Mistimed Feeding Disrupts Metabolic Rhythm and Increases Lipid Accumulation of Growing Rabbits in Winter
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Feeding and Sample Collection
2.2. Experimental Environment, Behavior Monitoring, and Rectal Temperature Detection
2.3. Serum Physiological Parameters Analysis
2.4. Lipid Analysis of the Liver
2.4.1. Frozen Sections and Oil Red O Staining of the Liver
2.4.2. Biochemical Detection
2.5. Histology and Electron Microscopy
2.6. RNA Extraction and Quantitative Real-Time Polymerase Chain Reaction Analyses
2.7. Protein Preparation and Western Blot Analyses
2.8. Muscle Fat Content Testing
2.9. Statistical Analysis
3. Results
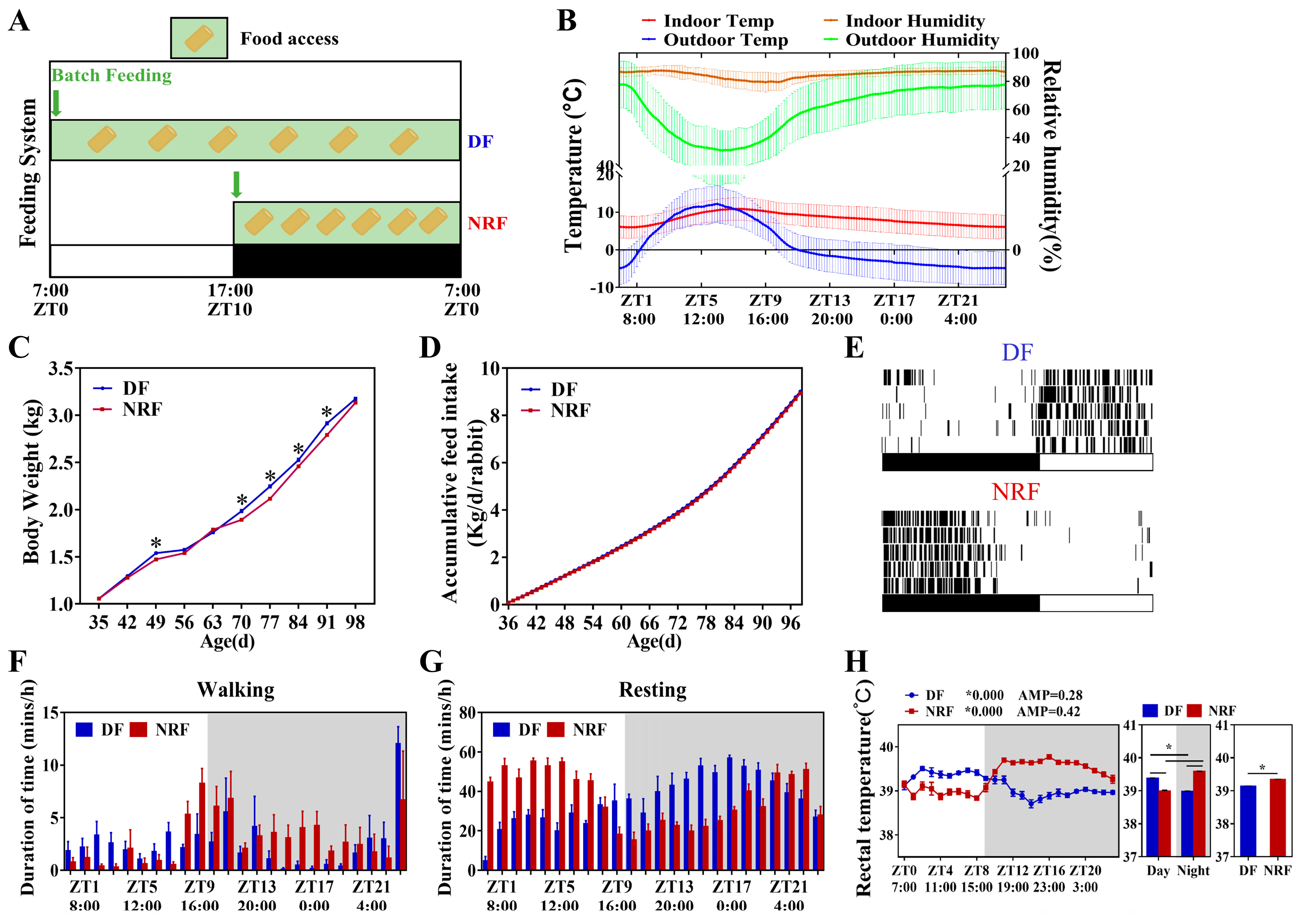
3.1. Daytime Feeding Resets Behavior Rhythm and Increases Weight Gain of Rabbits in Winter
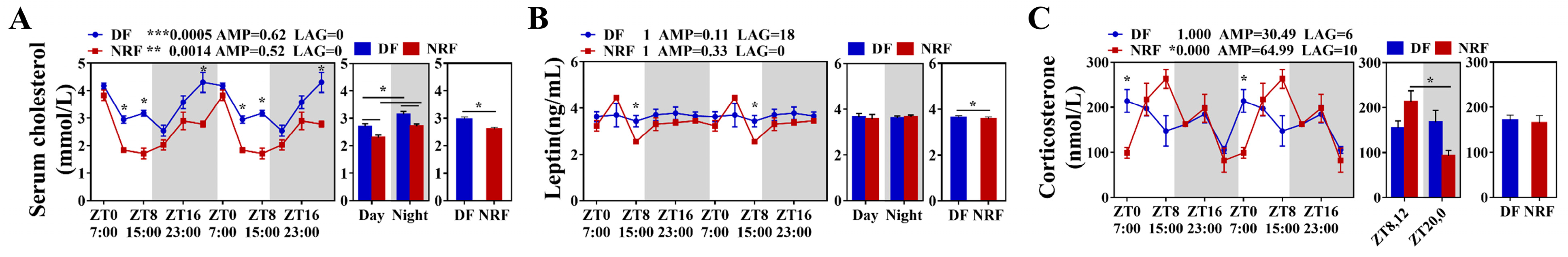
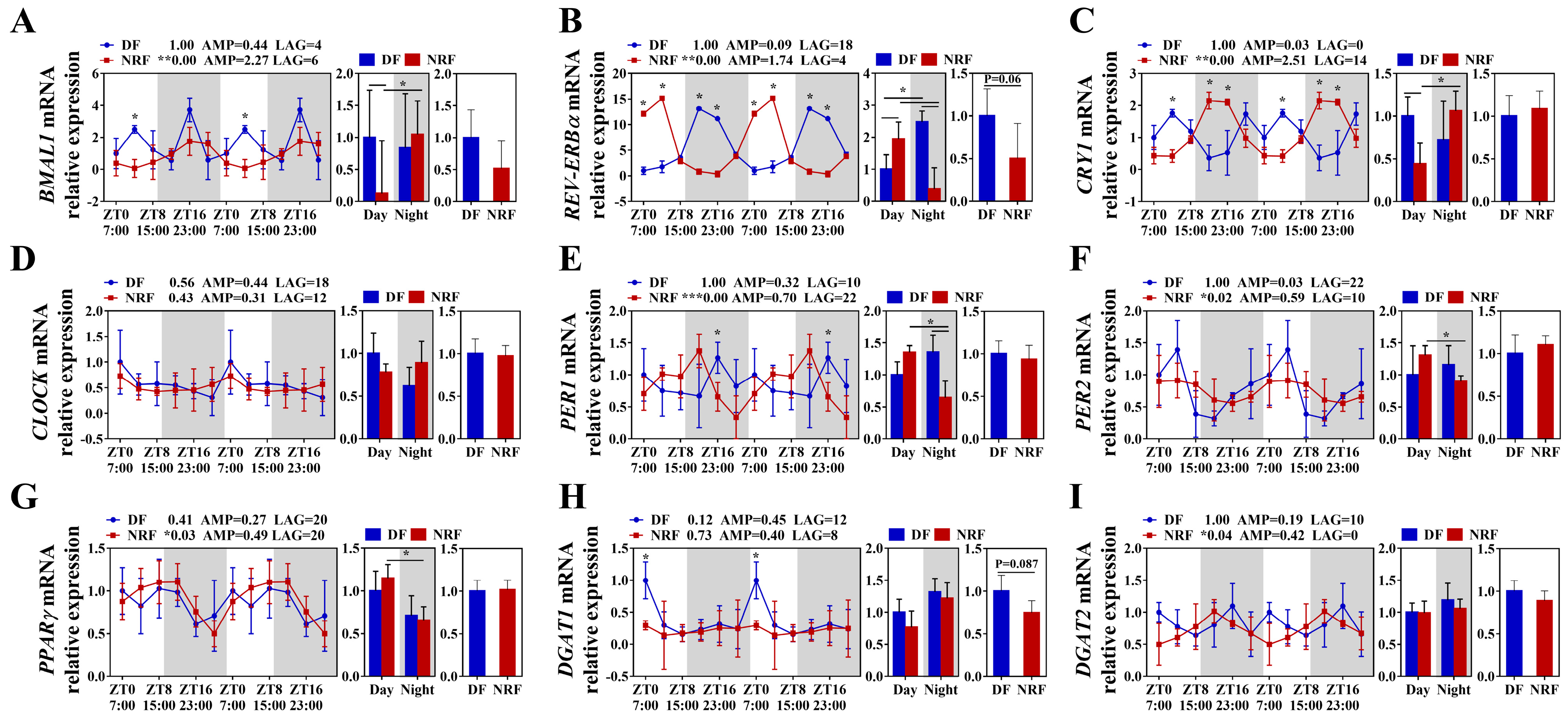
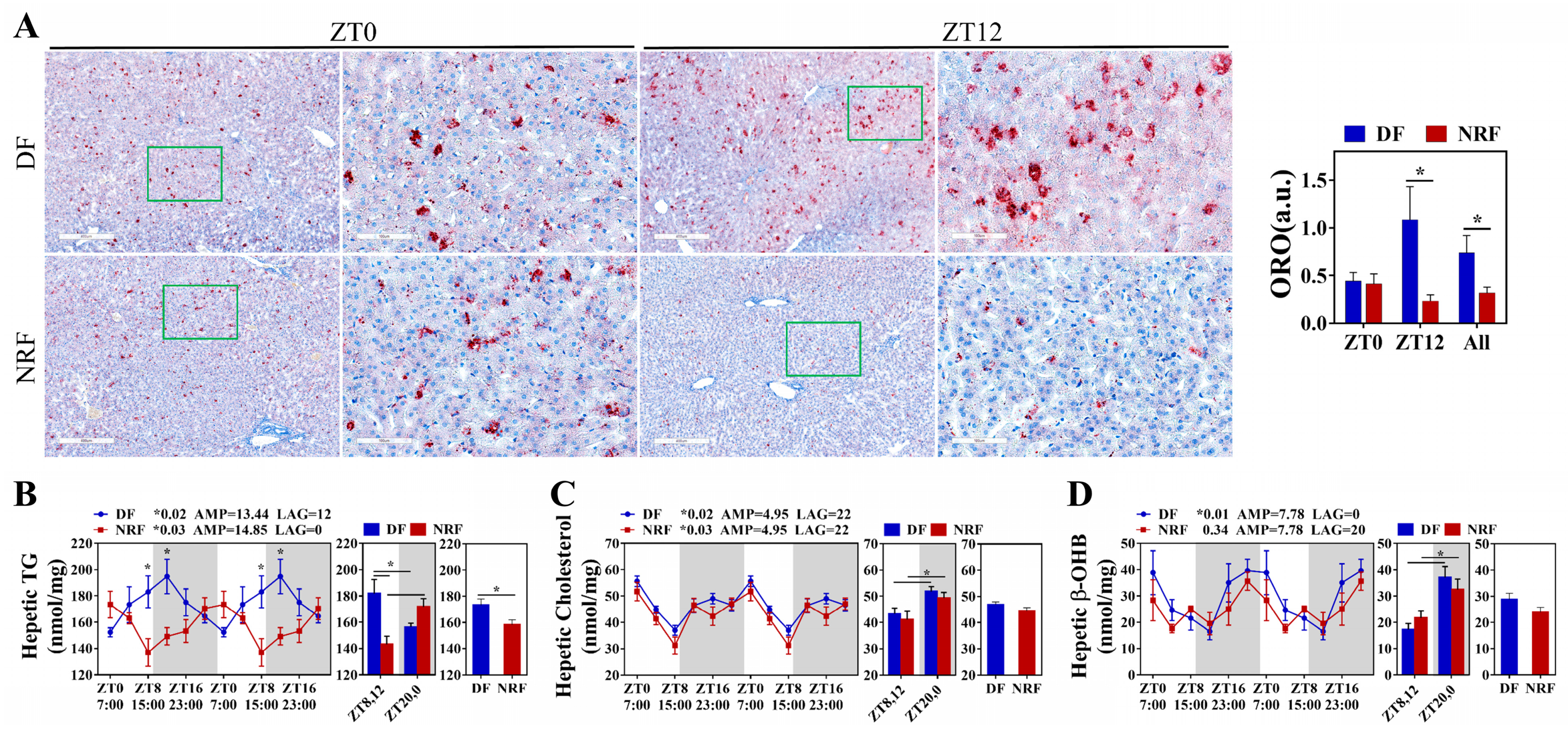
3.2. Daytime Feeding Disrupts Hepatic Lipid Metabolic Rhythms and Promotes Lipid Deposition in Winter
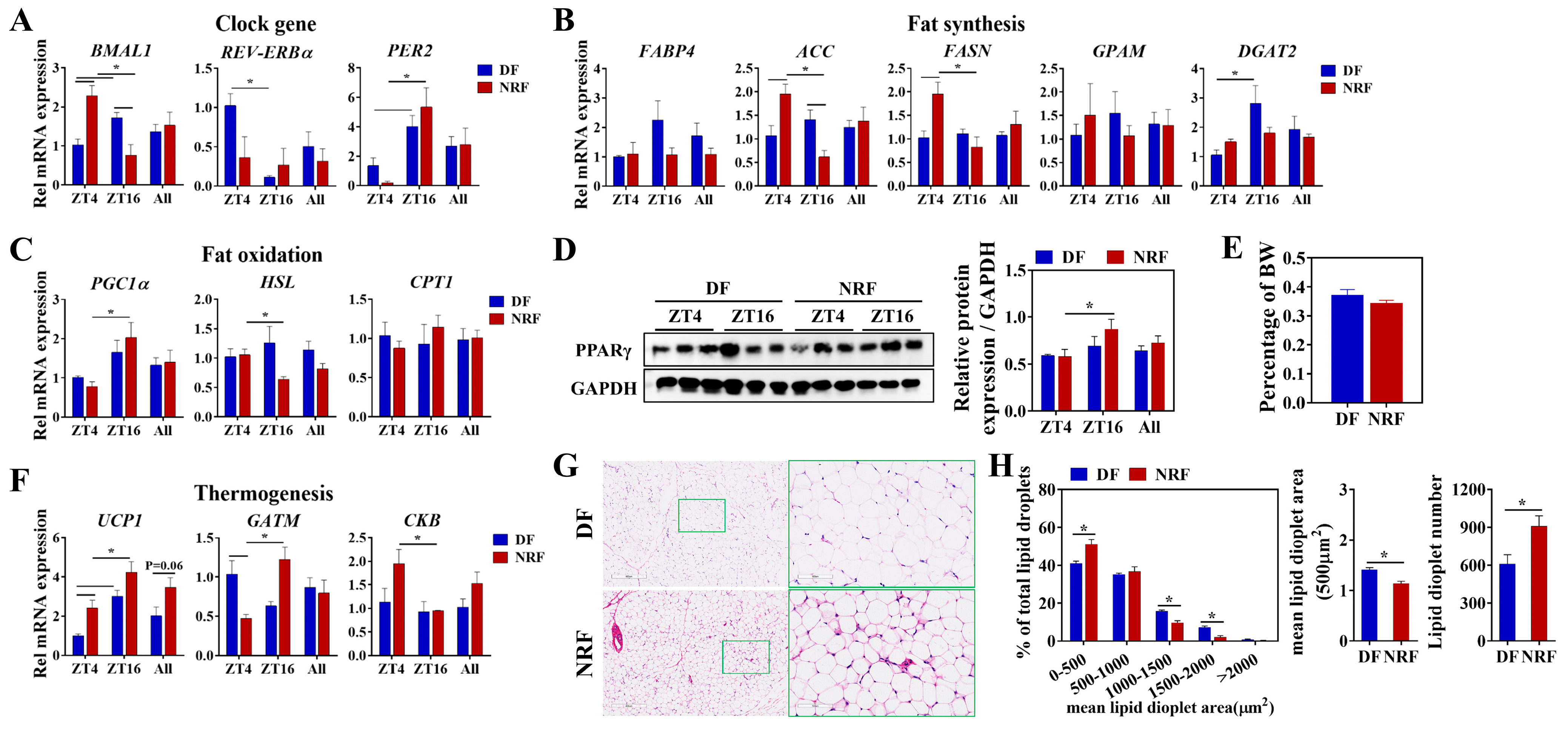
3.3. Daytime Feeding Disrupts the Rhythm of Thermogenesis and Promotes Lipid Deposition in Brown Adipose Tissue
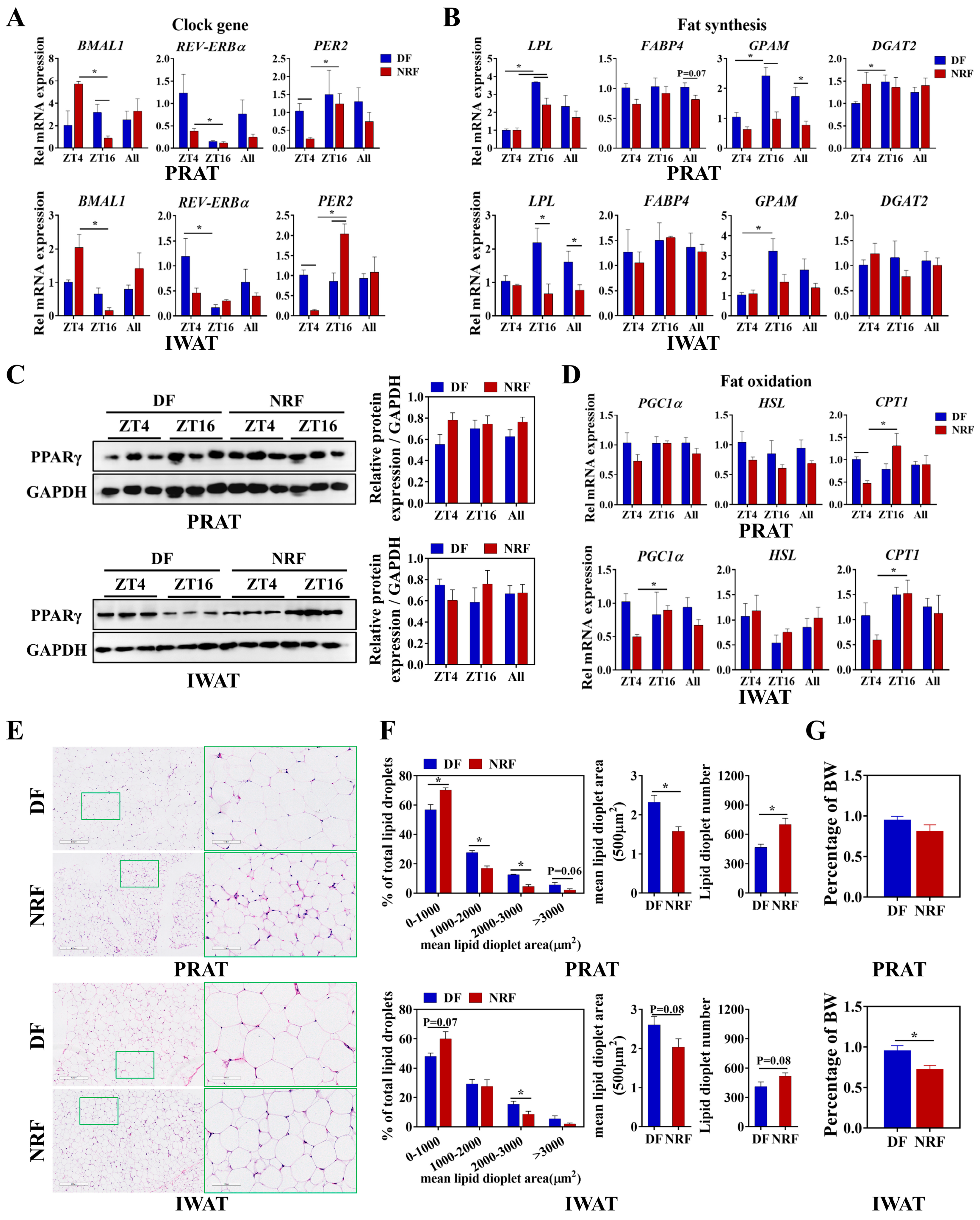
3.4. Daytime Feeding Disrupts the Metabolic Rhythms and Facilitates Lipid Deposition in White Adipose Tissue
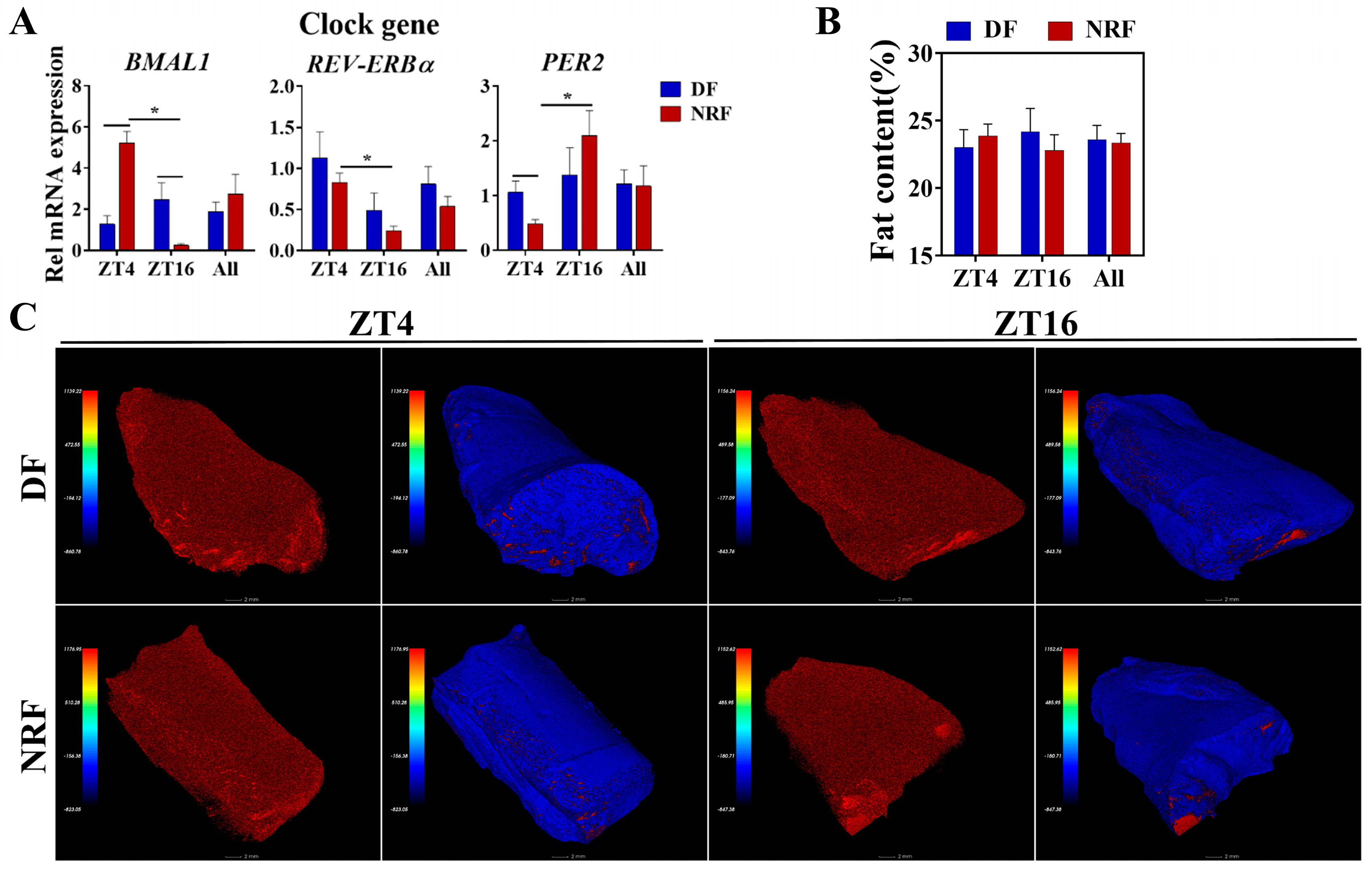
3.5. Daytime Feeding Disrupts Muscle Clock Gene Oscillations Without Affecting Fat Content
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, J.; Wang, Q.; Zhang, K.; He, S.; Liu, Z.; Li, M.; Liu, M.; Guo, Y.; Wu, Z. Optimizing feeding strategies for growing rabbits: Impact of timing and amount on health and circadian rhythms. Animals 2023, 13, 2742. [Google Scholar] [CrossRef] [PubMed]
