Role of Vanin-1 Gene Methylation in Fat Synthesis in Goose Liver: Effects of Betaine and 5-Azacytidine Treatments
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Approval and Consent to Participate
2.2. Experimental Design and Feeding Management
2.3. Sample Collection
2.4. Liver Slices
2.5. Serum Biochemical Indexes
2.6. DNA Methylation-Related Enzyme Activity
2.7. Bisulfite Sequencing PCR (BSP)
2.8. RNA Extraction and Real-Time Polymerase Chain Reaction
2.9. Data Analysis
3. Results
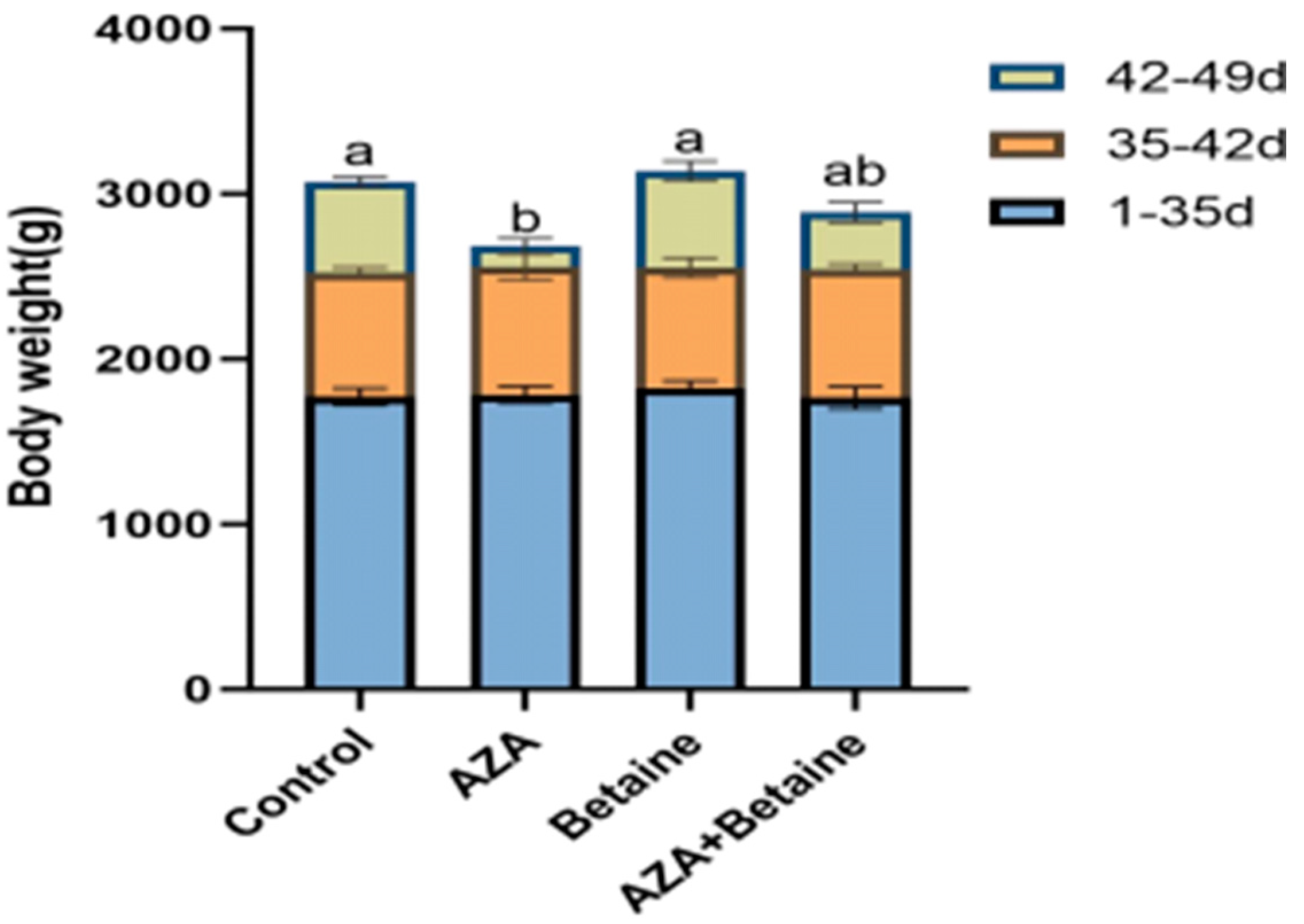
3.1. Effects of Different Treatments on Body Weight of Geese
3.2. Effects of Different Treatments on Serum TG, TC, HDL, and LDL of Geese
3.3. Effects of Different Treatments on DNA Methylation-Related Enzyme Activities in Liver of Geese
3.4. Effects of Different Treatments on Fat Morphology of Goose Liver
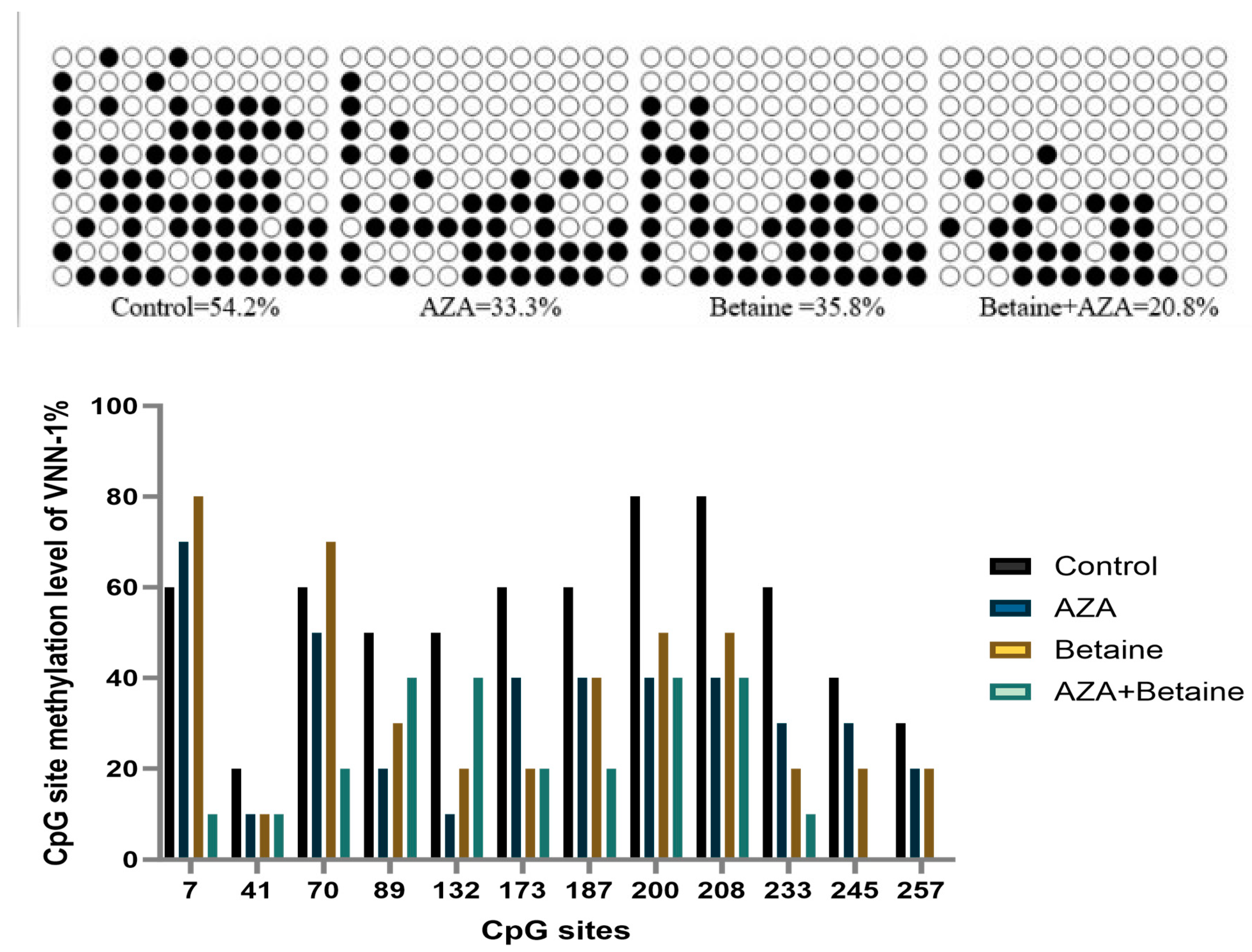
3.5. Effects of Different Treatments on DNA Methylation Level (in VNN1 Promoter Region) of Goose Liver
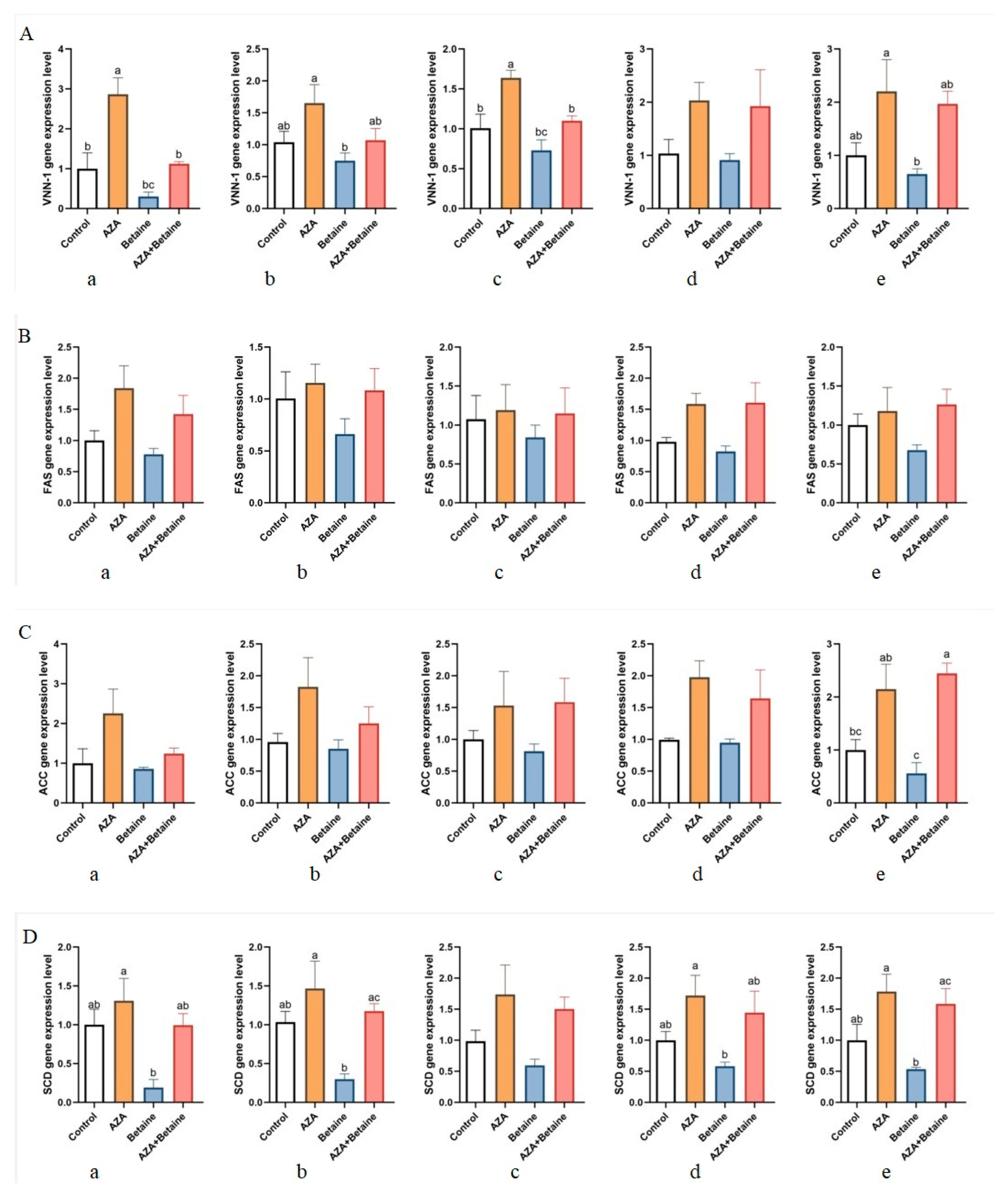
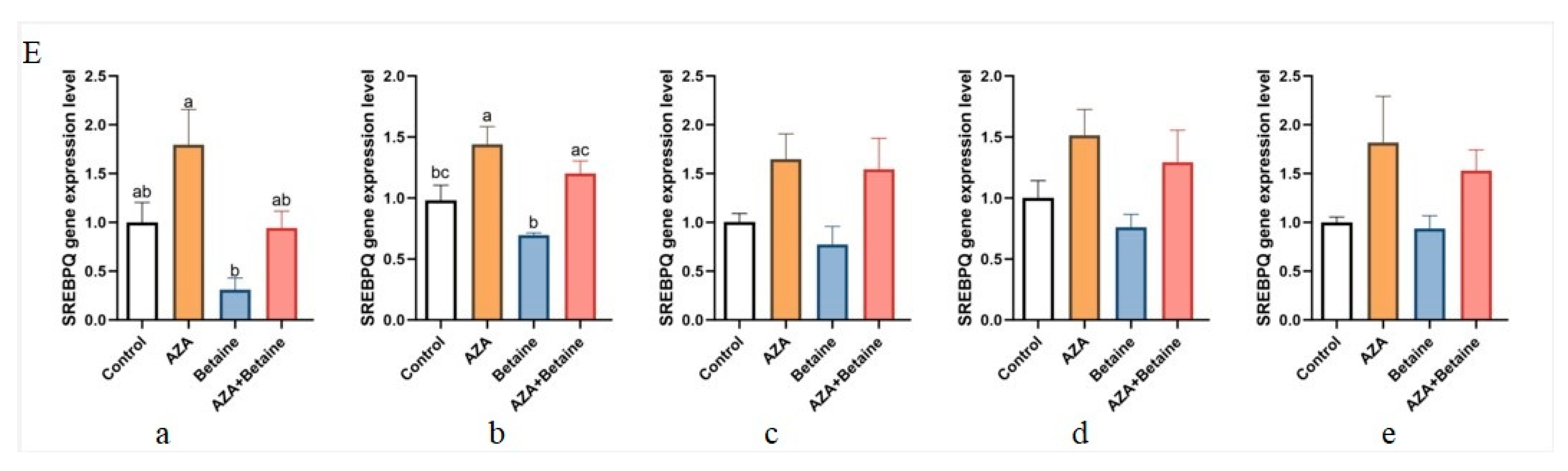
3.6. Effects of Different Treatments on the Expression of VNN1, ACC, FAS, SCD, and SREBPQ Genes in Different Organs of Geese
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Suzuki, S.; Kobayashi, M.; Murai, A.; Tsudzuki, M.; Ishikawa, A. Characterization of growth, fat deposition, and lipid metabolism-related gene expression in lean and obese meat-type chickens. J. Poult. Sci. 2019, 56, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wang, Z.; Song, Q.; Dong, B.; Bi, Y.; Bai, H.; Jiang, Y.; Chang, G.; Chen, G. Transcriptome sequencing to identify important genes and lncrnas regulating abdominal fat deposition in ducks. Animals 2022, 12, 1256. [Google Scholar] [CrossRef] [PubMed]
- Duprè, S.; Graziani, M.T.; Rosei, M.A.; Fabi, A.; Grosso, E.D. The enzymatic breakdown of pantethine to pantothenic acid and cystamine. Eur. J. Biochem. 1970, 16, 571–578. [Google Scholar] [CrossRef]
