Quill Mites of the Family Syringophilidae (Acariformes: Cheyletoidea) Parasitising Birds of the Subfamily Euphoninae (Passeriformes: Fringillidae)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Mites Collection, Preparation, Description, and Deposition
2.2. Statistics
2.3. Bird Systematics and Zoogeographical Regions
3. Results
3.1. Systematics
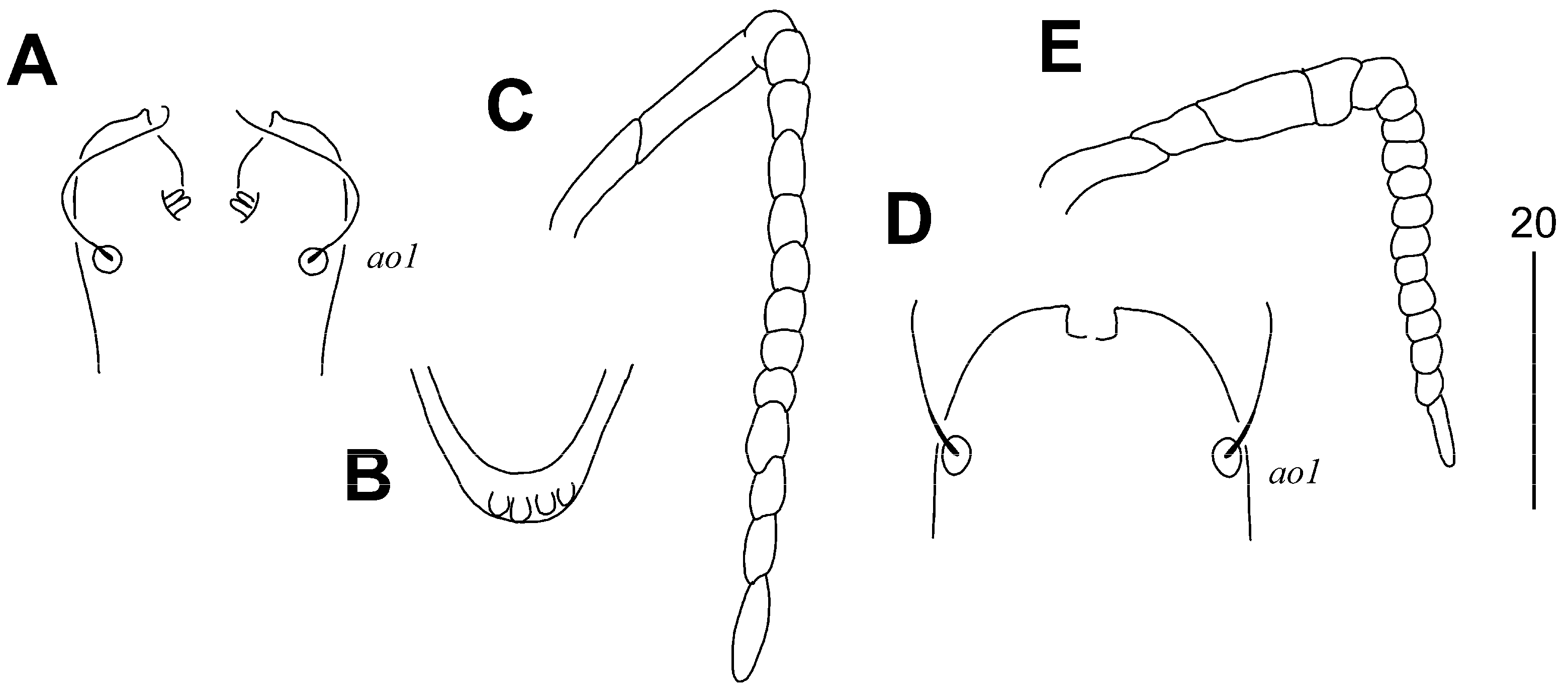
3.1.1. Aulonastus neotropicalis sp. n. (Figure 1 and Figure 2)

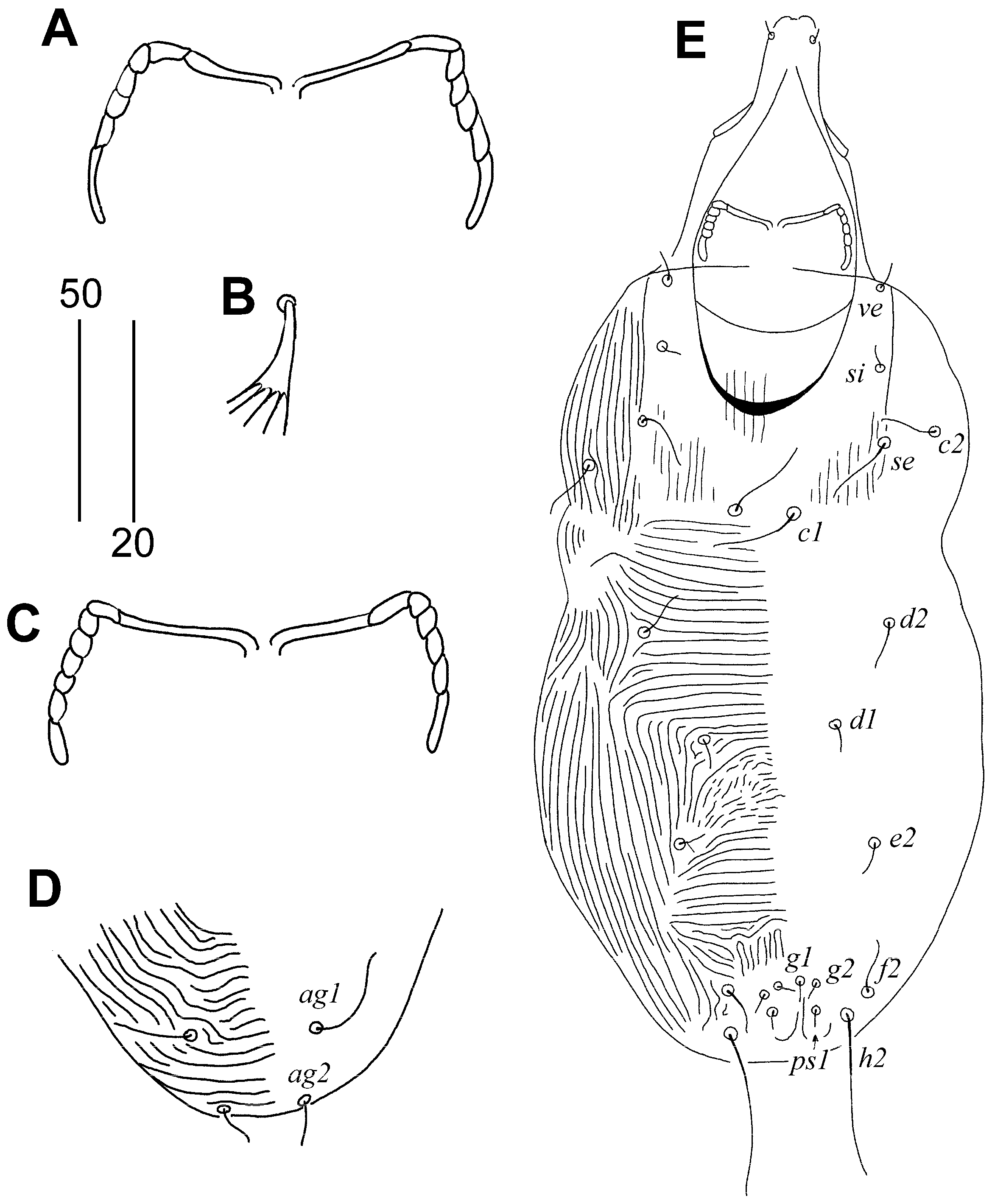
Type Material
Type Material Deposition
Additional Material
Differential Diagnosis
Etymology
3.1.2. Syringophilopsis euphonicus sp. n. (Figure 3, Figure 4 and Figure 5)
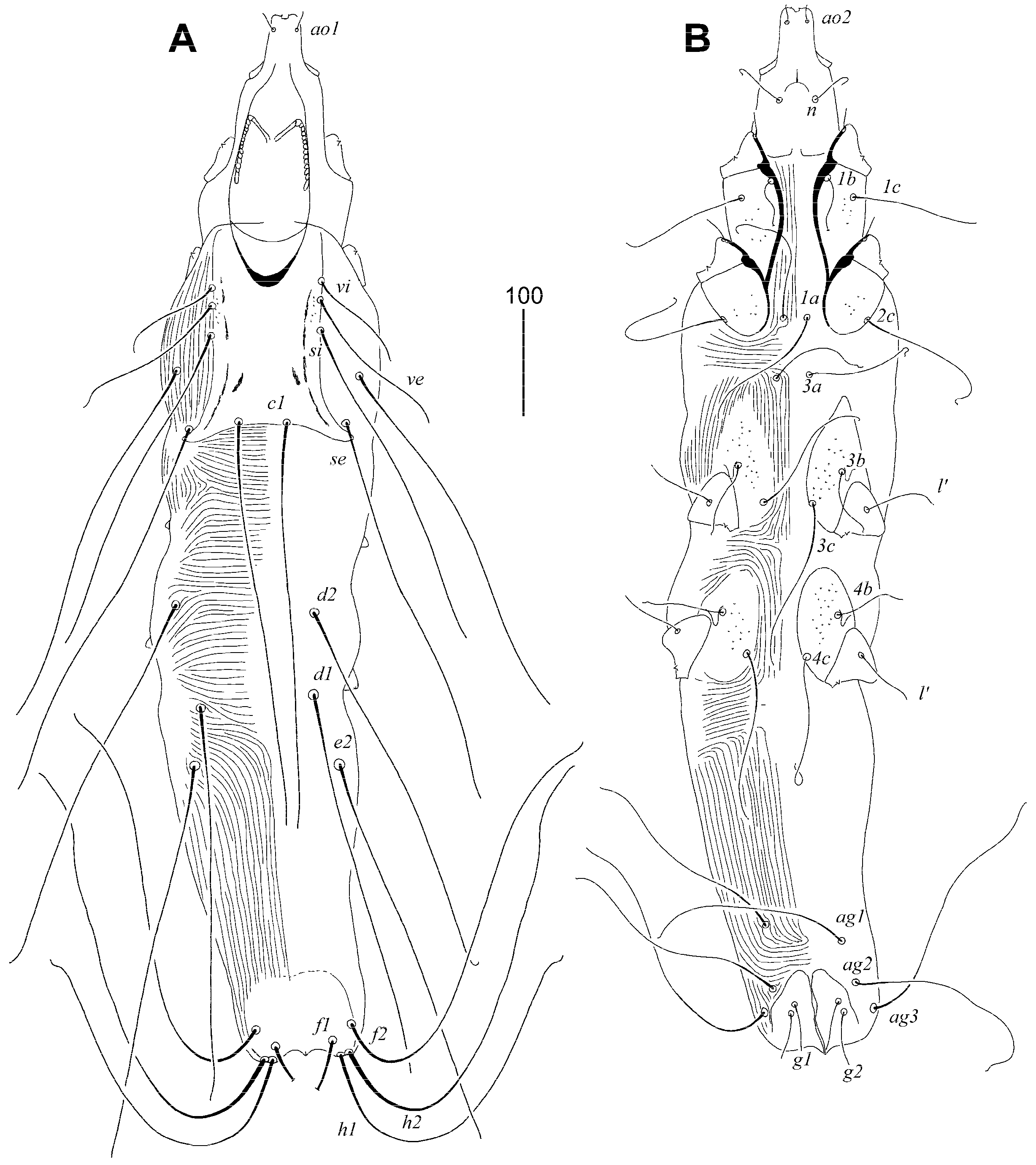
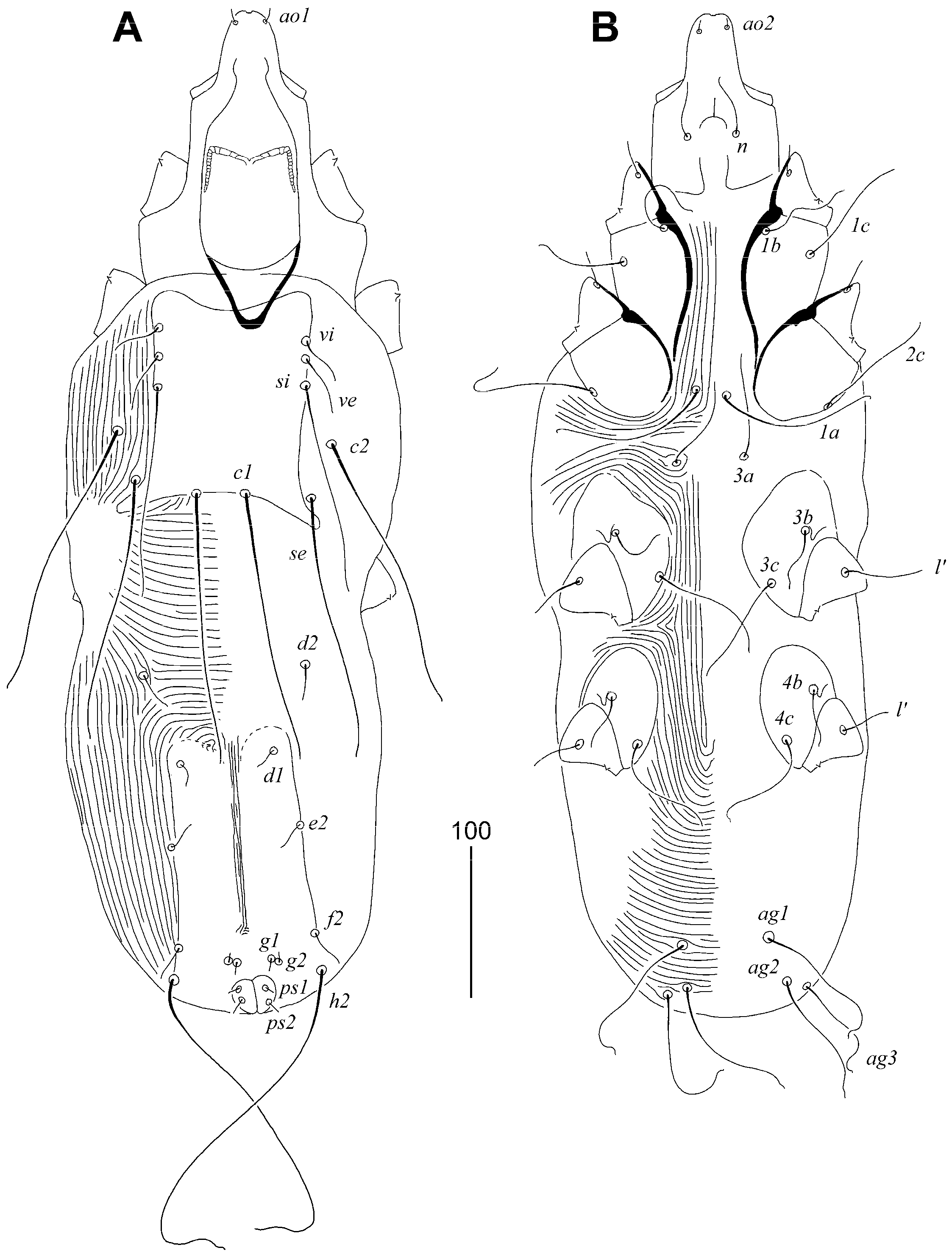
Type Material
