Molasses-Based Block Supplements for Cattle Fed Endophyte-Infected Tall Fescue (Festuca arundinacea) Seed: Effects on Growth Performance, Circulating Biomarkers, Heat Stress, and Coccygeal Artery Diameter
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Cattle Processing and Data Collection
2.3. Diet Preparation and Delivery
2.4. Statistical Analyses
3. Results and Discussion
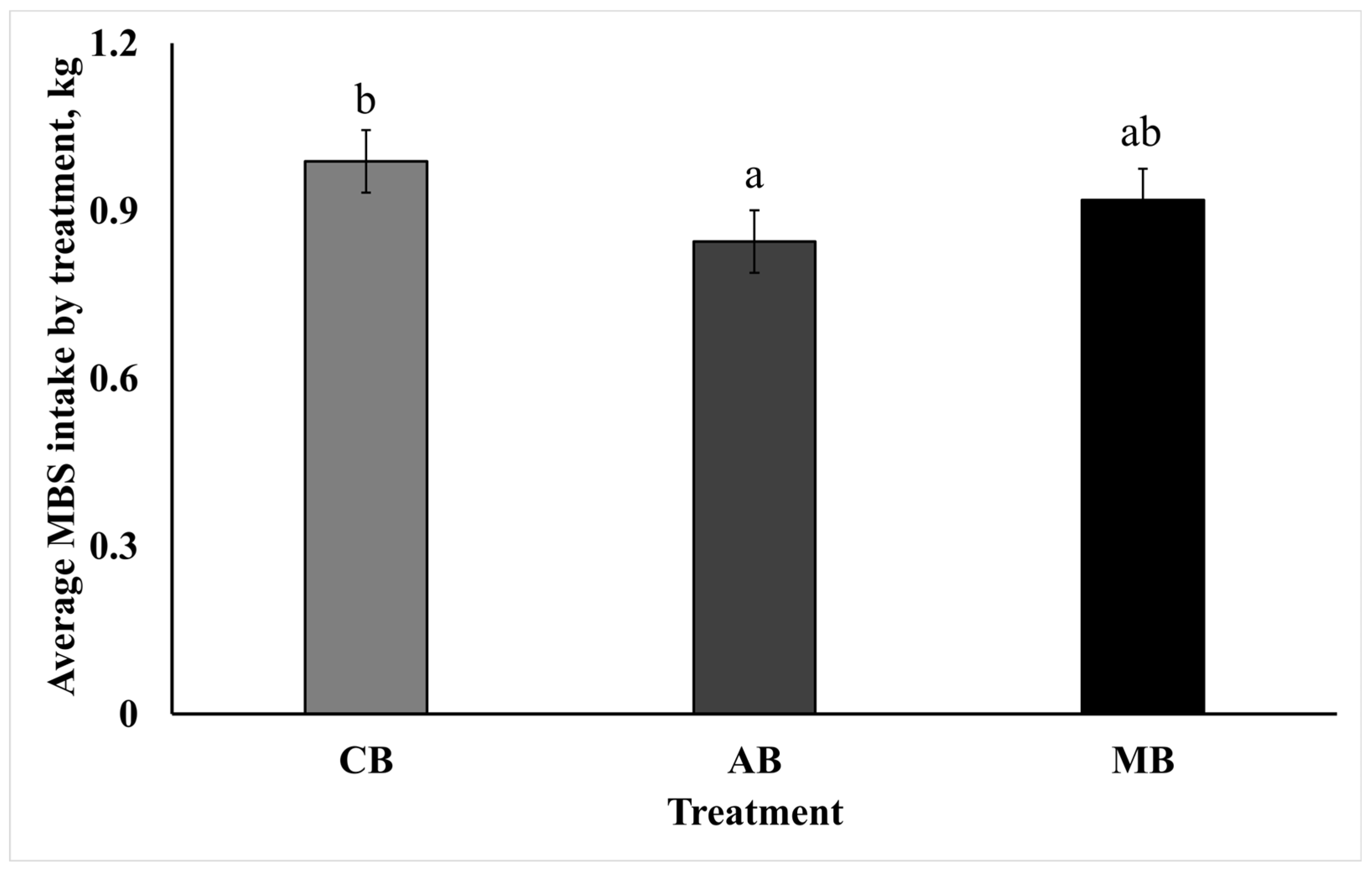
3.1. Molasses-Based Block Intake
3.2. Cattle Growth Performance
3.3. Coccygeal Artery Diameter Imaging
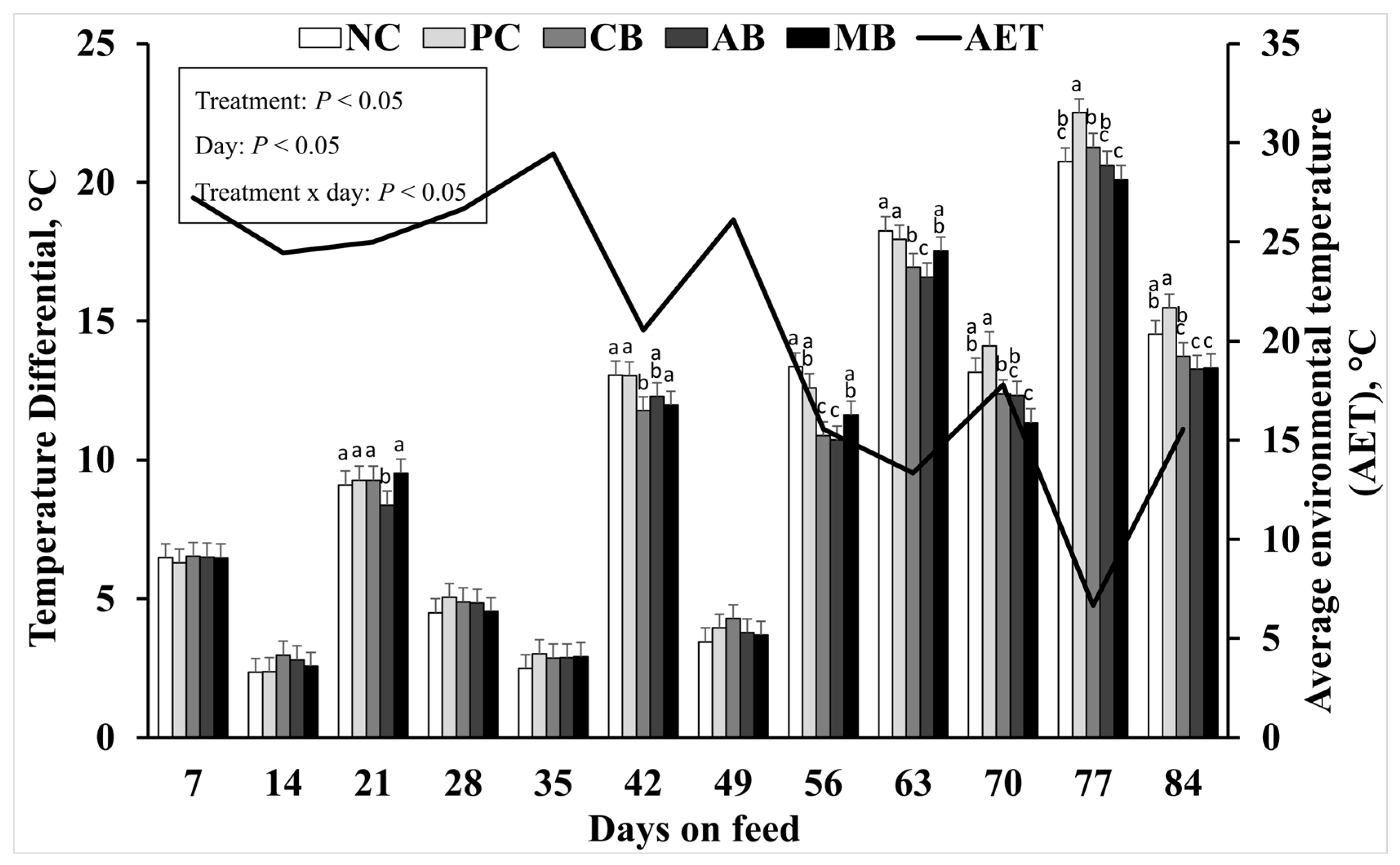
3.4. Thermography
3.5. Plasma Glucose and L-Lactate
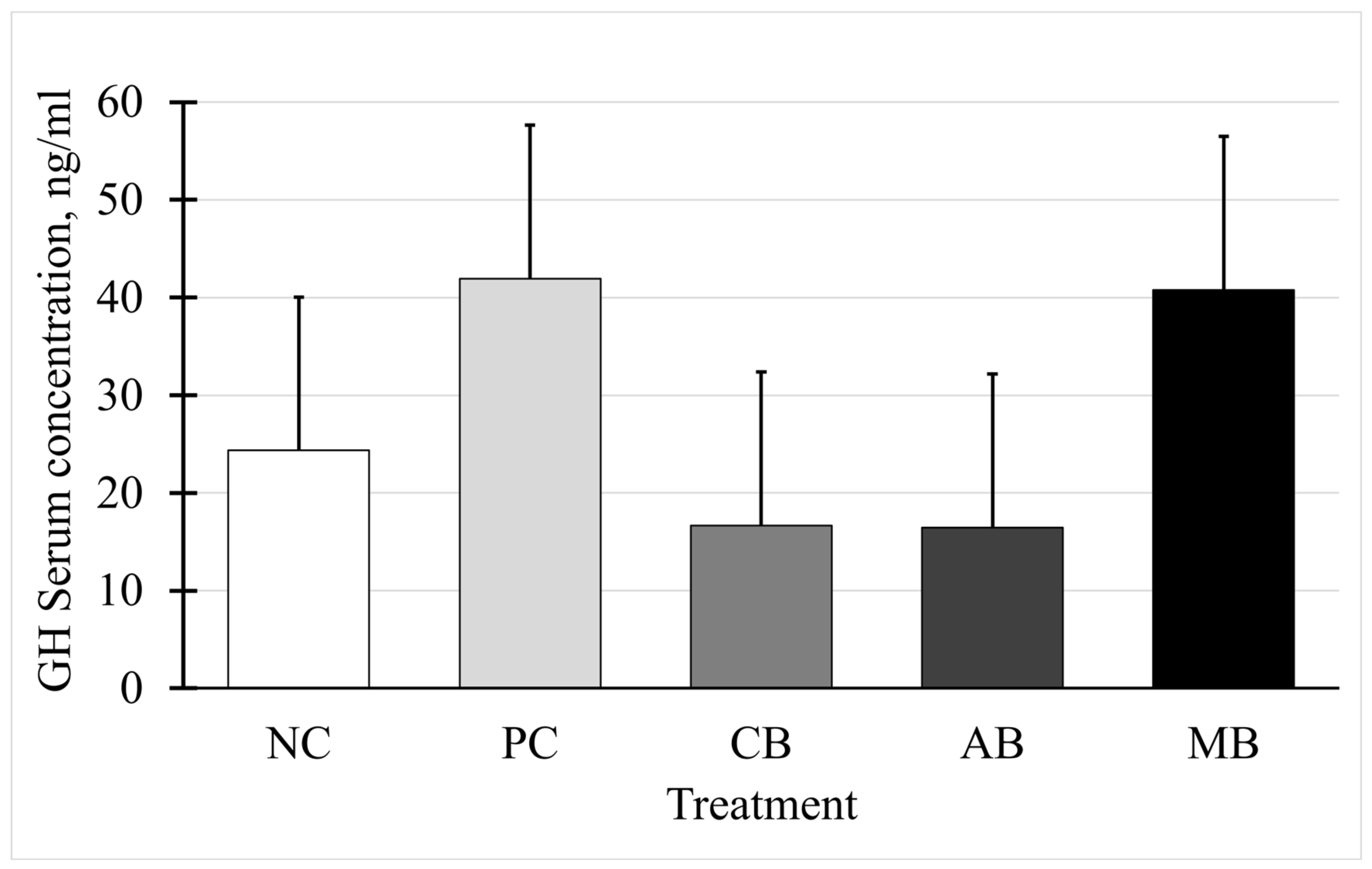
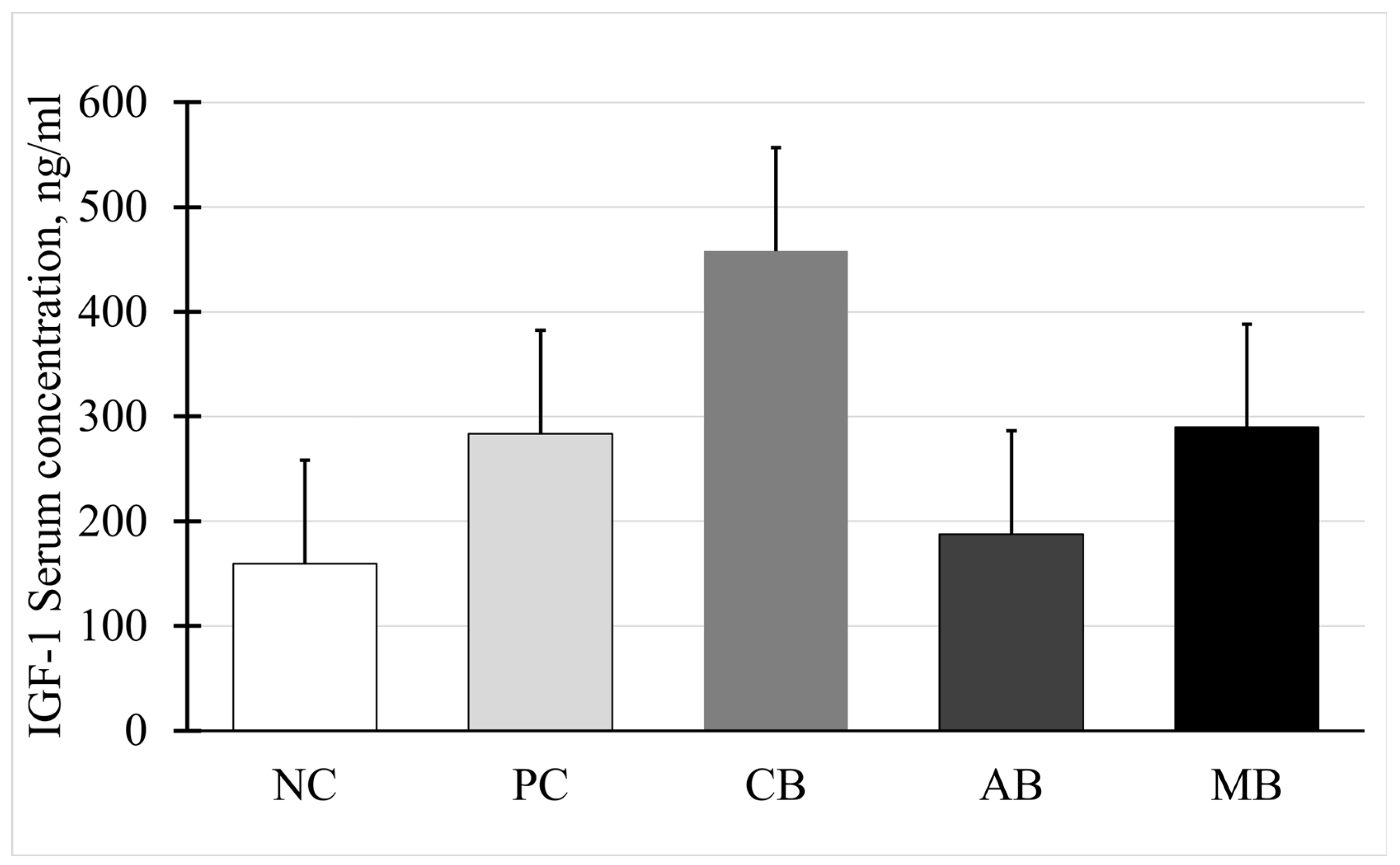
3.6. Somatotropin and Insulin-like Growth Factor I
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AB | 34% protein block containing a proprietary blend of mannan oligosaccharide and capsaicin |
| ADG | Average daily gain |
| AET | Average environmental temperature (°C) |
| BW | Body weight |
| CAD | Coccygeal artery diameter |
| CB | 34% protein block |
| CFDU | Color-flow Doppler ultrasound imaging |
| CV | Coefficient of variation |
| DE | Temperature of the distal ear |
| DM | Dry matter |
| DMI | Dry matter intake |
| DOF | Days on feed |
| eNOS | Endothelium nitric oxide synthase |
| G:F | Gain to feed |
| GH-1 | Bovine somatotropin |
| IGF-1 | Bovine insulin-like growth factor–1 |
| MB | 34% protein block containing 0.3% crystalline menthol |
| MBS | Cooked molasses-based block supplements |
| NC | Negative control |
| NO | Nitric oxide |
| OC | Temperature of ocular conjunctiva |
| PC | Positive control |