- Naquet, P.; Pitari, G.; Duprè, S. Role of the Vnn1 pantetheinase in tissue tolerance to stress. Biochem. Soc. Trans. 2014, 42, 1094–1100. [Google Scholar] [CrossRef] [PubMed]
- van Diepen, J.A.; Jansen, P.A.; Ballak, D.B.; Hijmans, A.; Hooiveld, G.J.; Rommelaere, S.; Galland, F.; Naquet, P.; Rutjes, F.P.; Mensink, R.P.; et al. PPAR-alpha dependent regulation of vanin-1 mediates hepatic lipid metabolism. J. Hepatol. 2014, 61, 366–372. [Google Scholar] [CrossRef]
- Naquet, P.; Kerr, E.W.; Vickers, S.D. Regulation of coenzyme A levels by degradation: The ‘Ins and Outs’. Prog. Lipid Res. 2020, 78, 101028. [Google Scholar] [CrossRef]
- Motomura, W.; Yoshizaki, T.; Takahashi, N.; Kumei, S.; Mizukami, Y.; Jang, S.-J.; Kohgo, Y. Analysis of vanin-1 upregulation and lipid accumulation in hepatocytes in response to a high-fat diet and free fatty acids. J. Clin. Biochem. Nutr. 2012, 51, 163–169. [Google Scholar] [CrossRef]
- Belinda, J.K.; Proffit, J.M.; John, B.; Eric, K.M.; Lawrence, J.A. Diverse biological activities of the vascular non-inflammatory molecules—The Vanin pantetheinases. Biochem. Biophys. Res. Commun. 2012, 417, 653–658. [Google Scholar]
- Yu, C.; Ji, S.-Y.; Dang, Y.-J.; Sha, Q.-Q.; Yuan, Y.-F.; Zhou, J.-J.; Yan, L.-Y.; Qiao, J.; Tang, F.; Fan, H.-Y. Oocyte-expressed yes-associated protein is a key activator of the early zygotic genome in mouse. Cell Res. 2016, 26, 275–287. [Google Scholar] [CrossRef]
- Rommelaere, S.; Millet, V.; Gensollen, T.; Bourges, C.; Eeckhoute, J.; Hennuyer, N.; Baugé, E.; Chasson, L.; Cacciatore, I.; Staels, B.; et al. PPARalpha regulates the production of serum Vanin-1 by liver. FEBS Lett. 2013, 587, 3742–3748. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, J.; Hua, N.; Li, J.; Xu, L.; Yao, W.; Gu, Z. Regulation of chicken vanin1 gene expression by peroxisome proliferators activated receptor α and miRNA-181a-5p. Anim. Biosci. 2021, 34, 172–184. [Google Scholar] [CrossRef]