- Van Erp, R.; de Vries, S.; van Kempen, T.; Den Hartog, L.A.; Gerrits, W. Circadian misalignment imposed by nocturnal feeding tends to increase fat deposition in pigs. Br. J. Nutr. 2020, 123, 529–536. [Google Scholar] [CrossRef]
- Jilge, B. Restricted feeding: A nonphotic zeitgeber in the rabbit. Physiol. Behav. 1992, 51, 157. [Google Scholar] [CrossRef] [PubMed]
- Sha, Y.; He, Y.; Liu, X.; Shao, P.; Wang, F.; Xie, Z.; Li, W.; Wang, J.; Li, S.; Zhao, S.; et al. Interactions of rumen microbiota and metabolites with meat quality-related genes to regulate meat quality and flavor of Tibetan sheep under nutrient stress in the cold season. J. Appl. Microbiol. 2023, 134, lxad182. [Google Scholar] [CrossRef]
- Grosjean, E.; Simonneaux, V.; Challet, E. Reciprocal Interactions between Circadian Clocks, Food Intake, and Energy Metabolism. Biology 2023, 12, 539. [Google Scholar] [CrossRef]
- Scheer, F.A.; Hilton, M.F.; Mantzoros, C.S.; Shea, S.A. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc. Natl. Acad. Sci. USA 2009, 106, 4453–4458. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, H.; Su, Y. New insights into the diurnal rhythmicity of gut microbiota and its crosstalk with host circadian rhythm. Animals 2022, 12, 1677. [Google Scholar] [CrossRef] [PubMed]
- Balsalobre, A.; Brown, S.A.; Marcacci, L.; Tronche, F.; Kellendonk, C.; Reichardt, H.M.; Schutz, G.; Schibler, U. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science 2000, 289, 2344–2347. [Google Scholar] [CrossRef]