Type Material Deposition
Additional Material
Differential Diagnosis
Etymology
3.1.3. Syringophiloidus stawarczyki Skoracki, 2004
New Material Examined
3.1.4. Picobia chloris Bochkov, Mironov & Kravtsova, 2000
New Material Examined
3.2. Prevalence, Host Specificity and Bipartite Network Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Winkler, D.W.; Billerman, S.M.; Lovette, I.J. Finches, Euphonias, and Allies (Fringillidae). In Birds of the World; Version 1.0; Billerman, S.M., Keeney, B.K., Rodewald, P.G., Schulenberg, T.S., Eds.; Cornell Lab of Ornithology: Ithaca, NY, USA, 2020. [Google Scholar] [CrossRef]
- Zuccon, D.; Prys-Jones, R.; Rasmussen, P.C.; Ericson, P.G.P. The phylogenetic relationships and generic limits of finches (Fringillidae). Mol. Phylogenet. Evol. 2012, 62, 581–596. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-López, M.; Ramírez-Barrera, S.M.; Terrones-Ramírez, A.K.; Robles-Bello, S.M.; Nieto-Montes de Oca, A.; Ruegg, K.; Hernández-Baños, B.E. Biogeographic factors contributing to the diversification of Euphoniinae (Aves, Passeriformes, Fringillidae): A phylogenetic and ancestral areas analysis. ZooKeys 2024, 1188, 169–195. [Google Scholar] [CrossRef] [PubMed]
- Chesser, R.T.; Billerman, S.M.; Burns, K.J.; Cicero, C.; Dunn, J.L.; Hernández-Baños, B.E.; Kratter, A.W.; Lovette, I.J.; Mason, N.M.; Rasmussen, P.C.; et al. Sixty-second Supplement to the American Ornithological Society’s Check-list of North American Birds. Ornithology 2021, 138, ukab037. [Google Scholar] [CrossRef]
- Kethley, J.B. A revision of the family Syringophilidae (Prostigmata: Acarina). Contrib. Am. Entomol. Inst. 1970, 5, 1–76. [Google Scholar]
- Skoracki, M. Quill mites (Acari: Syringophilidae) of the Palaearctic region. Zootaxa 2011, 2840, 1–414. [Google Scholar] [CrossRef]
- Glowska, E.; Chrzanowski, M.; Kaszewska, K. Checklist of the quill mites (Acariformes: Syringophilidae) of the World. Zootaxa 2015, 3968, 1–81. [Google Scholar] [CrossRef]
- Zmudzinski, M.; Skoracki, M.; Sikora, B. An Updated Checklist of Quill Mites of the Family Syringophilidae (Acariformes: Prostigmata). 2023. Available online: https://figshare.com/articles/dataset/An_updated_checklist_of_quill_mites_of_the_family_Syringophilidae_Acariformes_Prostigmata_/16529574 (accessed on 26 January 2025).