- Wang, S.; Zha, L.; Cui, X.; Yeh, Y.T.; Liu, R.; Jing, J.; Shi, H.; Chen, W.; Hanover, J.; Yin, J.; et al. Epigenetic regulation of hepatic lipid metabolism by dna methylation. Adv. Sci. 2023, 10, 2206068. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, R.; Perfilyev, A.; Männistö, V.; Ågren, J.; Nilsson, E.; Käkelä, P.; Ling, C.; de Mello, V.D.; Pihlajamäki, J. Liver saturated fat content associates with hepatic DNA methylation in obese individuals. Clin. Epigenetics 2023, 15, 21. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Sosa-Montes, E.; Yang, Y.; Yang, J.J.; Yang, H.M.; Wang, Z.Y. Dietary supplementation of betaine promotes lipolysis by regulating fatty acid metabolism in geese. Poult. Sci. 2021, 100, 10460. [Google Scholar] [CrossRef]
- Šorm, F.; Pískala, A.; Čihák, A.; Veselý, J. 5-Azacytidine, a new, highly effective cancerostatic. Experientia 1964, 20, 202–203. [Google Scholar] [CrossRef]
- Christman, J.K. 5-Azacytidine and 5-aza-2′-deoxycytidine as inhibitors of DNA methylation: Mechanistic studies and their implications for cancer therapy. Oncogene 2002, 21, 5483–5495. [Google Scholar] [CrossRef] [PubMed]
- Tada-aki, H. Induction of chromosome decondensation, sister-chromatid exchanges and endoreduplications by 5-azacytidine, an inhibitor of DNA methylation. Mutation Res. Lett. 1983, 121, 47–52. [Google Scholar]
- National Research Council. Nutrient Requirements of Poultry, 9th ed.; Natl. Acad. Press: Washington, DC, USA, 1994. [Google Scholar]
- Shi, S.R.; Wang, Z.Y.; Yang, H.M.; Zhang, Y.Y. Nitrogen requirement for maintenance in Yangzhou goslings. Br. Poult. Sci. 2007, 48, 205–208. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Shi, S.R.; Zhou, Q.Y.; Yang, H.M.; Zou, J.M.; Zhang, K.N.; Han, H.M. Response of growing goslings to dietary methionine from 28 to 70 days of age. Br. Poult. Sci. 2010, 51, 118–121. [Google Scholar] [CrossRef]
