.P.; Cheyne, S.M. Complexities of using wild versus captive activity budget comparisons for assessing captive primate welfare. J. Appl. Anim. Welf. Sci. 2019, 22, 78–96. [Google Scholar] [CrossRef]
- Jaman, M.F.; Huffman, M.A. The effect of urban and rural habitats and resource type on activity budgets of commensal rhesus macaques (Macaca mulatta) in Bangladesh. Primates 2013, 54, 49–59. [Google Scholar] [CrossRef]
- Kerridge, F.J. Environmental enrichment to address behavioral differences between wild and captive black-and-white ruffed lemurs (Varecia variegata). Am. J. Primatol. 2005, 66, 71–84. [Google Scholar] [CrossRef]
- Melfi, V.A.; Feistner, A.T.C. A comparison of the activity budgets of wild and captive Sulawesi crested black macaques (Macaca nigra). Anim. Welf. 2002, 11, 213–222. [Google Scholar] [CrossRef]
- Westergaard, G.C.; Fragaszy, D.M. Effects of manipulatable objects on the activity of captive capuchin monkeys (Cebus apella). Zoo Biol. 1985, 4, 317–327. [Google Scholar] [CrossRef]
- Seier, J.V.; Lange, P.W. A mobile cage facilitates periodic social contact and exercise for singly caged adult Vervet monkeys. J. Med. Primatol. 1996, 25, 64–68. [Google Scholar] [CrossRef]
- Bourgeois, S.R.; Vazquez, M.; Brasky, K. Combination therapy reduces self-injurious behavior in a chimpanzee (Pan troglodytes troglodytes): A case report. J. Appl. Anim. Welf. Sci. 2007, 10, 123–140. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.K.; Bass, T.; Flory, G.S.; Hankenson, C.F. Use of low-dose chlorpromazine in conjunction with environmental enrichment to eliminate self-injurious behavior in a rhesus macaque (Macaca mulatta). Comp. Med. 2005, 55, 282–288. [Google Scholar] [PubMed]
- Reamer, L.; Tooze, Z.; Coulson, C.; Semple, S. Correlates of self-directed and stereotypic behaviours in captive red-capped mangabeys (Cercocebus torquatus torquatus). Appl. Anim. Behav. Sci. 2010, 124, 68–74. [Google Scholar] [CrossRef]
- Kranendonk, G.; Schippers, E.P. A pilot study on the effects of a change in behavioural management on the behaviour of captive chimpanzees (Pan troglodytes). Appl. Anim. Behav. Sci. 2014, 160, 127–137. [Google Scholar] [CrossRef]
- Pascual, A.; Kalcher-Sommersguter, E.; Riba, D.; Crailsheim, D. Long-term assessment of captive chimpanzees: Influence of social group composition, seasonality and biographic background. Animals 2023, 13, 424. [Google Scholar] [CrossRef]

| Classification | Definition |
|---|---|
| Feeding | Protocol designed to alter feeding or foraging behaviour of the subject. Includes presentation of novel foods, foods presented in novel forms or times and foods presented in a manner which requires physical or cognitive effort (excluding interaction with another individual) by subjects in order to gain access. |
| Object | Protocol involves the presentation of non-nutritive fixed or mobile objects for subjects to interact with which are otherwise absent from the environment and do not pose an overt cognitive challenge. |
| Olfactory | Protocol presents uncontrollable olfactory stimuli, such as diffused odours or essential oils, as a means of enhancing the sensory environment. |
| Auditory | Protocol presents uncontrollable auditory stimuli, such as recordings of rainforest sounds or music, as a means of enhancing the sensory environment. |
| Visual | Protocol presents uncontrollable visual stimuli, such as video or images, as a means of enhancing the sensory environment. |
| Social | Protocol involves manipulation of social conditions. Includes changes to group size and composition and opportunities for conspecific social interaction. |
| Cognitive | Protocol presents subjects with an overt cognitive challenge or game which does not involve interaction between conspecific or heterospecific individuals. Food rewards may be involved as incentives to motivate cognition but feeding is not the primary aim. |
| Training | Protocol involves structured interaction sessions between a human trainer and a primate subject intended to modify the behaviour of the subject, typically to perform a specified behaviour on command. |
