Effects of Different Levels of Flea Infestation on Gut Microbiota of Brandt’s Voles (Lasiopodomys brandtii) in China
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Animals
2.2. Experimental Design
2.3. 16S rRNA Gene Amplicon Sequencing and Bioinformatics Analysis
2.4. Statistical Analyses
3. Results
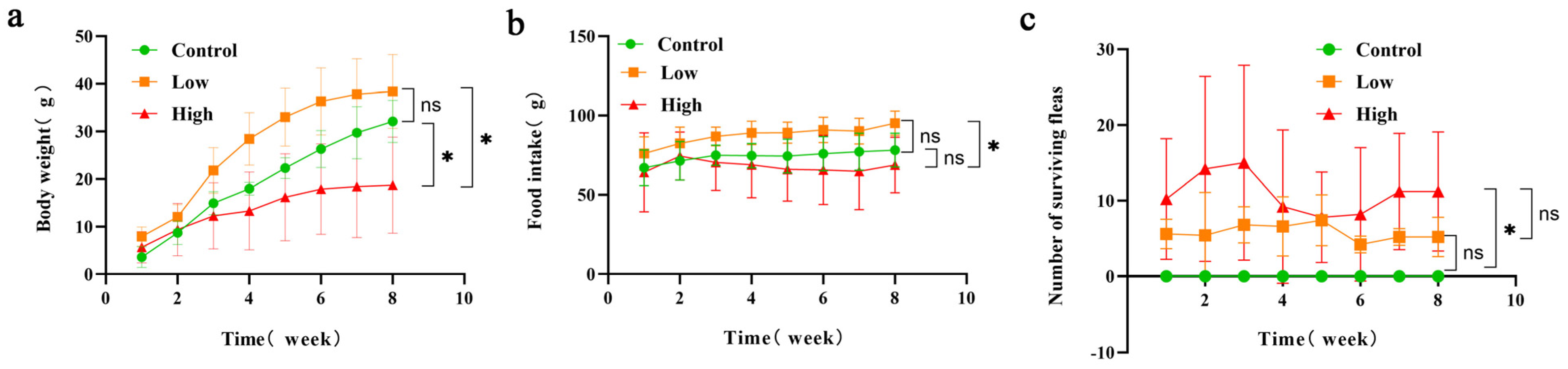
3.1. Changes in Average Body Weight and Food Intake of Brandt’s Voles and Number of Surviving Fleas
3.2. Results of 16S rRNA Sequencing
3.3. Analysis of Species Composition and Differences in Brandt’s Voles
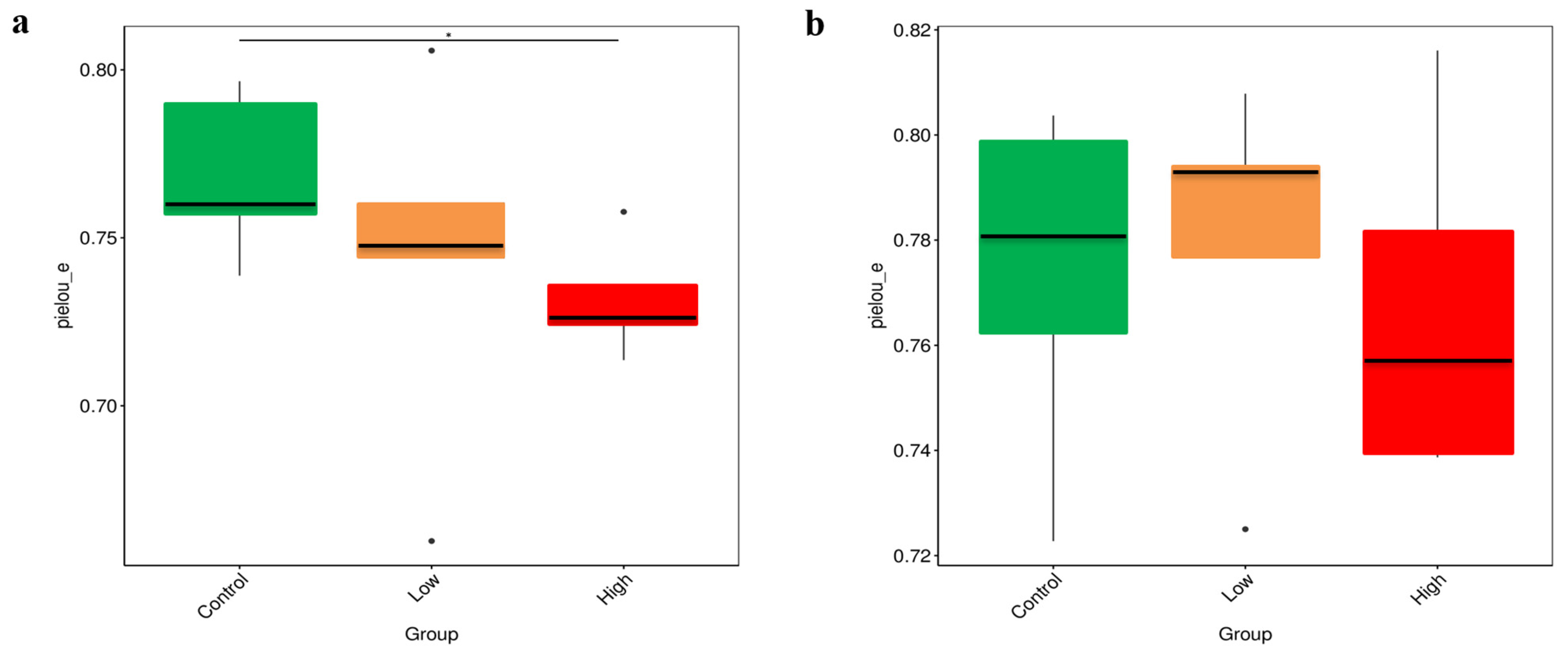
3.4. Alpha-Diversity Analysis
3.5. Beta-Diversity Analysis
3.6. LEfSe Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, Z.J.; Yin, J.X. Epidemic status of flea-borne natural epidemic diseases in China. J. Baotou Med. Coll. 2015, 31, 143–144. [Google Scholar]
- Liu, Q.Y. Reported cases of vector-borne diseases in China, 2005- 2020: Epidemic trend, challenges in prevention and control, and related coping strategies. Chin. J. Vector Biol. Control 2022, 33, 1–7. [Google Scholar]
- Zhang, L.; Wei, X.H.; Chang, N.; Li, H.Y.; Si, X.Y.; Bai, X.F.; Wang, J.; Liu, Q.Y. Composition of parasitic fleas on rodents and spatiotemporal distribution of flea index in Xilin Gol League, Inner Mongolia Autonomous Region, China, 2012–2021. Chin. J. Vector. Biol. Control 2023, 34, 82–90. [Google Scholar]
- Abdullah, S.; Helps, C.; Tasker, S.; Newbury, H.; Wall, R. Pathogens in fleas collected from cats and dogs: Distribution and prevalence in the UK. Parasit. Vectors 2019, 12, 71. [Google Scholar] [CrossRef]
- Shang, Z.; Chen, K.; Han, T.; Bu, F.; Sun, S.; Zhu, N.; Man, D.; Yang, K.; Yuan, S.; Fu, H. Natural Foraging Selection and Gut Microecology of Two Subterranean Rodents from the Eurasian Steppe in China. Animals 2024, 14, 2334. [Google Scholar] [CrossRef]
- Warburton, E.M.; Kam, M.; Bar-Shira, E.; Friedman, A.; Khokhlova, I.S.; Koren, L.; Asfur, M.; Geffen, E.; Kiefer, D.; Krasnov, B.R.; et al. Effects of parasite pressure on parasite mortality and reproductive output in a rodent-flea system: Inferring host defense trade-offs. Parasitol. Res. 2016, 115, 3337–3344. [Google Scholar] [CrossRef]
- Kam, M.; Degen, A.A.; Khokhlova, I.S.; Krasnov, B.R.; Geffen, E. Do fleas affect energy expenditure of their free-living hosts? PLoS ONE 2010, 5, e13686. [Google Scholar] [CrossRef]