- Sikora, B.; Unsoeld, M.; Melzer, R.R.; Friedrich, S.; Hromada, M. Revealing the Complexity of Host-Parasite Relationships Between Syringophilid Mites and Sunbirds in Their Global Range. Animals 2025, 15, 110. [Google Scholar] [CrossRef]
- Marcisova, I.; Skoracki, M.; Patan, M.; Hromada, M.; Sikora, B. Quill Mites of the Subfamily Syringophilinae (Acariformes: Syringophilidae) Parasitising Starlings (Passeriformes: Sturnidae). Animals 2024, 14, 2239. [Google Scholar] [CrossRef]
- Sikora, B.; Kosicki, J.Z.; Patan, M.; Marcisova, I.; Hromada, M.; Skoracki, M. Diversity and Interactions between Picobiine Mites and Starlings. Animals 2024, 14, 2517. [Google Scholar] [CrossRef]
- Marciniak-Musial, N.; Skoracki, M.; Kosicki, J.Z.; Unsöld, M.; Sikora, B. Host-parasite relationships of quill mites (Syringophilidae) and parrots (Psittaciformes). Diversity 2023, 15, 1. [Google Scholar] [CrossRef]
- Kaszewska-Gilas, K.; Kosicki, J.Z.; Hromada, M.; Skoracki, M. Global Studies of the Host-Parasite Relationships between Ectoparasitic Mites of the Family Syringophilidae and Birds of the Order Columbiformes. Animals 2021, 11, 3392. [Google Scholar] [CrossRef] [PubMed]
- Skoracki, M. A review of quill mites of the genus Syringophiloidus Kethley, 1970 (Acari: Prostigmata: Syringophilidae) parasitising quills of passeriform birds, with descriptions of four new species. Genus 2004, 15, 281–300. [Google Scholar]
- Walter, D.E.; Krantz, G.W. Collecting, rearing, and preparing specimens. In A Manual of Acarology; Krantz, G.W., Walter, D.E., Eds.; Texas Tech University Press: Lubbock, TX, USA, 2009; pp. 83–96. [Google Scholar]
- Grandjean, F. Les segments post-larvaires de l’hystérosoma chez les Oribates (Acariens). Bull. Soc. Zool. Fr. 1939, 64, 273–284. [Google Scholar]
- Kethley, J.B. Acarina: Prostigmata (Actinedida). In Soil Biology Guide; Dindal, D.L., Ed.; John Wiley & Sons: New York, NY, USA, 1990; pp. 667–756. [Google Scholar]
- Grandjean, F. Observations sur les Acariens de la famille des Stigmaeidae. Arch. Sci. Phys. Nat. 1944, 26, 103–131. [Google Scholar]
- Reiczigel, J.; Rozsa, L.; Reiczigel, A.; Fabian, I. Quantitative Parasitology (QPweb). 2021. Available online: http://www.zoologia.hu/qp/qp.html (accessed on 25 January 2025).
- Rozsa, L.; Reiczigel, J.; Majoros, G. Quantifying parasites in samples of hosts. J. Parasitol. 2000, 86, 228–232. [Google Scholar] [CrossRef]
- Reiczigel, J.; Marozzi, M.; Fábián, I.; Rózsa, L. Biostatistics for parasitologists—A primer to Quantitative Parasitology. Trends Parasitol. 2019, 35, 277–281. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 25 January 2025).
- Dormann, C.F.; Fründ, J.; Blüthgen, N.; Gruber, B. Indices, graphs and null models: Analysing bipartite ecological networks. Open Ecol. J. 2009, 2, 7–24. [Google Scholar] [CrossRef]
- Clements, J.F.; Rasmussen, P.C.; Schulenberg, T.S.; Iliff, M.J.; Fredericks, T.A.; Gerbracht, J.A.; Lepage, D.; Spencer, A.; Billerman, S.M.; Sullivan, B.L.; et al. The eBird/Clements Checklist of Birds of the World. 2024. Available online: https://www.birds.cornell.edu/clementschecklist/download/ (accessed on 25 January 2025).
- IUCN 2025. The IUCN Red List of Threatened Species. Version 2024-2. Available online: https://www.iucnredlist.org (accessed on 25 January 2025).
