Effects of Oxidative Stress and Antioxidant Activity in Plasma and Uterine Fluid During Early Postpartum on Subsequent Reproductive Performance of Japanese Black Cows
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Housing, and Feeding
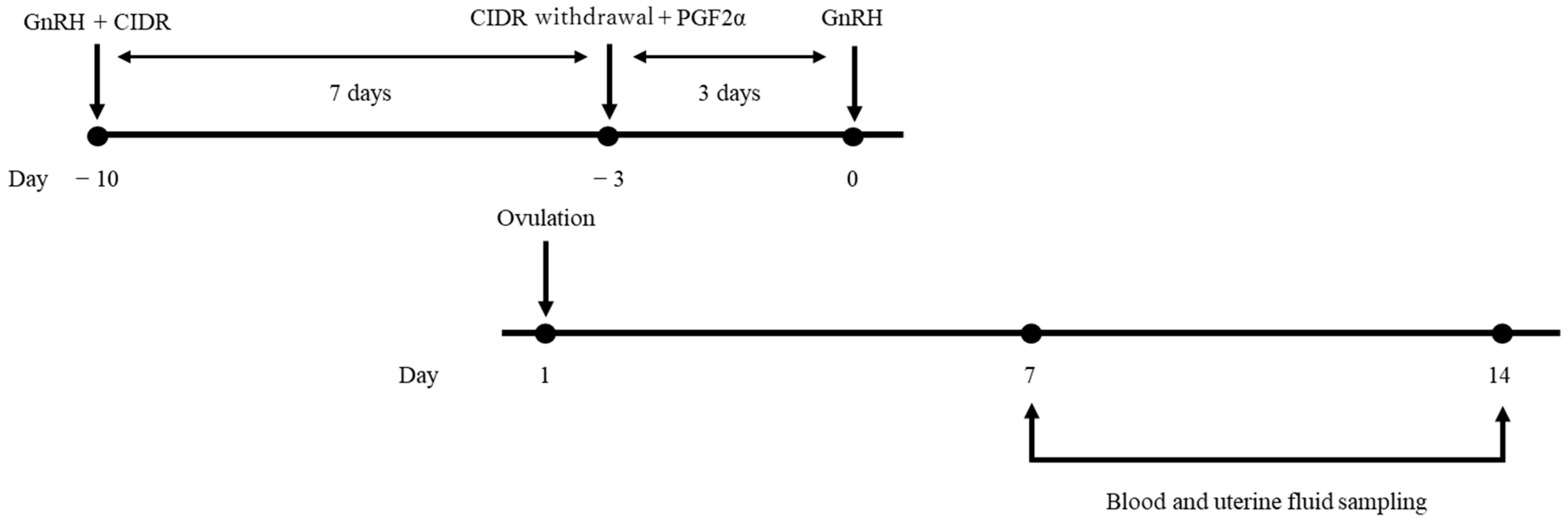
2.2. Study Design
2.3. Sampling of Plasma and Uterine Fluid
2.4. Reproductive Management
2.5. Measurement of d-ROMs and BAP
2.6. Classification
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Perry, G.A.; Cushman, R.A.; Perry, B.L.; Schiefelbein, A.K.; Northrop, E.J.; Rich, J.J.J.; Perkins, S.D. Role of preovulatory concentrations of estradiol on timing of conception and regulation of the uterine environment in beef cattle. Syst. Biol. Reprod. Med. 2020, 66, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Perry, G.A.; Smith, M.F.; Lucy, M.C.; Green, J.A.; Parks, T.E.; MacNeil, M.D.; Roberts, A.J.; Geary, T.W. Relationship between follicle size at insemination and pregnancy success. Proc. Natl. Acad. Sci. USA 2005, 102, 5268–5273. [Google Scholar] [CrossRef] [PubMed]
- Ricci, A.; Gallo, S.; Molinaro, F.; Dondo, A.; Zoppi, S.; Vincenti, L. Evaluation of subclinical endometritis and consequences on fertility in piedmontese beef cows. Reprod. Domest. Anim. 2015, 50, 142–148. [Google Scholar] [CrossRef]
- Nishimura, T.K.; Martins, T.; da Silva, M.I.; Lafuente, B.S.; Maio, J.R.d.G.; Binelli, M.; Pugliesi, G.; Saran Netto, A. Importance of body condition score and ovarian activity on determining the fertility in beef cows supplemented with long-acting progesterone after timed-AI. Anim. Reprod. Sci. 2018, 198, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Shoup, L.M.; Kloth, A.C.; Wilson, T.B.; González-Peña, D.; Ireland, F.A.; Rodriguez-Zas, S.; Felix, T.L.; Shike, D.W. Prepartum supplement level and age at weaning: I. Effects on pre- and postpartum beef cow performance and calf performance through weaning. J. Anim. Sci. 2015, 93, 4926–4935. [Google Scholar] [CrossRef]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef]
- Poprac, P.; Jomova, K.; Simunkova, M.; Kollar, V.; Rhodes, C.J.; Valko, M. Targeting Free Radicals in Oxidative Stress-Related Human Diseases. Trends Pharmacol. Sci. 2017, 38, 592–607. [Google Scholar] [CrossRef]
- Burton, G.J.; Jauniaux, E. Oxidative stress. Best Pract. Res. Clin. Obstet. Gynaecol. 2011, 25, 287–299. [Google Scholar] [CrossRef]
- Sharma, R.K.; Agarwal, A. Role of reactive oxygen species in gynecologic diseases. Reprod. Med. Biol. 2004, 3, 177–199. [Google Scholar] [CrossRef]