- Chen, R.; Zhuang, S.; Cheng, Y.; Wen, C.; Zhou, Y. Betaine improves the growth performance and muscle growth of partridge shank broiler chickens via altering myogenic gene expression and insulin-like growth factor-1 signaling pathway. Poult. Sci. 2018, 97, 4297–4305. [Google Scholar] [CrossRef]
- Colwell, M.; Flack, N.; Medida, R.L.; Drown, C.; Faulk, C.; Mauro, L.J. Effects of 5-aza-2′-deoxycytidine on DNA methylation within female mouse reproductive tissues. Res. Sq. 2022. [Google Scholar] [CrossRef]
- He, S.; Zhao, S.L.; Dai, S.J.; Liu, D.Y.; Bokhari, S.G. Effects of dietary betaine on growth performance, fat deposition and serum lipids in broilers subjected to chronic heat stress. Anim. Sci. J. 2015, 86, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.L.; Shen, J.; Yang, X.; Sun, Q. Folic acid reduced triglycerides deposition in primary chicken hepatocytes. J. Agric. Food Chem. 2018, 66, 13162–13172. [Google Scholar] [CrossRef]
- Leng, Z.X.; Fu, Q.Y.; Xue, Y.; Ding, L.R.; Cui, W.; Zhou, Y.M. Increased fatty acid β-oxidation as a possible mechanism for fat-reducing effect of betaine in broilers. Anim. Sci. J. 2016, 87, 1005–1010. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.N. Maternal Betaine Alleviates Glucocorticoid-Induced Non Alcoholic Fatty Liver Disease in Offspring Rats and Its Mechanism. Ph.D. Thesis, Nanjing Agricultural University, Nanjing, China, 2018. [Google Scholar]
- Ito, S.; D’Alessio, A.C.; Taranova, O.; Hong, K.; Sowers, L.C.; Zhang, Y. Role of Tet proteins in 5mC to 5hmC conversion, ES-cell self-renewal and inner cell mass specification. Nature 2010, 466, 1129–1133. [Google Scholar] [CrossRef] [PubMed]
- Singhal, N.K.; Sternbach, S.; Fleming, S.; Alkhayer, K.; Shelestak, J.; Popescu, D.; Weaver, A.; Clements, R.; Wasek, B.; Bottiglieri, T.; et al. Betaine restores epigenetic control and supports neuronal mitochondria in the cuprizone mouse model of multiple sclerosis. Epigenetics 2020, 15, 871–886. [Google Scholar] [CrossRef]