- Campbell, J.E.; Peckett, A.J.; D’Souza, A.M.; Hawke, T.J.; Riddell, M.C. Adipogenic and lipolytic effects of chronic glucocorticoid exposure. Am. J. Physiol.—Cell Physiol. 2011, 300, C198–C209. [Google Scholar] [CrossRef]
- Wehrens, S.; Christou, S.; Isherwood, C.; Middleton, B.; Gibbs, M.A.; Archer, S.N.; Skene, D.J.; Johnston, J.D. Meal timing regulates the human circadian system. Curr. Biol. 2017, 27, 1768–1775. [Google Scholar] [CrossRef] [PubMed]
- Woodie, L.N.; Oral, K.T.; Krusen, B.M.; Lazar, M.A. The circadian regulation of nutrient metabolism in Diet-Induced obesity and metabolic disease. Nutrients 2022, 14, 3136. [Google Scholar] [CrossRef]
- Melendez-Fernandez, O.H.; Liu, J.A.; Nelson, R.J. Circadian rhythms disrupted by light at night and mistimed food intake alter hormonal rhythms and metabolism. Int. J. Mol. Sci. 2023, 24, 3392. [Google Scholar] [CrossRef]
- Hatori, M.; Vollmers, C.; Zarrinpar, A.; Ditacchio, L.; Bushong, E.A.; Gill, S.; Leblanc, M.; Chaix, A.; Joens, M.S.; Fitzpatrick, J.A.J. Time-Restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a High-Fat diet. Cell Metab. 2012, 15, 848–860. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wang, Q.; Zhang, K.; Yao, C.; Huang, J.; Li, Q.; Liu, Z.; Zhang, Y.; Shan, C.; Liu, P.; et al. Night-restricted feeding improves locomotor activity rhythm and modulates nutrient utilization to accelerate growth in rabbits. FASEB J. 2021, 35, e21166. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Sharma, N.; Narnoliya, L.K.; Verma, A.K.; Umaraw, P.; Mehta, N.; Ismail-Fitry, M.R.; Kaka, U.; Yong-Meng, G.; Lee, S.J.; et al. Improving quality and consumer acceptance of rabbit meat: Prospects and challenges. Meat Sci. 2025, 219, 109660. [Google Scholar] [CrossRef] [PubMed]