- Bosio, C.F.; Viall, A.K.; Jarrett, C.O.; Gardner, D.; Rood, M.P.; Hinnebusch, B.J. Evaluation of the murine immune response to Xenopsylla cheopis flea saliva and its effect on transmission of Yersinia pestis. PLoS Negl. Trop. Dis. 2014, 8, e3196. [Google Scholar] [CrossRef]
- Sieng, S.; Chen, P.; Wang, N.; Xu, J.Y.; Han, Q. Toxocara canis-induced changes in host intestinal microbial communities. Parasites Vectors 2023, 16, 462. [Google Scholar] [CrossRef]
- Shao, D.Y.; Bai, X.; Tong, M.W.; Zhang, Y.Y.; Liu, X.L.; Zhou, Y.H.; Li, C.; Cai, W.; Gao, X.; Liu, M.; et al. Changes to the gut microbiota in mice induced by infection with Toxoplasma gondii. Acta Trop. 2020, 203, 105301. [Google Scholar] [CrossRef]
- Bian, J.H. Research advances and perspectives on mammal population ecology in China. Acta Theriol. Sin. 2021, 41, 556–570. [Google Scholar]
- Zhang, X.Y.; Sukhchuluun, G.; Bo, T.B.; Chi, Q.S.; Yang, J.J.; Chen, B.; Zhang, L.; Wang, D.H. Correction to: Huddling remodels gut microbiota to reduce energy requirements in a small mammal species during cold exposure. Microbiome 2018, 6, 126. [Google Scholar] [CrossRef] [PubMed]
- Bo, T.B.; Zhang, X.Y.; Wen, J.; Deng, K.; Qin, X.W.; Wang, D.H. The microbiota-gut-brain interaction in regulating host metabolic adaptation to cold in male Brandt’s voles (Lasiopodomys brandtii). ISME J. 2019, 13, 3037–3053. [Google Scholar] [CrossRef] [PubMed]
- Bo, T.B.; Zhang, X.Y.; Kohl, K.D.; Wen, J.; Tian, S.J.; Wang, D.H. Coprophagy prevention alters microbiome, metabolism, neurochemistry, and cognitive behavior in a small mammal. ISME J. 2020, 14, 2625–2645. [Google Scholar] [CrossRef] [PubMed]
- Khakisahneh, S.; Zhang, X.Y.; Nouri, Z.; Wang, D.H. Gut Microbiota and Host Thermoregulation in Response to Ambient Temperature Fluctuations. mSystems 2020, 5, e00514-20. [Google Scholar] [CrossRef]
- Rapin, A.; Chuat, A.; Lebon, L.; Zaiss, M.M.; Marsland, B.J.; Harris, N.L. Infection with a small intestinal helminth, Heligmosomoides polygyrus bakeri, consistently alters microbial communities throughout the murine small and large intestine. Int. J. Parasitol. 2020, 50, 35–46. [Google Scholar] [CrossRef]
- Wang, Z.; Chang, N.; Lun, X.; Wang, L.; Wang, Z.; Guo, C.; Wang, X.; Ji, H.; Liu, P.; Liu, Q. Impacts of ectoparasite flea infestation on gut microbiota of the Meriones unguiculatus in a rodent-flea system. Parasitol. Res. 2024, 123, 361. [Google Scholar] [CrossRef]
