Aberrant Expression Levels of Androgen Receptor and SRD5A2 in Epididymal Epithelial Cells of Crossbred Infertile Cattle–Yak
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal and Sample Collection
2.2. Tissue Processing
2.3. Immunohistochemistry
2.4. Semi-Quantification of Immunolabelling in Epididymis Epithelial Cells
2.5. Statistical Analysis
3. Results
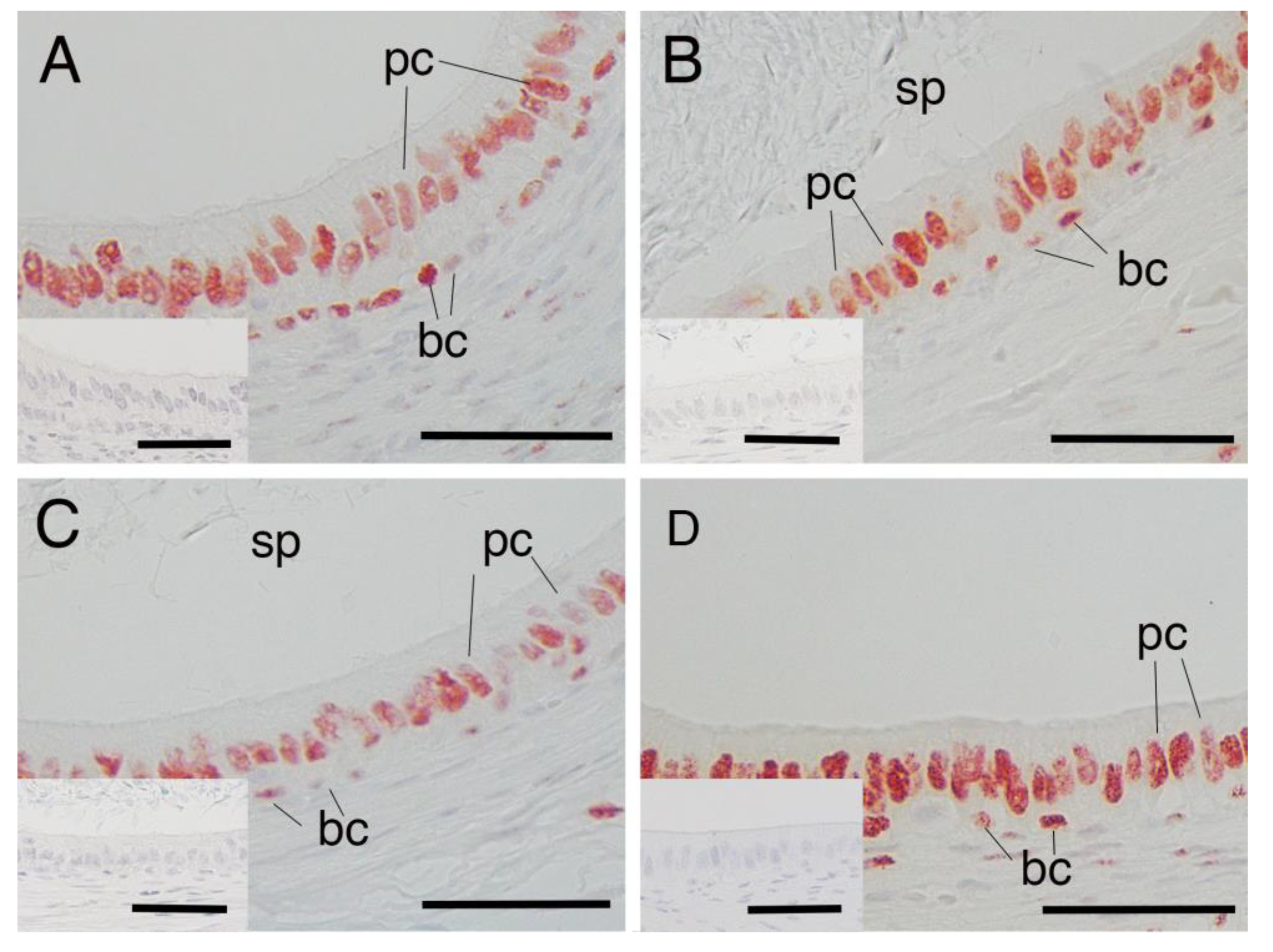
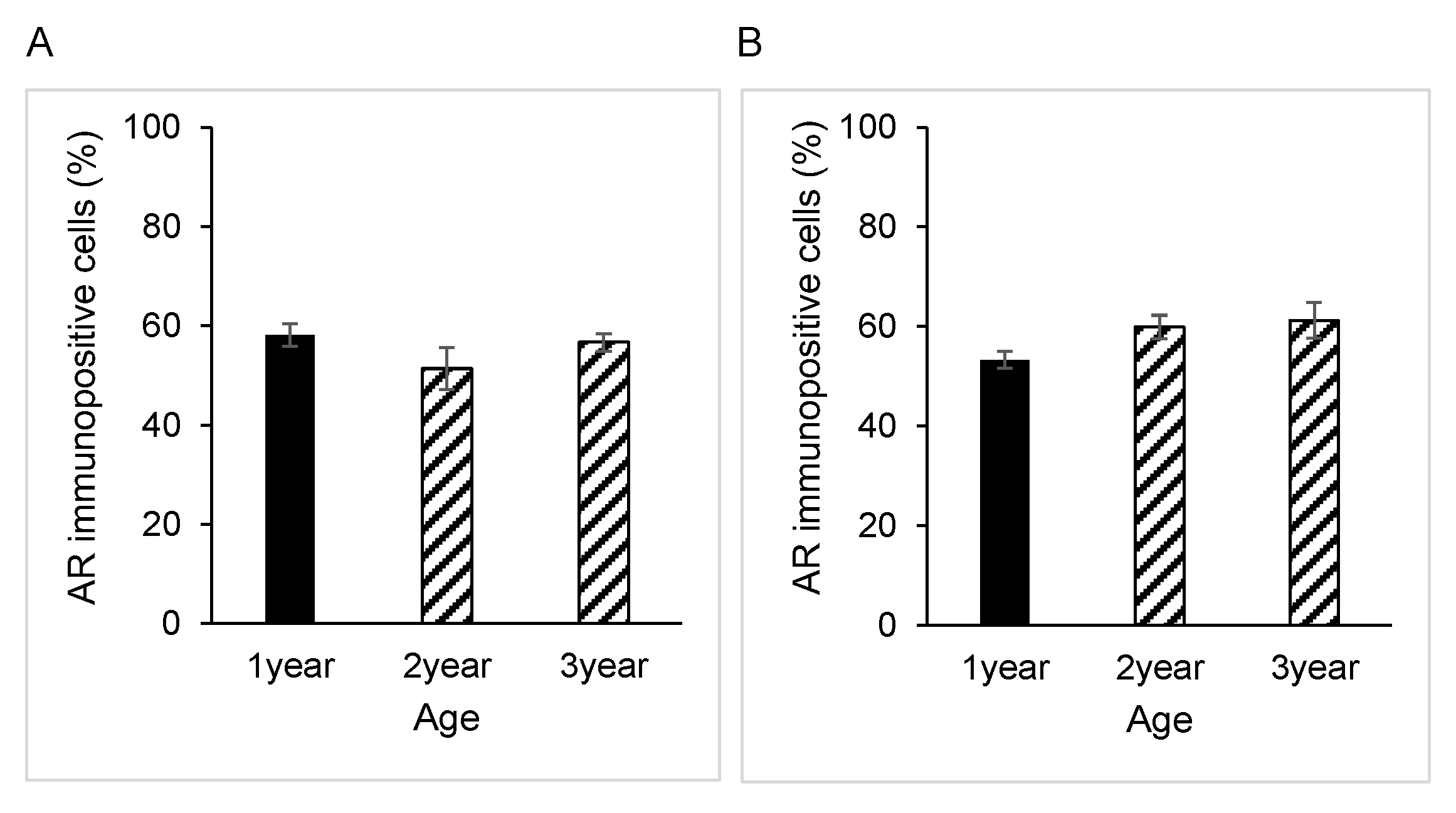
3.1. AR Expression in Epididymal