- Anzengruber, J.; Bublin, M.; Bönisch, E.; Janesch, B.; Tscheppe, A.; Braun, M.L.; Varga, E.; Hafner, C.; Breiteneder, H.; Schäffer, C. Lactobacillus buchneri S-layer as carrier for an Ara h 2-derived peptide for peanut allergen-specific immunotherapy. Mol. Immunol. 2017, 85, 81–88. [Google Scholar] [CrossRef]
- Gandia, K.M.; Herrelko, E.S.; Kessler, S.E.; Buchanan-Smith, H.M. Understanding Circadian and Circannual Behavioral Cycles of Captive Giant Pandas (Ailuropoda melanoleuca) Can Help to Promote Good Welfare. Animals 2023, 13, 2401. [Google Scholar] [CrossRef] [PubMed]
- Morgado, E.; Juarez, C.; Melo, A.I.; Dominguez, B.; Lehman, M.N.; Escobar, C.; Meza, E.; Caba, M. Artificial feeding synchronizes behavioral, hormonal, metabolic and neural parameters in mother-deprived neonatal rabbit pups. Eur. J. Neurosci. 2011, 34, 1807–1816. [Google Scholar] [CrossRef]
- Wang, C.; Niimi, M.; Kitajima, S.; Matsuhisa, F.; Yan, H.; Dong, S.; Liang, J.; Fan, J. Sex hormones affect endothelial lipase-mediated lipid metabolism and atherosclerosis. Lipids Health Dis. 2019, 18, 226. [Google Scholar] [CrossRef] [PubMed]
- Committee for the Update of the Guide for the Care and Use of Laboratory Animals; Institute for Laboratory Animal Research. Guide for the Care and Use of Laboratory Animals, 8th ed.; National Academies Press (US): Washington, DC, USA, 2011. [CrossRef]
- Gidenne, T.; Combes, S.; Fortun-Lamothe, L. Feed intake limitation strategies for the growing rabbit: Effect on feeding behaviour, welfare, performance, digestive physiology and health: A review. Animal 2012, 6, 1407–1419. [Google Scholar] [CrossRef]