- Weickhmann, A.K.; Keller, H.; Wurm, J.P.; Strebitzer, E.; A Juen, M.; Kremser, J.; Weinberg, Z.; Kreutz, C.; Duchardt-Ferner, E.; Wöhnert, J. The structure of the SAM/SAH-binding riboswitch. Nucleic Acids Res. 2019, 47, 2654–2665. [Google Scholar] [CrossRef]
- Dai, X.; Liu, S.; Cheng, L.; Huang, T.; Guo, H.; Wang, D.; Xia, M.; Ling, W.; Xiao, Y. Betaine supplementation attenuates s-adenosylhomocysteine hydrolase-deficiency-accelerated atherosclerosis in apolipoprotein e-deficient mice. Nutrients 2022, 14, 718. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, X.; Wu, W.J.; Wang, X.; Wang, Y. FTO-dependent function of N6-methyladenosine is involved in the hepatoprotective effects of betaine on adolescent mice. J. Physiol. Biochem. 2015, 71, 405–413. [Google Scholar] [CrossRef]
- Alirezaei, M.; Jelodar, G.; Niknam, P.; Ghayemi, Z.; Nazifi, S. Betaine prevents ethanol-induced oxidative stress and reduces total homocysteine in the rat cerebellum. J. Physiol. Biochem. 2011, 67, 605–612. [Google Scholar] [CrossRef]
- Lin, Q.; Ge, X.; Gao, L.; Chen, Y.; Su, T.; Ma, M.; Wang, H.; Chen, C.; Han, B.; Liu, D. Betaine alleviates spermatogenic cells apoptosis of oligoasthenozoospermia rat model by up-regulating methyltransferases and affecting DNA methylation. Phytomedicine 2024, 129, 155713. [Google Scholar] [CrossRef]
- Jüttermann, R.; En, L.; Rudolf, J. Toxicity of 5-aza-2′-deoxycytidine to mammalian cells is mediated primarily by covalent trapping of DNA methyltransferase rather than DNA demethylation. Proc. Natl. Acad. Sci. USA 1994, 91, 11791–11801. [Google Scholar] [CrossRef]
- Yang, Z.; Yang, H.; Gong, D.; Rose, S.; Pirgozliev, V.; Chen, X.; Wang, Z. Transcriptome analysis of hepatic gene expression and DNA methylation in methionine- and betaine-supplemented geese (Anser cygnoides domesticus). Poult. Sci. 2018, 97, 3463–3477. [Google Scholar] [CrossRef] [PubMed]