- Shea-Donohue, T.; Qin, B.; Smith, A. Parasites, nutrition, immune responses and biology of metabolic tissues. Parasite Immunol. 2017, 39, e12422. [Google Scholar] [CrossRef]
- Hawlena, H.; Bashary, D.; Abramsky, Z.; Krasnov, B.R. Benefits, Costs and Constraints of Anti-Parasitic Grooming in Adult and Juvenile Rodents. Ethology 2007, 113, 394–402. [Google Scholar] [CrossRef]
- Khokhlova, I.S. Immune response to fleas in a wild desert rodent: Effect of parasite species, parasite burden, sex of host and host parasitological experience. J. Exp. Biol. 2004, 207, 2725–2733. [Google Scholar] [CrossRef]
- Devevey, G.; Hélène, N.; Biollaz, F.; Yvon, C.; Chapuisat, M.; Christe, P. Developmental, metabolic and immunological costs of flea infestation in the common vole. Funct. Ecol. 2008, 22, 1091–1098. [Google Scholar] [CrossRef]
- Zhou, S.; Lu, Y.; Chen, J.; Pan, Z.; Pang, L.; Wang, Y.; Zhang, Q.; Strand, M.R.; Chen, X.X.; Huang, J. Parasite reliance on its host gut microbiota for nutrition and survival. ISME J. 2022, 16, 2574–2586. [Google Scholar] [CrossRef]
- Grondin, J.A.; Jamal, A.; Mowna, S.; Seto, T.; Khan, W.I. Interaction between Intestinal Parasites and the Gut Microbiota: Implications for the Intestinal Immune Response and Host Defence. Pathogens 2024, 13, 608. [Google Scholar] [CrossRef]
- Maizels, R.M.; McSorley, H.J. Regulation of the host immune system by helminth parasites. J. Allergy Clin. Immunol. 2016, 138, 666–675. [Google Scholar] [CrossRef]
- Yoo, J.Y.; Groer, M.; Dutra, S.V.O.; Sarkar, A.; McSkimming, D.I. Gut Microbiota and Immune System Interactions. Microorganisms 2020, 8, 1587. [Google Scholar] [CrossRef]
- Giorgi, M.S.; Arlettaz, R.; Vogel, C.P. The energetic grooming costs imposed by a parasitic mite (Spinturnix myoti) upon its bat host (Myotis myotis). Proc. Biol. 2001, 268, 2071–2075. [Google Scholar] [CrossRef]
- Liu, C.; Chen, C.; Yan, X.; Gu, S.; Jia, X.; Fu, W.; Meng, X.; Xue, W. Assessment of immune responses and intestinal flora in BALB/c mice model of wheat food allergy via different sensitization methods. Food Sci. Hum. Wellness 2023, 12, 871–881. [Google Scholar] [CrossRef]
- Méndez-Salazar, E.O.; Ortiz-López, M.G.; Granados-Silvestre, M.L.Á.; Palacios-González, B.; Menjivar, M. Altered Gut Microbiota and Compositional Changes in Firmicutes and Proteobacteria in Mexican Undernourished and Obese Children. Front. Microbiol. 2018, 9, 2494. [Google Scholar]