- Holt, B.G.; Lessard, J.P.; Borregaard, M.K.; Fritz, S.A.; Araújo, M.B.; Dimitrov, D.; Fabre, P.H.; Graham, C.H.; Graves, G.R.; Jønsson, K.A.; et al. An update of Wallace’s zoogeographic regions of the World. Science 2013, 339, 74–78. [Google Scholar] [CrossRef]
- Ficetola, G.; Mazel, F.; Thuiller, W. Global determinants of zoogeographical boundaries. Nat. Ecol. Evol. 2017, 1, 89. [Google Scholar] [CrossRef]
- Zmudzinski, M.; Unsoeld, M. Quill mites (Acariformes: Syringophilidae) parasitising birds in Germany: New host records and descriptions of two new species from Limosa lapponica (L.) (Aves: Scolopacidae). Syst. Appl. Acarol. 2019, 24, 362–376. [Google Scholar] [CrossRef]
- Bochkov, A.V.; Mironov, S.V.; Kravtsova, N.T. Two new syringophilid mites (Acari: Syringophilidae) from the Greenfinch Carduelis chloris (Passeriformes: Fringillidae) from Kirghizia. Genus 2000, 11, 351–358. [Google Scholar]
- Fritsch, W. Die milbengattung Syringophilus Heller, 1880 (subordo Trombidiformes, Fam. Myobiidae Megnin, 1877). Zool. Jahrb. Syst. 1958, 86, 227–234. [Google Scholar]
- Bochkov, A.V.; Mironov, S.V. Quill mites of the family Syringophilidae Lavoipierre, 1953 (Acariformes: Prostigmata) parasitic on birds (Aves) of the fauna of the former USSR. Acarina 1998, 6, 3–16. [Google Scholar]
- Glowska, E.; Skoracki, M.; Khourly, F. A new species and new records of syringophilid mites (Acari: Prostigmata: Syringophilidae) from birds of Jordan. Zootaxa 2007, 1635, 63–68. [Google Scholar] [CrossRef]
- Skoracki, M. Quill mites of the genus Syringophilopsis (Acari, Syringophilidae) from passeriform birds of Poland with descriptions of five new species. Acta Parasitol. 2004, 49, 45–62. [Google Scholar]
- Bochkov, A.V.; Apanaskevich, D. Two new species of the family Syringophilidae (Acari: Cheyletoidea) from passeriform birds collected in the Altai. Folia Parasitol. 2001, 48, 321–325. [Google Scholar] [CrossRef]
- Bochkov, A.V.; Fain, A.; Skoracki, M. New quill mites of the family Syringophilidae (Acari: Cheyletoidea). Syst. Parasitol. 2004, 57, 135–150. [Google Scholar] [CrossRef]
- Skoracki, M.; Bochkov, A.V. Quill mites from Kazakhstan. Zootaxa 2010, 2546, 52–68. [Google Scholar]
- Skoracki, M.; Hendricks, S.; Spicer, G.S. Systematics of the ectoparasitic quill mites of the genus Aulobia Kethley, 1970 (Acari: Syringophilidae) with the description of a new species. Zootaxa 2010, 2399, 31–41. [Google Scholar] [CrossRef]
- Skoracki, M.; Hendricks, S.; Spicer, G.S. Four new species of Aulonastus Kethley, 1970 (Acari: Syringophilidae) from North American passerines. Syst. Parasitol. 2010, 76, 131–144. [Google Scholar] [CrossRef]
- Skoracki, M.; Sikora, B.; Spicer, G.S. A review of the subfamily Picobiinae Johnston and Kethley, 1973 (Acariformes: Prostigmata: Syringophilidae). Zootaxa 2016, 4113, 1–95. [Google Scholar] [CrossRef] [PubMed]
- Bochkov, A.V.; Mironov, S.V. New quill mite species of the family Syringophilidae (Acari: Cheyletoidea) from the European part of Russia. Acarina 1999, 7, 35–45. [Google Scholar]