- Diamantopoulos, P.; Zervakis, K.; Papadopoulou, V.; Iliakis, T.; Kalala, F.; Giannakopoulou, N.; Rougala, N.; Galanopoulos, A.; Bakarakos, P.; Variami, E.; et al. 5-Azacytidine in the treatment of intermediate-2 and high-risk myelodysplastic syndromes and acute myeloid leukemia. a five-year experience with 44 consecutive patients. Anticancer Res. 2015, 35, 5141–5147. [Google Scholar]
- Rosen, M.B.; Chernoff, N. 5-Aza-2′-deoxycytidine-induced cytotoxicity and limb reduction defects in the mouse. Birth Defects Res. 2002, 65, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.-B.; Long, C.-L.; Liu, X.-Q.; Chen, X.-M.; Guo, L.-R.; Xia, Y.-Y.; He, J.-L.; Wang, Y.-X. 5-Aza-2′-deoxycytidine leads to reduced embryo implantation and reduced expression of dna methyltransferases and essential endometrial genes. PLoS ONE 2012, 7, e45364. [Google Scholar] [CrossRef] [PubMed]
- Csáki, L.; Karen, R. Lipins: Multifunctional Lipid Metabolism Proteins. Annu. Rev. Nutr. 2010, 30, 257–272. [Google Scholar] [CrossRef]
- Xing, J.K.; Li, K.; Jiang, Y.L. Effect of dietary betaine supplementation on lipogenesis gene expression and CpG methylation of lipoprotein lipase gene in broilers. Mol. Biol. Rep. 2010, 38, 1975–1981. [Google Scholar] [CrossRef]
- Cordero, P.; Gómez-Úriz, A.M.; Campión, J. Dietary supplementation with methyl donors reduces fatty liver and modifies the fatty acid synthase DNA methylation profile in rats fed an obesogenic diet. Genes Nutr. 2012, 8, 105–113. [Google Scholar] [CrossRef]
- Dahlhoff, C.; Worsch, S.; Sailer, M.; Hummel, B.A.; Fiamoncini, J.; Uebel, K.; Obeid, R.; Scherling, C.; Geisel, J.; Bader, B.L.; et al. Methyl-donor supplementation in obese mice prevents the progression of NAFLD, activates AMPK and decreases acyl-carnitine levels. Mol. Metab. 2014, 3, 565–580. [Google Scholar] [CrossRef]
- Cheng, J.; Chan, C.; Kaplowitz, N. Predominant role of sterol response element binding proteins (SREBP) lipogenic pathways in hepatic steatosis in the murine intragastric ethanol feeding model. J. Hepatol. 2006, 45, 717–724. [Google Scholar]