| PPB | Parts per billion |
| TFS | Tall fescue seed |
| TRP | Transient receptor potential channel class |
| TRPM8 | Transient receptor potential cation channel subfamily Melastatin-related member 8 |
| TRPV1 | Transient receptor potential channel class subfamily identified as vanilloid 1 |
| VFA | Volatile fatty acids |
| ΔT | Temperature differential |
References
- Klotz, J.L. Activities and effects of ergot alkaloids on livestock physiology and production. Toxins 2015, 7, 2801–2821. [Google Scholar] [CrossRef] [PubMed]
- Klotz, J.L.; Aiken, G.E.; Bussard, J.R.; Foote, A.P.; Harmon, D.L.; Goff, B.M.; Schrick, F.N.; Strickland, J.R. Vasoactivity and vasoconstriction changes in cattle related to time off toxic Endophyte-infected tall Fescue. Toxins 2016, 8, 271. [Google Scholar] [CrossRef] [PubMed]
- Klotz, J.L.; McDowell, K.J. Tall fescue ergot alkaloids are vasoactive in equine vasculature. J. Anim. Sci. 2017, 95, 5151–5160. [Google Scholar] [CrossRef] [PubMed]
- Rumsey, T.S.I.; Stuedemann, J.A.; Wilkinson, S.R.; Williams, D.J. Chemical composition of necrotic fat lesions in beef cows grazing fertilized “Kentucky—31” Tall fescue. J. Anim. Sci. 1979, 48, 673–682. [Google Scholar] [CrossRef]
- Schmidt, S.P.; Osborn, T.G. Effects of endophyte-infected tall fescue on animal performance. Agric. Ecosyst. Environ. 1993, 44, 233–262. [Google Scholar] [CrossRef]
- Walls, J.R. Skin temperature and blood flow in the tail of dairy heifers administered extracts of toxic tall fescue. J. Anim. Sci. 1970, 30, 420–423. [Google Scholar] [CrossRef]