| Interaction-based | Intentional and/or supervised interactions initiated by humans between heterospecific individuals (typically involves gestural or physical interaction between animals and a human) which do not serve an overt training or veterinary function. Interactions may be direct (e.g., physical contact) or indirect (e.g., interaction via a computer-based system or through a protective barrier such as a fence or window). |
| Enclosure modification | A large-scale qualitative and/or quantitative change in the physical housing environment. Includes the provision of naturalistic elements, novel substrates or structural elements of the housing environment. |
| Combination | Protocol involves the application of two or more enrichment classifications simultaneously or in a fashion which prevents the effects of the protocol elements from being independently identified. |
| Other | Any enrichment protocol or design which does not clearly adhere to the classifications above. |
| Domain of Change | Criteria for Beneficial Welfare State |
|---|---|
| Behavioural | Relative decrease in abnormal behavioural expression (e.g., stereotypy, abnormal posturing or gait, coprophagia). |
| Relative increase in ‘desirable’ behavioural expression (e.g., play, social interaction, activity patterns approximating free-ranging conspecifics). | |
| Relative decrease in pathological behavioural expression (e.g., self-injurious behaviour, trichotillomania). | |
| Relative decrease in agonistic behavioural expression † (e.g., aggression, threat display). | |
| Relative qualitative improvement in behavioural expression (e.g., increased diversity of ‘desired’ behaviour, decreased diversity of abnormal, pathological or aggressive behaviour). | |
| Relative decrease in self-narcotisation (e.g., reduced self-administration of cocaine or consumption of alcohol). | |
| Neurocognitive | Relatively improved cognitive/perceptual functioning (e.g., problem solving ability, learning and/or memory capacity). |
| Relative increase in brain mass and/or neural complexity. | |
| Developmental | Adherence to species-typical physical and psychological/cognitive development or aging. |
| Physiological/Clinical | Relative decrease in hormonal measures associated with acute and chronic stress as determined through direct measures (e.g., blood sampling) or indirect measures (e.g., urinary or faecal metabolites). |
| Relative improvement in physical condition (e.g., increased body mass for the same quantity of food, decreased prevalence of physical pathology or illness). Relative decrease in veterinary interventions and/or time required for veterinary treatment and/or recovery. | |
| Relative increase in measures of immunological function (e.g., immune cell or cytokine assays) in the absence of infection or disease. | |
| Reproductive | Relative increase in reproductive output. |
| Relative decrease in reproductive abnormalities or pathologies (e.g., stillbirths, premature births, congenital defects or disorders). | |
| Survival | Relative reduction in non-age-related deaths, increases in longevity. |
| Least Concern | Near Threatened | Vulnerable | Endangered | Critically Endangered | ||
|---|---|---|---|---|---|---|
| Protocols | Total | 102 | 7 | 21 | 75 | 43 |
| % | 41.13 | 2.82 | 8.47 | 30.24 | 17.34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duncan, L.M.; Pillay, N. A Metric-Based, Meta-Analytic Appraisal of Environmental Enrichment Efficacy in Captive Primates. Animals 2025, 15, 799. https://doi.org/10.3390/ani15060799
Duncan LM, Pillay N. A Metric-Based, Meta-Analytic Appraisal of Environmental Enrichment Efficacy in Captive Primates. Animals. 2025; 15(6):799. https://doi.org/10.3390/ani15060799
Chicago/Turabian StyleDuncan, Luke Mangaliso, and Neville Pillay. 2025. "A Metric-Based, Meta-Analytic Appraisal of Environmental Enrichment Efficacy in Captive Primates" Animals 15, no. 6: 799. https://doi.org/10.3390/ani15060799
APA StyleDuncan, L. M., & Pillay, N. (2025). A Metric-Based, Meta-Analytic Appraisal of Environmental Enrichment Efficacy in Captive Primates. Animals, 15(6), 799. https://doi.org/10.3390/ani15060799