- Nishizawa, Y.; Ikeda, R.; Yamamoto, M.; Kawahara, K.; Shinsato, Y.; Minami, K.; Nitta, M.; Terazono, H.; Akiyama, S.-I.; Furukawa, T.; et al. 5-Aza-2-deoxycytidine Enhances the Sensitivity of 5-Fluorouracil by Demethylation of the Thymidine Phosphorylase Promoter. Anticancer Res. 2019, 39, 4129–4136. [Google Scholar] [CrossRef] [PubMed]

| Ingredients | % |
|---|---|
| Corn | 62.31 |
| Soybean meal | 29.27 |
| Wheat bran | 2.57 |
| Rice husk | 2.16 |
| Limestone | 0.90 |
| Calcium hydrogen phosphate | 1.27 |
| DL-methionine | 0.19 |
| Lysine | 0.03 |
| Salt | 0.30 |
| Vitamin and trace mineral premix 1 | 1.00 |
| Total | 100.00 |
| Nutritional level 2 | |
| Metabolizable energy (MJ/kg) | 11.55 |
| Crude protein (%) | 18.11 |
| Crude fiber (%) | 4.28 |
| Lysine (%) | 0.97 |
| Methionine (%) | 0.48 |
| Calcium (%) | 0.82 |
| Available phosphorus (%) | 0.38 |
| Gene | Primer Sequence (5′-3′) | Product Size (bp) | Reference |
|---|---|---|---|
| β-actin | F: GAAATCGTGCGTGACATCAA R: GCAGGACTCCATACCCAAGA F: | 198 | XM_066977989.1 |
| VNN1 | GAACTCAAGCACGAAGATGGA A R: CAGGTCTGTGCTCCTACACTTG A F: | 189 | XM-048075766.1 |
| FAS | ATGCTTCAGGAGATGGGTATTG R: CCATCAGTGTTACTCCCAGCA F: TCCAGCAGAACCGCATTGACA | 118 | XM-048050305.1 |
| ACC | C R:GTATGAGCAGGCAGGACTTG GC | 187 | XM-048074039.1 |
| SCD | F: TAACGGCTGGATCTCATCGC R: AGAGAACTTGTGGTGGACGC | 149 | XM-048069234.1 |
| SREBPQ | F: GGTCCGGGCCATGTTGA R: CAGGTTGGTGCGGGTGA | 175 | XM-048066212.1 |
| Groups 2 | Betaine (g/kg) | AZA (mg/kg) | TC (mmol/L) | TG (mmol/L) | HDL (mmol/L) | LDL (mmol/L) |
|---|---|---|---|---|---|---|
| A | 0 | 0 | 4.15 b | 0.48 bc | 1.80 a | 1.42 b |
| B | 0 | 2 | 4.50 a | 0.70 a | 1.29 b | 1.83 a |
| C | 1.2 | 0 | 3.50 c | 0.44 c | 2.00 a | 1.29 b |
| D | 1.2 | 2 | 4.30 bc | 0.61 ab | 1.32 b | 1.59 b |
| Betaine | SEM | 0.10 | 0.03 | 0.09 | 0.06 | |