- Kamatou, G.P.P.; Vermaak, I.; Viljoen, A.M.; Lawrence, B.M. Phytochemistry Menthol : A simple monoterpene with remarkable biological properties. Phytochemistry 2013, 96, 15–25. [Google Scholar] [CrossRef]
- Andersen, H.H.; Olsen, R.V.; Møller, H.G.; Eskelund, P.W.; Gazerani, P.; Arendt-nielsen, L. A review of topical high-concentration L-menthol as a translational model of cold allodynia and hyperalgesia. Eur. J. Pain 2014, 18, 315–325. [Google Scholar] [CrossRef]
- Craighead, D.H.; McCartney, N.B.; Tumlinson, J.H.; Alexander, L.M. Mechanisms and time course of menthol-induced cutaneous vasodilation. Microvasc. Res. 2017, 110, 43–47. [Google Scholar] [CrossRef]
- Szolcsányi, J. Effect of capsaicin on thermoregulation: An update with new aspects. Temperature 2015, 2, 277–296. [Google Scholar] [CrossRef]
- Mccarty, M.F.; Dinicolantonio, J.J.; Keefe, J.H.O. Capsaicin may have important potential for promoting vascular and metabolic health. Open Heart 2015, 2, e000262. [Google Scholar] [CrossRef] [PubMed]
- Bollwein, H.; Heppelmann, M.; Lüttgenau, J. Ultrasonographic Doppler Use for Female Reproduction Management. Vet. Clin. North Am.—Food Anim. Pract. 2016, 32, 149–164. [Google Scholar] [CrossRef]
- Beltrame, R.T.; Covre, C.; Littig, L.B.; de Barros Martins, A.; Quirino, C.R.; Junior, A.B.; da Costa, R.L.D. Transrectal Doppler sonography of uterine blood flow in ewes during pregnancy. Theriogenology 2017, 91, 55–61. [Google Scholar] [CrossRef]
- Arashiro, E.K.N.; Ungerfeld, R.; Clariget, R.P.; Pinto, P.H.N.; Balaro, M.F.A.; Bragança, G.M.; Ribeiro, L.S.; da Fonseca, J.F.; Brandão, F.Z. Early pregnancy diagnosis in ewes by subjective assessment of luteal vascularisation using colour