- Zhang, L.; Yao, C.Y.; Wang, Q.J.; Shan, C.H.; Zhang, K.H.; Guo, Y.; Li, Q.; Liu, Z.Y.; Liu, P.; Geng, S.X.; et al. Night-restricted feeding improves colonic barrier function and reduces inflammation by optimizing gut microbial composition in growing pigs during the hot season. FASEB J. 2024, 38, e23752. [Google Scholar] [CrossRef]
- Yamamuro, D.; Takahashi, M.; Nagashima, S.; Wakabayashi, T.; Yamazaki, H.; Takei, A.; Takei, S.; Sakai, K.; Ebihara, K.; Iwasaki, Y.; et al. Peripheral circadian rhythms in the liver and white adipose tissue of mice are attenuated by constant light and restored by time-restricted feeding. PLoS ONE 2020, 15, e234439. [Google Scholar] [CrossRef]
- Cone, R.D. The corticotropin-releasing hormone system and feeding behavior--a complex web begins to unravel. Endocrinology 2000, 141, 2713–2714. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Oster, H.; Challet, E.; Ott, V.; Arvat, E.; de Kloet, E.R.; Dijk, D.; Lightman, S.; Vgontzas, A.; Van Cauter, E. The functional and clinical significance of the 24-Hour rhythm of circulating glucocorticoids. Endocr. Rev. 2017, 38, 3–45. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Androulakis, I.P. Light entrainment of the SCN circadian clock and implications for personalized alterations of corticosterone rhythms in shift work and jet lag. Sci. Rep. 2021, 11, 17929. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.J.; Chen, S.W.; Xu, X.; Zhang, H.L.; Yan, J.Y. The effect of cold exposure on the levels of glucocorticoids, 11-hydroxysteroid dehydrogenase 2, and placental vascularization in a rat model. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 11961–11974. [Google Scholar] [CrossRef] [PubMed]