| 0 | 4.32 a | 0.59 | 1.55 | 1.66 a | ||
| 1.2 | 3.90 b | 0.52 | 1.66 | 1.44 b | ||
| AZA | 0 | 3.82 b | 0.46 b | 1.90 a | 1.36 b | |
| 2 | 4.40 a | 0.66 b | 1.30 b | 1.71 b | ||
| p-value | Betaine | <0.001 | 0.058 | 0.313 | 0.023 | |
| AZA | <0.001 | <0.001 | <0.001 | <0.001 | ||
| Betaine × AZA | 0.008 | 0.385 | 0.478 | 0.431 | ||
| Groups 2 | Betaine (g/kg) | AZA (mg/kg) | VNN1 (pg/mL) | SAM/SAH | DNMT (U/L) |
|---|---|---|---|---|---|
| A | 0 | 0 | 17.93 b | 4.64 b | 109.24 ab |
| B | 0 | 2 | 21.09 a | 3.93 c | 80.86 c |
| C | 1.2 | 0 | 17.04 c | 5.06 a | 115.77 a |
| D | 1.2 | 2 | 19.54 ab | 4.56 b | 105.84 b |
| Betaine | SEM | 0.47 | 0.11 | 3.51 | |
| 0 | 19.51 | 0.86 b | 95.05 b | ||
| 1.2 | 18.29 | 0.96 a | 110.81 a | ||
| AZA | 0 | 17.48 b | 0.97 a | 112.50 a | |
| 2 | 20.32 a | 0.85 b | 93.35 b | ||
| p-value | Betaine | 0.05 | <0.001 | <0.001 | |
| AZA | <0.001 | <0.001 | <0.001 | ||
| Betaine × AZA | 0.562 | 0.260 | <0.001 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Shao, Y.; Yang, Z.; Yang, H.; Wang, Z. Role of Vanin-1 Gene Methylation in Fat Synthesis in Goose Liver: Effects of Betaine and 5-Azacytidine Treatments. Animals 2025, 15, 719. https://doi.org/10.3390/ani15050719
Wang X, Shao Y, Yang Z, Yang H, Wang Z. Role of Vanin-1 Gene Methylation in Fat Synthesis in Goose Liver: Effects of Betaine and 5-Azacytidine Treatments. Animals. 2025; 15(5):719. https://doi.org/10.3390/ani15050719
Chicago/Turabian StyleWang, Xinfang, Yu Shao, Zhi Yang, Haiming Yang, and Zhiyue Wang. 2025. "Role of Vanin-1 Gene Methylation in Fat Synthesis in Goose Liver: Effects of Betaine and 5-Azacytidine Treatments" Animals 15, no. 5: 719. https://doi.org/10.3390/ani15050719
APA StyleWang, X., Shao, Y., Yang, Z., Yang, H., & Wang, Z. (2025). Role of Vanin-1 Gene Methylation in Fat Synthesis in Goose Liver: Effects of Betaine and 5-Azacytidine Treatments. Animals, 15(5), 719. https://doi.org/10.3390/ani15050719