Doppler ultrasonography. Theriogenology 2018, 106, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Ciriaco, F.M.; Henry, D.D.; Mercadante, V.R.G.; Schulmeister, T.M.; Ruiz-Moreno, M.; Lamb, G.C.; DiLorenzo, N. Effects of molasses and crude glycerol combined in a liquid supplement on ruminal fermentation in beef steers consuming bermudagrass hay. J. Anim. Sci. 2016, 94, 3851–3863. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, R.H.; Titgemeyer, E.C.; Drouillard, J.S. Effects of base ingredient in cooked molasses blocks on intake and digestion of prairie hay by beef steers. J. Anim. Sci. 2000, 78, 167–172. [Google Scholar] [CrossRef]
- Olson, K.C.; Cochran, R.C.; Jones, T.J.; Vanzant, E.S.; Titgemeyer, E.C.; Johnson, D.E. Effects of ruminal administration of supplemental degradable intake protein and starch on utilization of low-quality warm-season grass hay by beef steers. J. Anim. Sci. 1999, 77, 1016–1025. [Google Scholar] [CrossRef]
- Beaty, J.L.; Cochran, R.C.; Lintzenich, B.A.; Vanzant, E.S.; Morrill, J.L.; Brandt Jr, R.T.; Johnson, D.E. Effect of frequency of supplementation and protein concentration in supplements on performance and digestion characteristics of beef cattle consuming low-quality forages. J. Anim. Sci. 1994, 72, 2475–2486. [Google Scholar] [CrossRef]
- Van Bibber-Krueger, C.L.; Miller, K.A.; Aperce, C.C.; Alvarado-Gilis, C.A.; Higgins, J.J.; Drouillard, J.S. Effects of crystalline menthol on blood metabolites in Holstein steers and in vitro volatile fatty acid and gas production. J. Anim. Sci. 2016, 94, 1170–1178. [Google Scholar] [CrossRef]