- So, A.Y.; Bernal, T.U.; Pillsbury, M.L.; Yamamoto, K.R.; Feldman, B.J. Glucocorticoid regulation of the circadian clock modulates glucose homeostasis. Proc. Natl. Acad. Sci. USA 2009, 106, 17582–17587. [Google Scholar] [CrossRef]
- Cho, H.; Zhao, X.; Hatori, M.; Yu, R.T.; Barish, G.D.; Lam, M.T.; Chong, L.W.; Ditacchio, L.; Atkins, A.R.; Glass, C.K.; et al. Regulation of circadian behaviour and metabolism by REV-ERB-alpha and REV-ERB-beta. Nature 2012, 485, 123–127. [Google Scholar] [CrossRef]
- Chen, Y.; Jing, Y.; Hu, L.; Xi, Z.; Lu, Z.; Loor, J.J.; Wang, M. Overexpression of PER2 promotes de novo fatty acid synthesis, fatty acid desaturation, and triglyceride accumulation in bovine mammary epithelial cells. Int. J. Mol. Sci. 2024, 25, 9785. [Google Scholar] [CrossRef] [PubMed]
- Coste, H.; Rodriguez, J.C. Orphan nuclear hormone receptor Rev-erbalpha regulates the human apolipoprotein CIII promoter. J. Biol. Chem. 2002, 277, 27120–27129. [Google Scholar] [CrossRef]
- Yoda, T.; Crawshaw, L.I.; Yoshida, K.; Su, L.; Hosono, T.; Shido, O.; Sakurada, S.; Fukuda, Y.; Kanosue, K. Effects of food deprivation on daily changes in body temperature and behavioral thermoregulation in rats. Am. J. Physiol.—Regul. Integr. Comp. Physiol. 2000, 278, R134–R139. [Google Scholar] [CrossRef] [PubMed]
- Scheer, F.A.; Pirovano, C.; Van Someren, E.J.; Buijs, R.M. Environmental light and suprachiasmatic nucleus interact in the regulation of body temperature. Neuroscience 2005, 132, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Yau, W.W.; Singh, B.K.; Lesmana, R.; Zhou, J.; Sinha, R.A.; Wong, K.A.; Wu, Y.; Bay, B.H.; Sugii, S.; Sun, L.; et al. Thyroid hormone (T(3)) stimulates brown adipose tissue activation via mitochondrial biogenesis and MTOR-mediated mitophagy. Autophagy 2019, 15, 131–150. [Google Scholar] [CrossRef] [PubMed]