- Liang, C.; Wang, L.; Wang, X.; Jia, Y.; Xie, Q.; Zhao, L.; Yuan, H. Altered ocular surface microbiota in obesity: A case-control study. Front. Cell Infect. Microbiol. 2024, 14, 1356197. [Google Scholar] [CrossRef]
- Swanson, K.; Blakeslee, A.M.H.; Fowler, A.E.; Roozbehi, S.; Field, E.K. Microbial communities are indicators of parasite infection status. Environ. Microbiol. 2023, 25, 3423–3434. [Google Scholar] [CrossRef]
- Lai, X.; Liu, S.; Miao, J.; Shen, R.; Wang, Z.; Zhang, Z.; Gong, H.; Li, M.; Pan, Y.; Wang, Q. Eubacterium siraeum suppresses fat deposition via decreasing the tyrosine-mediated PI3K/AKT signaling pathway in high-fat diet-induced obesity. Microbiome 2024, 12, 223. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Fang, S.; Wei, H.; He, M.; Fu, H.; Xiong, X.; Zhou, Y.; Wu, J.; Gao, J.; Yang, H.; et al. Prevotella copri increases fat accumulation in pigs fed with formula diets. Microbiome 2021, 9, 175. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Zhang, J.; Chen, L.; Yu, J.; Zhang, J.; Yin, H.; Shang, Q.; Yu, G. Discovery of Bacteroides uniformis F18-22 as a Safe and Novel Probiotic Bacterium for the Treatment of Ulcerative Colitis from the Healthy Human Colon. Int. J. Mol. Sci. 2023, 24, 14669. [Google Scholar] [CrossRef] [PubMed]
- Barcenilla, A.; Pryde, S.E.; Martin, J.C.; Duncan, S.H.; Stewart, C.S.; Henderson, C.; Flint, H.J. Phylogenetic relationships of butyrate-producing bacteria from the human gut. Appl. Environ. Microbiol. 2000, 66, 1654–1661. [Google Scholar] [CrossRef]
- Louis, P.; Flint, H.J. Formation of propionate and butymicee by the human colonic microbiota. Environ. Microbiol. 2017, 19, 29–41. [Google Scholar] [CrossRef]
- Turroni, S.; Rampelli, S.; Biagi, E.; Consolandi, C.; Severgnini, M.; Peano, C.; Quercia, S.; Soverini, M.; Carbonero, F.G.; Bianconi, G.; et al. Temporal dynamics of the gut microbiota in people sharing a confined environment, a 520-day ground-based space simulation, MARS500. Microbiome 2017, 5, 39. [Google Scholar] [CrossRef]
- Inoki, K.; Li, Y.; Zhu, T.; Wu, J.; Guan, K.L. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat. Cell Biol. 2002, 4, 648–657. [Google Scholar] [CrossRef]
- Degirmenci, U.; Wang, M.; Hu, J. Targeting Aberrant RAS/RAF/MEK/ERK Signaling for Cancer Therapy. Cells 2020, 9, 198. [Google Scholar] [CrossRef]