- Cardozo, P.W.; Calsamiglia, S.; Ferret, A.; Kamel, C. Effects of alfalfa extract, anise, capsicum, and a mixture of cinnamaldehyde and eugenol on ruminal fermentation and protein degradation in beef heifers fed a high-concentrate diet. J. Anim. Sci. 2006, 84, 2801–2808. [Google Scholar] [CrossRef]
- Rodríguez-Prado, M.; Ferret, A.; Zwieten, J.; Gonzalez, L.; Bravo, D.; Calsamiglia, S. Effects of dietary addition of capsicum extract on intake, water consumption, and rumen fermentation of fattening heifers fed a high-concentrate diet. J. Anim. Sci. 2012, 90, 1879–1884. [Google Scholar] [CrossRef] [PubMed]
- Calixto, J.B.; Beirith, A.; Ferreira, J.; Santos, A.R.S.; Filho, V.C.; Yunes, R.A. Naturally occurring antinociceptive substances from plants. Phytother. Res. 2000, 14, 401–418. [Google Scholar] [CrossRef] [PubMed]
- Melanaphy, D.; Johnson, C.D.; Kustov, M.V.; Watson, C.A.; Borysova, L.; Burdyga, T.V.; Zholos, A.V. Ion channel mechanisms of rat tail artery contraction-relaxation by menthol involving, respectively, TRPM8 activation and L-type Ca2+ channel inhibition. Am. J. Physiol.—Heart Circ. Physiol. 2016, 311, H1416–H1430. [Google Scholar] [CrossRef]
- Fortney, S.M.; Wenger, C.B.; Bove, J.R.; Nadel, E.R. Effect of hyperosmolality on control of blood flow and sweating. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1984, 57, 1688–1695. [Google Scholar] [CrossRef] [PubMed]
- Nose, H.; Kamijo, Y.i.; Masuki, S. Interactions between body fluid homeostasis and thermoregulation in humans. Handb. Clin. Neurol. 2018, 156, 417–429. [Google Scholar] [CrossRef]