- Hasan, N.; Nagata, N.; Morishige, J.I.; Islam, M.T.; Jing, Z.; Harada, K.I.; Mieda, M.; Ono, M.; Fujiwara, H.; Daikoku, T.; et al. Brown adipocyte-specific knockout of Bmal1 causes mild but significant thermogenesis impairment in mice. Mol. Metab. 2021, 49, 101202. [Google Scholar] [CrossRef]
- Gerhart-Hines, Z.; Feng, D.; Emmett, M.J.; Everett, L.J.; Loro, E.; Briggs, E.R.; Bugge, A.; Hou, C.; Ferrara, C.; Seale, P.; et al. The nuclear receptor Rev-erbalpha controls circadian thermogenic plasticity. Nature 2013, 503, 410–413. [Google Scholar] [CrossRef]
- Giuliani, G.; Rosina, M.; Reggio, A. Signaling pathways regulating the fate of fibro/adipogenic progenitors (FAPs) in skeletal muscle regeneration and disease. FEBS J. 2022, 289, 6484–6517. [Google Scholar] [CrossRef]
- Li, X.; Fu, X.; Yang, G.; Du, M. Review: Enhancing intramuscular fat development via targeting fibro-adipogenic progenitor cells in meat animals. Animal 2020, 14, 312–321. [Google Scholar] [CrossRef]
- Morishima, Y.; Murphy, P.J.M.; Li, D.; Sanchez, E.R.; Pratt, W.B. Stepwise Assembly of a Glucocorticoid Receptor·hsp90 Heterocomplex Resolves Two Sequential ATP-dependent Events Involving First hsp70 and then hsp90 in Opening of the Steroid Binding Pocket. J. Biol. Chem. 2000, 275, 18054–18060. [Google Scholar] [CrossRef]
- Luo, G.; Hu, S.; Lai, T.; Wang, J.; Wang, L.; Lai, S. MiR-9-5p promotes rabbit preadipocyte differentiation by suppressing leptin gene expression. Lipids Health Dis. 2020, 19, 126. [Google Scholar] [CrossRef]