- Savova, M.S.; Mihaylova, L.V.; Tews, D.; Wabitsch, M.; Georgiev, M.I. Targeting PI3K/AKT signaling pathway in obesity. Biomed. Pharmacother. 2023, 159, 114244. [Google Scholar] [CrossRef]
- Yang, T.; Li, G.; Xu, Y.; He, X.; Song, B.; Cao, Y. Characterization of the gut microbiota in polycystic ovary syndrome with dyslipidemia. BMC Microbiol. 2024, 24, 169. [Google Scholar] [CrossRef]
- Kim, H.S.; Oh, S.J.; Kim, B.K.; Kim, J.E.; Kim, B.H.; Park, Y.K.; Yang, B.G.; Lee, J.Y.; Bae, J.W.; Lee, C.K. Dysbiotic signatures and diagnostic potential of gut microbial markers for inflammatory bowel disease in Korean population. Sci. Rep. 2024, 14, 23701. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, P.; Krausz, M.; Han, Y.; Mitsuiki, N.; Gabrysch, A.; Nöltner, C.; Proietti, M.; Heller, T.; Grou, C.; Calderon, V.; et al. The intestinal microbiome and metabolome discern disease severity in cytotoxic T-lymphocyte-associated protein 4 deficiency. Microbiome 2025, 13, 51. [Google Scholar] [CrossRef] [PubMed]



| Variable | Estimate | Standard Error | Wald χ2 | p Value |
|---|---|---|---|---|
| Intercept | −20.522 | 3.414 | 36.1 | 0.000 *** |
| Group | −1.651 | 0.519 | 10.1 | 0.002 ** |
| Time | 3.152 | 0.248 | 161.9 | 0.000 *** |
| Food intake | 0.368 | 0.044 | 70.2 | 0.000 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Wang, L.; Guo, C.; Wang, Z.; Lun, X.; Ji, H.; Shang, M.; Wang, X.; Liu, Q. Effects of Different Levels of Flea Infestation on Gut Microbiota of Brandt’s Voles (Lasiopodomys brandtii) in China. Animals 2025, 15, 669. https://doi.org/10.3390/ani15050669
Wang Z, Wang L, Guo C, Wang Z, Lun X, Ji H, Shang M, Wang X, Liu Q. Effects of Different Levels of Flea Infestation on Gut Microbiota of Brandt’s Voles (Lasiopodomys brandtii) in China. Animals. 2025; 15(5):669. https://doi.org/10.3390/ani15050669
Chicago/Turabian StyleWang, Zhenxu, Lu Wang, Chenran Guo, Zihao Wang, Xinchang Lun, Haoqiang Ji, Meng Shang, Xiaoxu Wang, and Qiyong Liu. 2025. "Effects of Different Levels of Flea Infestation on Gut Microbiota of Brandt’s Voles (Lasiopodomys brandtii) in China" Animals 15, no. 5: 669. https://doi.org/10.3390/ani15050669
APA StyleWang, Z., Wang, L., Guo, C., Wang, Z., Lun, X., Ji, H., Shang, M., Wang, X., & Liu, Q. (2025). Effects of Different Levels of Flea Infestation on Gut Microbiota of Brandt’s Voles (Lasiopodomys brandtii) in China. Animals, 15(5), 669. https://doi.org/10.3390/ani15050669