- Buczinski, S.; Rademacher, R.D.; Tripp, H.M.; Edmonds, M.; Johnson, E.G.; Dufour, S. Assessment of l-lactatemia as a predictor of respiratory disease recognition and severity in feedlot steers. Prev. Vet. Med. 2015, 118, 306–318. [Google Scholar] [CrossRef]
- Owens, F.N.; Secrist, D.S.; Hill, W.J.; Gill, D.R. Acidosis in Cattle: A Review. J. Anim. Sci. 1998, 76, 275–286. [Google Scholar] [CrossRef]
- Sébédio, J.L.; Pujos-Guillot, E.; Ferrara, M. Metabolomics in evaluation of glucose disorders. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 412–418. [Google Scholar] [CrossRef]
- Muruganathan, U.; Srinivasan, S.; Vinothkumar, V. Antidiabetogenic efficiency of menthol, improves glucose homeostasis and attenuates pancreatic β-cell apoptosis in streptozotocin–nicotinamide induced experimental rats through ameliorating glucose metabolic enzymes. Biomed. Pharmacother. 2017, 92, 229–239. [Google Scholar] [CrossRef]
- Houseknecht, K.L.; Boggs, D.L.; Campion, D.R.; Sartin, J.L.; Kiser, T.E.; Rampacek, G.B.; Amos, H.E. Effect of dietary energy source and level on serum growth hormone, insulin-like growth factor 1, growth and body composition in beef heifers. J. Anim. Sci. 1988, 66, 2916–2923. [Google Scholar] [CrossRef]
- Micke, G.C.; Sullivan, T.M.; Gatford, K.L.; Owens, J.A.; Perry, V.E.A. Nutrient intake in the bovine during early and mid-gestation causes sex-specific changes in progeny plasma IGF-I, liveweight, height and carcass traits. Anim. Reprod. Sci. 2010, 121, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Elsasser, T.H.; Rumsey, T.S.; Hammond, A.C. Influence of diet on basal and growth hormone-stimulated plasma concentrations of IGF-I in beef cattle. J. Anim. Sci. 1989, 67, 128–141. [Google Scholar] [CrossRef] [PubMed]