- Ou, C.Y.; Chen, T.C.; Lee, J.V.; Wang, J.C.; Stallcup, M.R. Coregulator cell cycle and apoptosis regulator 1 (CCAR1) positively regulates adipocyte differentiation through the glucocorticoid signaling pathway. J. Biol. Chem. 2014, 289, 17078–17086. [Google Scholar] [CrossRef]
- Baker, J.D.; Ozsan, I.; Rodriguez, O.S.; Gulick, D.; Blair, L.J. Hsp90 heterocomplexes regulate steroid hormone receptors: From stress response to psychiatric disease. Int. J. Mol. Sci. 2018, 20, 79. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, K.-H.; He, S.; Wang, Q.-G.; Li, J.-J.; Yao, C.-Y.; Shan, C.-H.; Zhang, L.; Liu, Z.-Y.; Liu, P.; Li, M.-Y.; et al. Mistimed Feeding Disrupts Metabolic Rhythm and Increases Lipid Accumulation of Growing Rabbits in Winter. Animals 2025, 15, 692. https://doi.org/10.3390/ani15050692
Zhang K-H, He S, Wang Q-G, Li J-J, Yao C-Y, Shan C-H, Zhang L, Liu Z-Y, Liu P, Li M-Y, et al. Mistimed Feeding Disrupts Metabolic Rhythm and Increases Lipid Accumulation of Growing Rabbits in Winter. Animals. 2025; 15(5):692. https://doi.org/10.3390/ani15050692
Chicago/Turabian StyleZhang, Ke-Hao, Shuai He, Quan-Gang Wang, Jun-Jiao Li, Chun-Yan Yao, Chun-Hua Shan, Lei Zhang, Zhong-Ying Liu, Peng Liu, Ming-Yong Li, and et al. 2025. "Mistimed Feeding Disrupts Metabolic Rhythm and Increases Lipid Accumulation of Growing Rabbits in Winter" Animals 15, no. 5: 692. https://doi.org/10.3390/ani15050692
APA StyleZhang, K.-H., He, S., Wang, Q.-G., Li, J.-J., Yao, C.-Y., Shan, C.-H., Zhang, L., Liu, Z.-Y., Liu, P., Li, M.-Y., Guo, Y., & Wu, Z.-H. (2025). Mistimed Feeding Disrupts Metabolic Rhythm and Increases Lipid Accumulation of Growing Rabbits in Winter. Animals, 15(5), 692. https://doi.org/10.3390/ani15050692