- Hussain, T.; Murtaza, G.; Metwally, E.; Kalhoro, D.H.; Kalhoro, M.S.; Rahu, B.A.; Tan, B. The role of oxidative stress and antioxidant balance in pregnancy. Mediat. Inflamm. 2021, 2021, 11. [Google Scholar] [CrossRef]
- Cesarone, M.R.; Belcaro, G.; Carratelli, M.; Cornelli, U.; De Sanctis, M.T.; Incandela, L.; Barsotti, A.; Terranova, R.; Nicolaides, A. A simple test to monitor oxidative stress. Int. Angiol. 1999, 18, 127–130. [Google Scholar] [PubMed]
- Trotti, R.; Carratelli, M.; Barbieri, M. Performance and clinical application of a new, fast method for the detection of hydroperoxides in serum. Panminerva Medica 2002, 44, 37–40. [Google Scholar]
- Fukui, T.; Yamauchi, K.; Maruyama, M.; Yasuda, T.; Kohno, M.; Abe, Y. Significance of measuring oxidative stress in lifestyle-related diseases from the viewpoint of correlation between d-ROMs and BAP in Japanese subjects. Hypertens. Res. 2011, 34, 1041–1045. [Google Scholar] [CrossRef] [PubMed]
- Abuelo, A.; Hernández, J.; Benedito, J.L.; Castillo, C. The importance of the oxidative status of dairy cattle in the periparturient period: Revisiting antioxidant supplementation. J. Anim. Physiol. Anim. Nutr. 2015, 99, 1003–1016. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.K.; Pasqualotto, F.F.; Nelson, D.R.; Thomas Jr, A.J.; and Agarwal, A. The reactive oxygen species—Total antioxidant capacity score is a new measure of oxidative stress to predict male infertility. Hum. Reprod. 1999, 14, 2801–2807. [Google Scholar] [CrossRef]
- Tinning, H.; Edge, J.C.; DeBem, T.H.C.; Deligianni, F.; Giovanardi, G.; Pensabene, V.; Meirelles, F.V.; Forde, N. Review: Endometrial function in pregnancy establishment in cattle. Animal 2023, 17 (Suppl. S1), 100751. [Google Scholar] [CrossRef]
- Ribeiro, E.S.; Gomes, G.; Greco, L.F.; Cerri, R.L.A.; Vieira-Neto, A.; Monteiro, P.L.J., Jr.; Lima, F.S.; Bisinotto, R.S.; Thatcher, W.W.; Santos, J.E.P. Carryover effect of postpartum inflammatory diseases on developmental biology and fertility in lactating dairy cows. J. Dairy Sci. 2016, 99, 2201–2220. [Google Scholar] [CrossRef]
- Funeshima, N.; Miura, R.; Katoh, T.; Yaginuma, H.; Kitou, T.; Yoshimura, I.; Shirasuna, K. Metabolomic profiles of plasma and uterine luminal fluids from healthy and repeat breeder Holstein cows. BMC Vet. Res. 2021, 17, 54. [Google Scholar] [CrossRef]
- Alberti, A.; Bolognini, L.; Macciantelli, D.; Caratelli, M. The radical cation of N, N-diethyl-para-phenylendiamine: A possible indicator of oxidative stress in biological samples. Res. Chem. Intermed. 2000, 26, 253–267. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Celi, P.; Merlo, M.; Barbato, O.; Gabai, G. Relationship between oxidative stress and the success of artificial insemination in dairy cows in a pasture-based system. Vet. J. 2012, 193, 498–502. [Google Scholar] [CrossRef] [PubMed]