Epithelial Cells During Yak Development
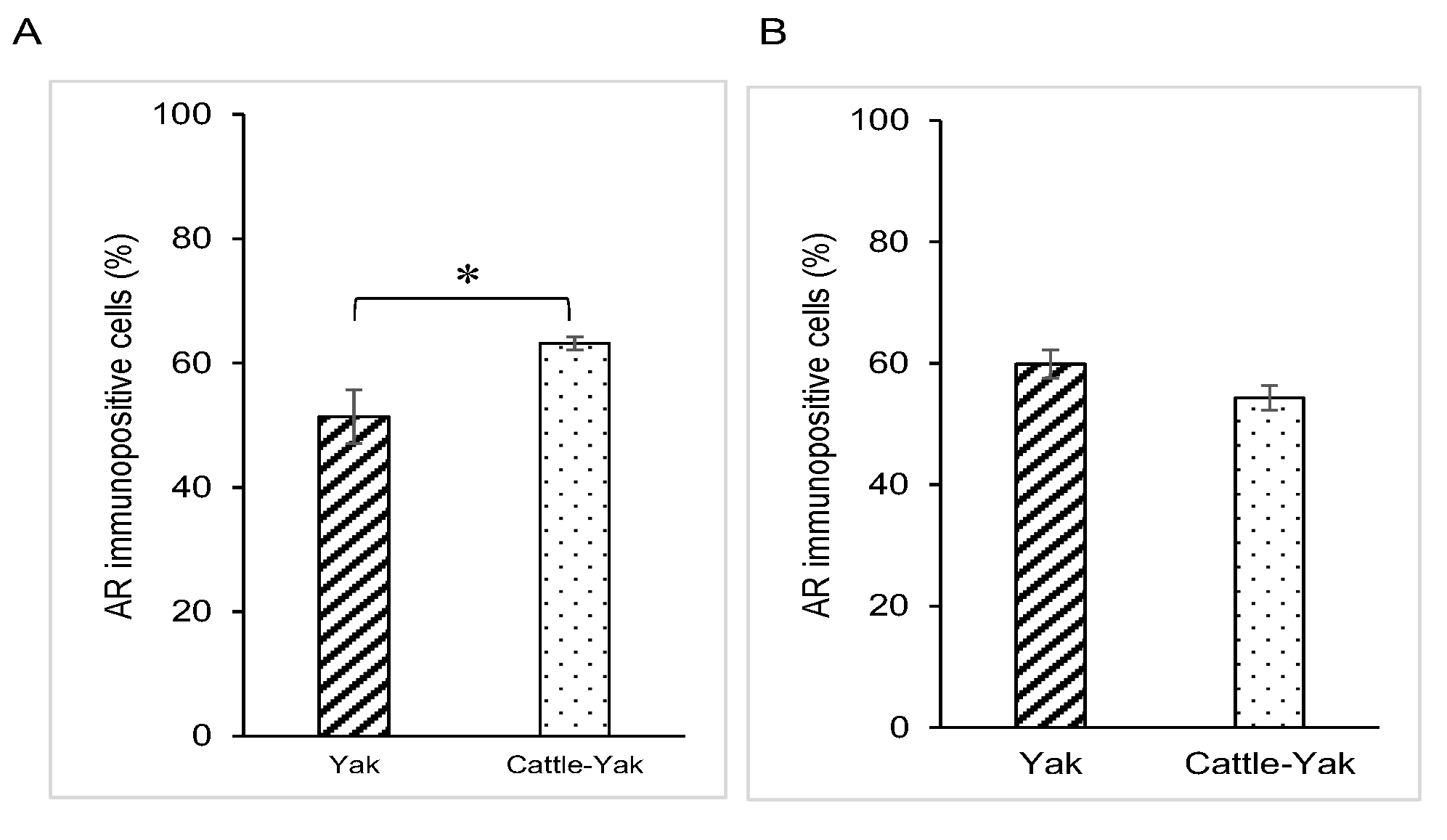
3.2. AR Expression in Epididymal Epithelial Cells of Mature Yak and Cattle–Yak