- Bochkov, A.V.; Flannery, M.E.; Spicer, G.S. Mites of the Genus Torotrogla (Prostigmata: Syringophilidae) from North American passerines. J. Med. Entomol. 2009, 46, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Imfeld, T.S.; Barker, F.K.; Brumfield, R.T. Mitochondrial genomes and thousands of ultraconserved elements resolve the taxonomy and historical biogeography of the Euphonia and Chlorophonia finches (Passeriformes: Fringillidae). Auk 2020, 137, ukaa016. [Google Scholar] [CrossRef]
- Skoracki, M.; Hromada, M.; Zmudzinski, M.; Unsoeld, M.; Sikora, B. Parasitic quill mites of the family Syringophilidae (Acariformes: Prostigmata) associated with sub-Saharan sunbirds (Passeriformes: Nectariniidae): Species composition and host-parasite relationships. J. Med. Entomol. 2018, 55, 1464–1477. [Google Scholar] [CrossRef]
- Cierocka, K.; Izdebska, J.N.; Rolbiecki, L. The Co-Occurrence of Demodecidae and Psorergatidae (Acariformes: Prostigmata) in the Yellow-Necked Field Mouse Apodemus f lavicollis (Rodentia: Muridae) with a Description of Two New Species and a NewHostRecord. Diversity 2024, 16, 550. [Google Scholar] [CrossRef]
- Skoracki, M.; Zabludovskaya, S.A.; Bochkov, A.V. A review of Prostigmata (Acariformes: Trombidiformes) permanently associated with birds. Acarina 2012, 20, 67–107. [Google Scholar]
- Izdebska, J.N.; Rolbiecki, L. The Biodiversity of Demodecid Mites (Acariformes: Prostigmata), Specific Parasites of Mammals with a Global Checklist and a New Finding for Demodex sciurinus. Diversity 2020, 12, 261. [Google Scholar] [CrossRef]
- Fajfer, M. Acari (Chelicerata)—Parasites of reptiles. Acarina 2012, 20, 108–129. [Google Scholar]
- Deunff, J. Observations on Spinturnicidae of occidental Paleartic region (Acarina, Mesostigmata)—Specificity, distribution and repartition. Acarologia 1977, 18, 602–617. [Google Scholar]
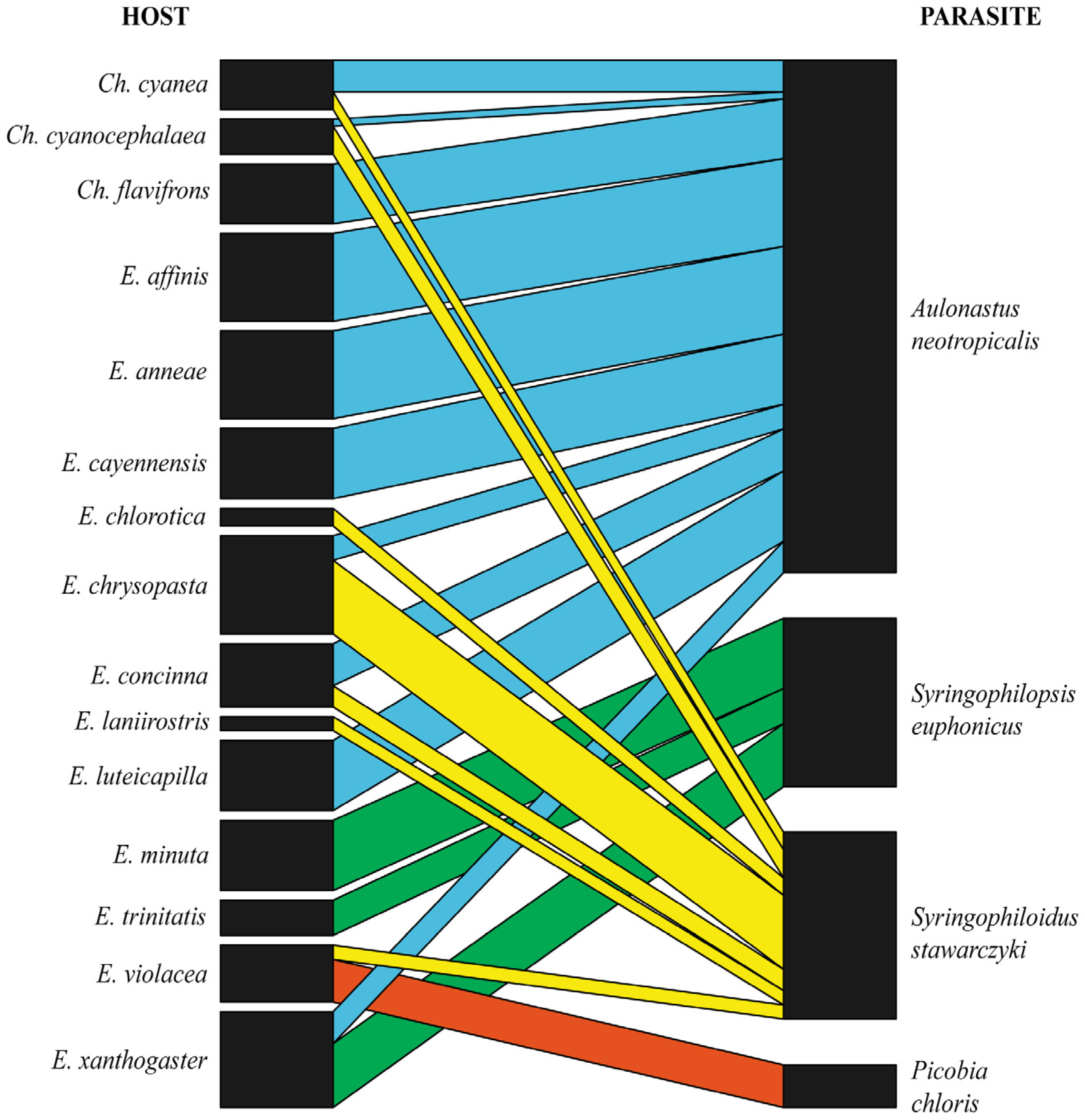
| Host Species and No. of Examined Individuals | No of Infested | Prevalence and CI(Sterne method) | Mite Species | Locality |
|---|---|---|---|---|
| Chlorophonia cyanea (44) | 4 | 9% (2.5–21.7) | Aulonastus neotropicalis | Bolivia, Venezuela |
| “ | 2 | 5% (0.6–15.5) | Syringophiloidus stawarczyki | Brazil, Colombia |
| Chlorophonia cyanocephala (40) | 1 | 2% (0.1–13.2) | Aulonastus neotropicalis | Colombia |
| “ | 3 | 8% (1.6–20.4) | Syringophiloidus stawarczyki | Colombia, Paraguay |
| Chlorophonia flavifrons (6) | 1 | 17% (0.4–64.1) | Aulonastus neotropicalis | Guadeloupe |
| Euphonia affinis (8) | 2 | 25% (3.2–65.1) | Aulonastus neotropicalis | Colombia |
| Euphonia concinna (16) | 2 | 12% (1.6–38.3) | Aulonastus neotropicalis | Colombia |
| “ | 1 | 6% (0.2–30.2) | Syringophiloidus stawarczyki | Colombia |
| Euphonia luteicapilla (5) | 1 | 20% (0.5–71.6) | Aulonastus neotropicalis | Panama |
| Euphonia anneae (4) | 1 | 25% (0.6–80.6) | Aulonastus neotropicalis | Costa Rica |
| Euphonia xanthogaster (22) | 2 | 9% (1.1–27.6) | Aulonastus neotropicalis + Syringophilopsis euphonicus | Peru |
| “ | 2 | 9% (1.1–27.6) | Syringophilopsis euphonicus | Peru |