- Preutthipan, S.; Chen, S.H.; Tilly, J.L.; Kugu, K.; Lareu, R.R.; Dharmarajan, A.M. Inhibition of nitric oxide synthesis potentiates apoptosis in the rabbit corpus luteum. Reprod. Biomed. Online 2004, 9, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Bruinjé, T.C.; Morrison, E.I.; Ribeiro, E.S.; Renaud, D.L.; LeBlanc, S.J. Associations of inflammatory and reproductive tract disorders postpartum with pregnancy and early pregnancy loss in dairy cows. J. Dairy Sci. 2024, 107, 1630–1644. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid. Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef]
- Takasaki, A.; Tamura, H.; Taniguchi, K.; Asada, H.; Taketani, T.; Matsuoka, A.; Yamagata, Y.; Shimamura, K.; Morioka, H.; Sugino, N. Luteal blood flow and luteal function. J. Ovarian Res. 2009, 2, 1. [Google Scholar] [CrossRef]
- Ledee-Bataille, N.; Olivennes, F.; Lefaix, J.L.; Chaouat, G.; Frydman, R.; Delanian, S. Combined treatment by pentoxifylline and tocopherol for recipient women with a thin endometrium enrolled in an oocyte donation programme. Hum. Reprod. 2002, 17, 1249–1253. [Google Scholar] [CrossRef]
- Burton, G.J.; Hempstock, J.; Jauniaux, E. Oxygen, early embryonic metabolism and free radical-mediated embryopathies. Reprod. Biomed. Online 2003, 6, 84–96. [Google Scholar] [CrossRef]
- Taru, M.; Katoh, T.; Koshimizu, K.; Kuribayashi, S.; Miura, R.; Hamano, S.; Shirasuna, K. Inflammatory uterine microenvironment in long-term infertility repeat breeder cows compared with normal fertile cows. Vet. Anim. Sci. 2024, 25, 100369. [Google Scholar] [CrossRef]
- Zhang, B.F.; Jiang, H.; Chen, J.; Guo, X.; Li, Y.; Hu, Q.; Yang, S. Nobiletin ameliorates myocardial ischemia and reperfusion injury by attenuating endoplasmic reticulum stress-associated apoptosis through regulation of the PI3K/AKT signal pathway. Int. Immunopharmacol. 2019, 73, 98–107. [Google Scholar] [CrossRef]
- Malik, S.; Bhatia, J.; Suchal, K.; Gamad, N.; Dinda, A.K.; Gupta, Y.K.; Arya, D.S. Nobiletin ameliorates cisplatin-induced acute kidney injury due to its anti-oxidant, anti-inflammatory and anti-apoptotic effects. Exp. Toxicol. Pathol. 2015, 67, 427–433. [Google Scholar] [CrossRef] [PubMed]
| D7 Serum | D14 Serum | |||||
|---|---|---|---|---|---|---|
| Pregnant (n = 9) | Nonpregnant (n = 8) | p | Pregnant (n = 9) | Nonpregnant (n = 8) | p | |