| Item, % | Prairie Hay | TFS † |
|---|---|---|
| Dry matter | 93.09 | 90.46 |
| Crude protein | 5.84 | 12.60 |
| Acid detergent fiber | 47.44 | 17.02 |
| Neutral detergent fiber | 66.52 | 30.12 |
| Calcium | 0.50 | 0.39 |
| Phosphorus | 0.15 | 0.35 |
| Potassium | 1.13 | 0.18 |
| Magnesium | 0.18 | - |
| Item | Concentration, Parts per Billion (ppb) 1 |
|---|---|
| Ergosine | 18,700 |
| Ergotamine | 10,560 |
| Ergocornine | 4560 |
| Ergocryptine | 7600 |
| Ergocristine | 5800 |
| Ergovaline | 2010 |
| Total | 49,230 |
| Block Supplement † | |||
|---|---|---|---|
| Item, % | CB | AB | MB |
| Dry matter | 94.76 | 94.78 | 94.53 |
| Crude protein | 34.21 | 34.44 | 34.35 |
| Crude protein equivalent as non-protein nitrogen | 15.22 | 14.75 | 15.85 |
| Ash | 15.10 | 15.93 | 15.21 |
| Calcium | 1.57 | 1.61 | 1.55 |
| Phosphorus | 1.00 | 0.97 | 0.99 |
| Potassium | 3.19 | 3.13 | 3.07 |
| Treatment † | SEM | p-Value | |||||
|---|---|---|---|---|---|---|---|
| Item | NC | PC | CB | AB | MB | ||
| Initial BW, kg | 286.3 | 288.1 | 288.0 | 286.2 | 287.5 | 6.38 | 0.40 |
| Final BW, kg | 284.9 a | 287.4 a | 326.6 b | 325.9 b | 328.4 b | 9.01 | <0.01 |
| ADG, kg | −0.15 a | −0.03 a | 0.46 b | 0.43 b | 0.60 b | 0.117 | <0.01 |
| DMI, kg/d | 4.98 a | 5.24 a | 7.19 b | 7.05 b | 7.29 b | 0.247 | <0.01 |
| gain/feed | −0.017 a | −0.003 a | 0.029 b | 0.027 b | 0.037 b | 0.009 | <0.01 |
| Treatment † | p-Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| NC | PC | CB | AB | MB | SEM | Trt | Day | Trt × Day | |
| Overall | 0.299 a | 0.317 a | 0.326 b | 0.338 b | 0.329 b | 0.009 | 0.04 | 0.10 | 0.67 |