| Euphonia chrysopasta (14) | 1 | 7% (0.2–33.9) | Aulonastus neotropicalis | Venezuela |
| “ | 3 | 21% (4.7–50.8) | Syringophiloidus stawarczyki | Bolivia, Venezuela |
| Euphonia cayennensis (5) | 1 | 20% (0.5–71.6) | Aulonastus neotropicalis | French Guiana |
| Euphonia trinitatis (10) | 1 | 10% (0.3–44.5) | Syringophilopsis euphonicus | Venezuela |
| Euphonia minuta (10) | 2 | 20% (2.5–55.6) | Syringophilopsis euphonicus | Colombia |
| Euphonia violacea (26) | 1 | 4% (0.1–19.6) | Syringophiloidus stawarczyki | Trinidad and Tobago |
| “ | 3 | 12% (2.4–30.2) | Picobia chloris | Brazil, French Guiana, Trinidad and Tobago |
| Euphonia laniirostris (25) | 1 | 4% (0.1–20.4) | Syringophiloidus stawarczyki | Venezuela |
| Euphonia chlorotica (19) | 1 | 5% (0.1–26.0) | Syringophiloidus stawarczyki | Paraguay |
| Chlorophonia callophrys (Cabanis), N = 8 | Euphonia gouldi Sclater, N = 1 |
| Chlorophonia occipitalis (Gisignies), N = 2 | Euphonia mesochrysa Salvadori, N = 7 |
| Chlorophonia pyrrhophrys (Sclater), N = 7 | Euphonia pectoralis (Latham), N = 7 |
| Euphonia chalybea (Mikan), N = 4 | Euphonia plumbea Gisignies, N = 1 |
| Euphonia fulvicrissa (Sclater), N = 3 | Euphonia rufiventris (Vieillot), N = 4 |
| Mite Genus | Euphoninae | Fringillinae | Carduelinae |
|---|---|---|---|
| Aulobia | - | - | 2/7 (distribution: Holarctic) |
| Aulonastus | 1/10 (Neotropical) | 1/1 (Palaearctic) | 1/2 (Palaearctic) |
| Syringophiloidus | 1/8 (Neotropical) | - | 4/5 (Palaearctic, Afrotropical) |
| Syringophilopsis | 1/3 (Neotropical) | 1/2 (Palaearctic) | 1/5 (Palaearctic, Afrotropical) |
| Torotrogla | - | 1/2 (Palaearctic) | 3/9 (Holarctic) |
| Neopicobia | - | - | 2/2 (Holarctic) |
| Picobia | 1/1 (Neotropical) | - | 1/1 (Palaearctic) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sikora, B.; Unsoeld, M.; Melzer, R.R.; Friedrich, S.; Hromada, M.; Skoracki, M. Quill Mites of the Family Syringophilidae (Acariformes: Cheyletoidea) Parasitising Birds of the Subfamily Euphoninae (Passeriformes: Fringillidae). Animals 2025, 15, 764. https://doi.org/10.3390/ani15050764
Sikora B, Unsoeld M, Melzer RR, Friedrich S, Hromada M, Skoracki M. Quill Mites of the Family Syringophilidae (Acariformes: Cheyletoidea) Parasitising Birds of the Subfamily Euphoninae (Passeriformes: Fringillidae). Animals. 2025; 15(5):764. https://doi.org/10.3390/ani15050764
Chicago/Turabian StyleSikora, Bozena, Markus Unsoeld, Roland R. Melzer, Stefan Friedrich, Martin Hromada, and Maciej Skoracki. 2025. "Quill Mites of the Family Syringophilidae (Acariformes: Cheyletoidea) Parasitising Birds of the Subfamily Euphoninae (Passeriformes: Fringillidae)" Animals 15, no. 5: 764. https://doi.org/10.3390/ani15050764
APA StyleSikora, B., Unsoeld, M., Melzer, R. R., Friedrich, S., Hromada, M., & Skoracki, M. (2025). Quill Mites of the Family Syringophilidae (Acariformes: Cheyletoidea) Parasitising Birds of the Subfamily Euphoninae (Passeriformes: Fringillidae). Animals, 15(5), 764. https://doi.org/10.3390/ani15050764