| d-ROMs | 110.2 ± 17.1 | 108.1 ± 22.9 | 0.83 | 120.0 ± 24.3 | 117.3 ± 15.2 | 0.79 |
| BAP | 3548.6 ± 771.9 | 3703.4 ± 590.8 | 0.65 | 3847.8 ± 781.8 | 4029.5 ± 474.3 | 0.58 |
| OSI | 3.2 ± 0.8 | 2.9 ± 0.6 | 0.41 | 3.2 ± 0.6 | 2.9 ± 0.4 | 0.36 |
| D7 uterine fluid | D14 uterine fluid | |||||
| Pregnant (n = 9) | Nonpregnant (n = 8) | p | Pregnant (n = 9) | Nonpregnant (n = 8) | p | |
| d-ROMs | 27.3 ± 3.2 | 28.1 ± 2.0 | 0.55 | 27.8 ± 2.8 | 27.1 ± 0.8 | 0.53 |
| BAP | 948.7 ± 105.5 | 856.1 ± 70.7 | 0.05 | 936.1 ± 131.1 | 931.3 ± 153.2 | 0.95 |
| OSI | 2.9 ± 0.4 | 3.3 ± 0.4 | 0.07 | 3.0 ± 0.7 | 3.0 ± 0.5 | 0.83 |
| D7 Serum | D14 Serum | |||||
|---|---|---|---|---|---|---|
| <120 (n = 10) | ≥120 (n = 7) | p | <120 (n = 10) | ≥120 (n = 7) | p | |
| d-ROMs | 113.3 ± 16.7 | 103.4 ± 22.8 | 0.32 | 125.8 ± 22.0 | 108.6 ± 11.8 | 0.08 |
| BAP | 3592.4 ± 737.9 | 3662.9 ± 631.0 | 0.84 | 4000.6 ± 666.9 | 3837.1 ± 644.4 | 0.62 |
| OSI | 3.3 ± 0.7 | 2.9 ± 0.6 | 0.25 | 3.2 ± 0.6 | 2.9 ± 0.4 | 0.23 |
| D7 uterine fluid | D14 uterine fluid | |||||
| <120 (n = 10) | ≥120 (n = 7) | p | <120 (n = 10) | ≥120 (n = 7) | p | |
| d-ROMs | 27.0 ± 3.1 | 28.7 ± 1.6 | 0.20 | 27.7 ± 2.6 | 27.1 ± 0.9 | 0.60 |
| BAP | 940.6 ± 102.4 | 854.4 ± 76.8 | 0.08 | 891.2 ± 102.3 | 994.7 ± 165.1 | 0.13 |
| OSI | 2.9 ± 0.4 | 3.4 ± 0.4 | <0.05 | 3.2 ± 0.6 | 2.8 ± 0.5 | 0.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hagita, Y.; Miura, R.; Shirasuna, K.; Ajito, T.; Matsumoto, H. Effects of Oxidative Stress and Antioxidant Activity in Plasma and Uterine Fluid During Early Postpartum on Subsequent Reproductive Performance of Japanese Black Cows. Animals 2025, 15, 767. https://doi.org/10.3390/ani15060767
Hagita Y, Miura R, Shirasuna K, Ajito T, Matsumoto H. Effects of Oxidative Stress and Antioxidant Activity in Plasma and Uterine Fluid During Early Postpartum on Subsequent Reproductive Performance of Japanese Black Cows. Animals. 2025; 15(6):767. https://doi.org/10.3390/ani15060767
Chicago/Turabian StyleHagita, Yujiro, Ryotaro Miura, Koumei Shirasuna, Tadaharu Ajito, and Hirotaka Matsumoto. 2025. "Effects of Oxidative Stress and Antioxidant Activity in Plasma and Uterine Fluid During Early Postpartum on Subsequent Reproductive Performance of Japanese Black Cows" Animals 15, no. 6: 767. https://doi.org/10.3390/ani15060767
APA StyleHagita, Y., Miura, R., Shirasuna, K., Ajito, T., & Matsumoto, H. (2025). Effects of Oxidative Stress and Antioxidant Activity in Plasma and Uterine Fluid During Early Postpartum on Subsequent Reproductive Performance of Japanese Black Cows. Animals, 15(6), 767. https://doi.org/10.3390/ani15060767