| Treatment † | p-Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Item | NC | PC | CB | AB | MB | SEM | Trt | Day | Trt × Day |
| D-glucose | |||||||||
| Overall | 61.05 a | 60.90 a | 62.63 ab | 64.72 ab | 66.04 b | 2.437 | 0.04 | 0.91 | 0.27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feitoza, L.F.B.B.; White, B.J.; Drouillard, J.S. Molasses-Based Block Supplements for Cattle Fed Endophyte-Infected Tall Fescue (Festuca arundinacea) Seed: Effects on Growth Performance, Circulating Biomarkers, Heat Stress, and Coccygeal Artery Diameter. Animals 2025, 15, 717. https://doi.org/10.3390/ani15050717
Feitoza LFBB, White BJ, Drouillard JS. Molasses-Based Block Supplements for Cattle Fed Endophyte-Infected Tall Fescue (Festuca arundinacea) Seed: Effects on Growth Performance, Circulating Biomarkers, Heat Stress, and Coccygeal Artery Diameter. Animals. 2025; 15(5):717. https://doi.org/10.3390/ani15050717
Chicago/Turabian StyleFeitoza, Luis F. B. B., Brad J. White, and James S. Drouillard. 2025. "Molasses-Based Block Supplements for Cattle Fed Endophyte-Infected Tall Fescue (Festuca arundinacea) Seed: Effects on Growth Performance, Circulating Biomarkers, Heat Stress, and Coccygeal Artery Diameter" Animals 15, no. 5: 717. https://doi.org/10.3390/ani15050717
APA StyleFeitoza, L. F. B. B., White, B. J., & Drouillard, J. S. (2025). Molasses-Based Block Supplements for Cattle Fed Endophyte-Infected Tall Fescue (Festuca arundinacea) Seed: Effects on Growth Performance, Circulating Biomarkers, Heat Stress, and Coccygeal Artery Diameter. Animals, 15(5), 717. https://doi.org/10.3390/ani15050717