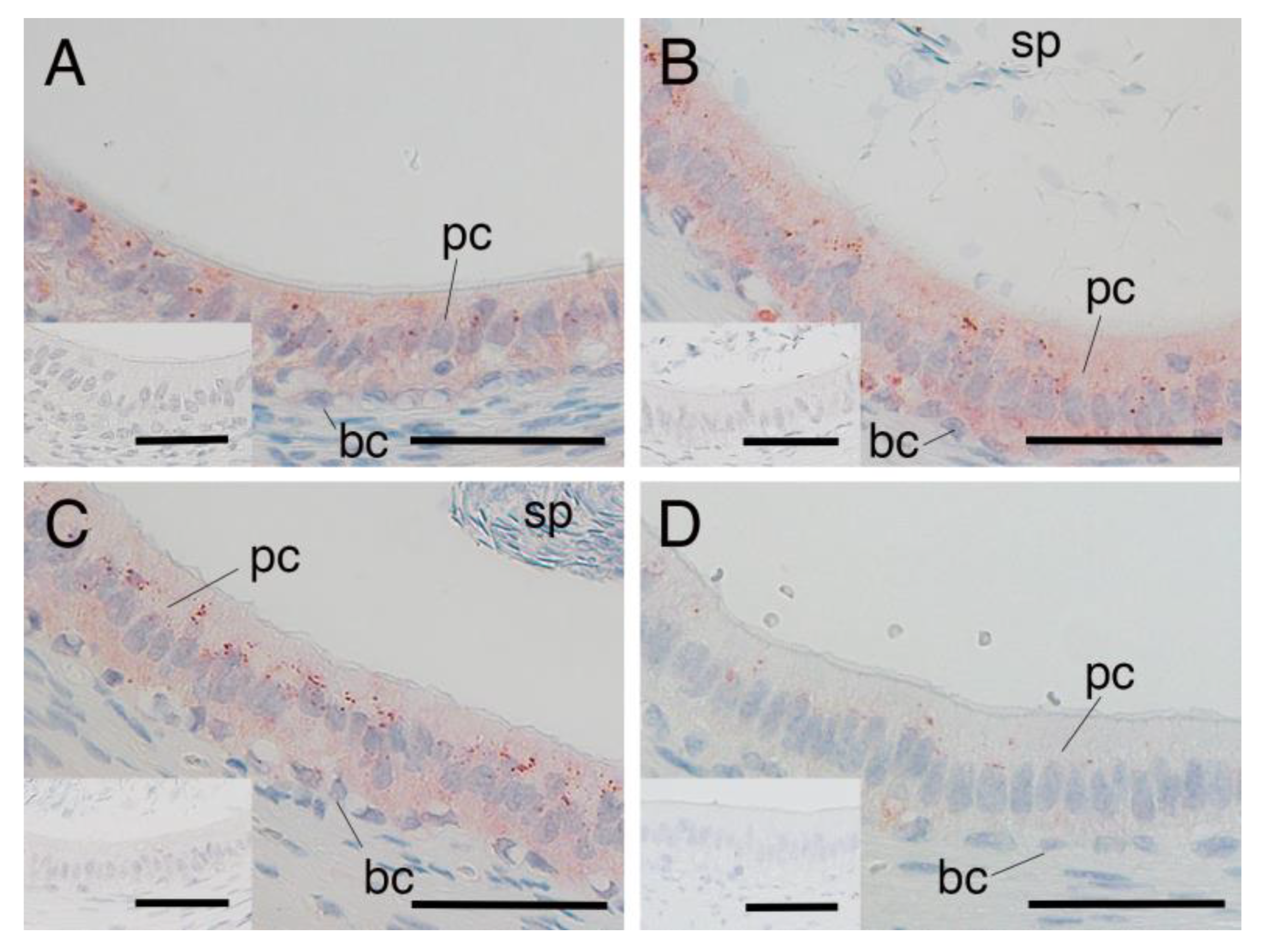
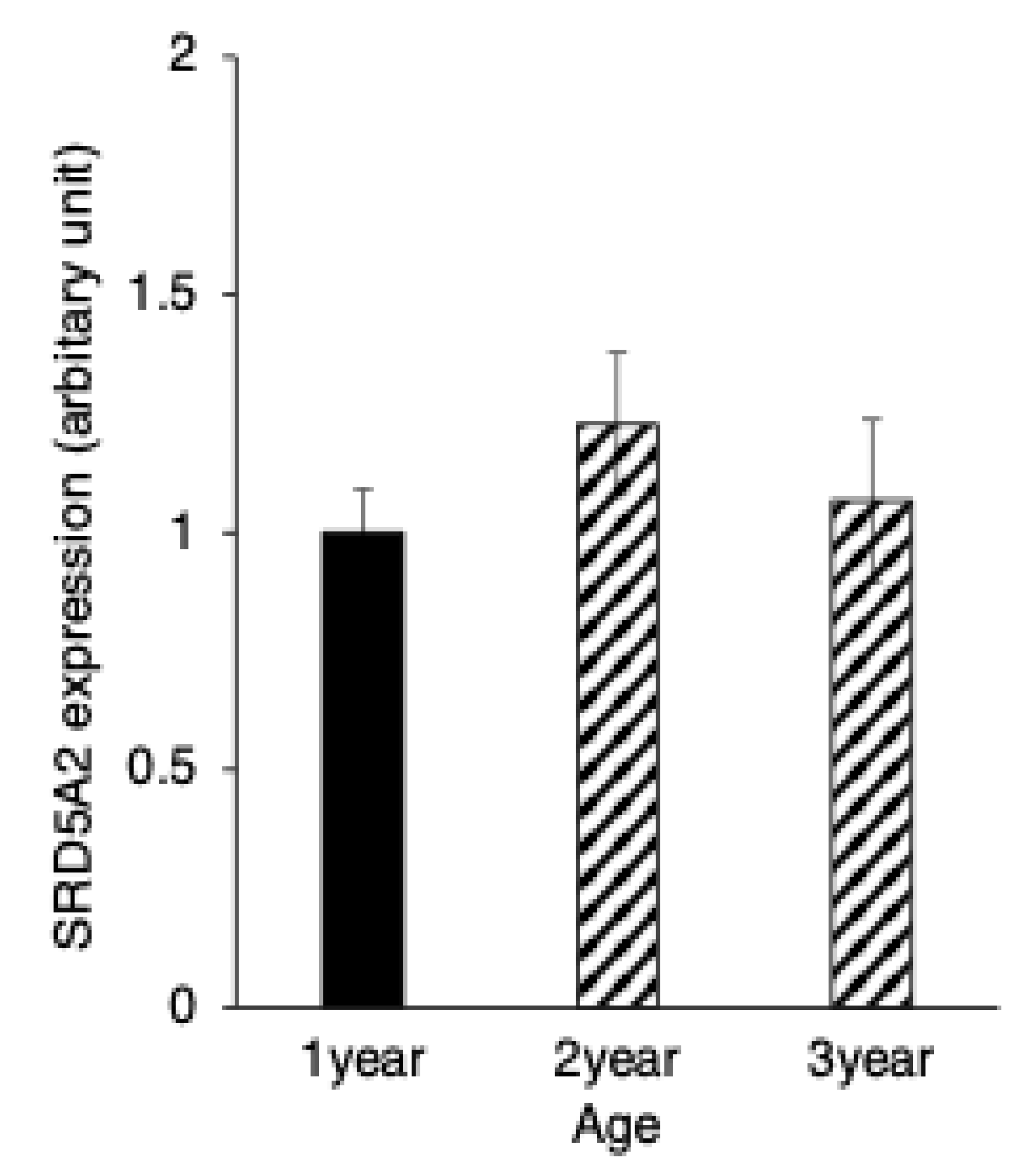
3.3. SRD5A2 Expression in Epididymal Epithelial Cells During Yak Development
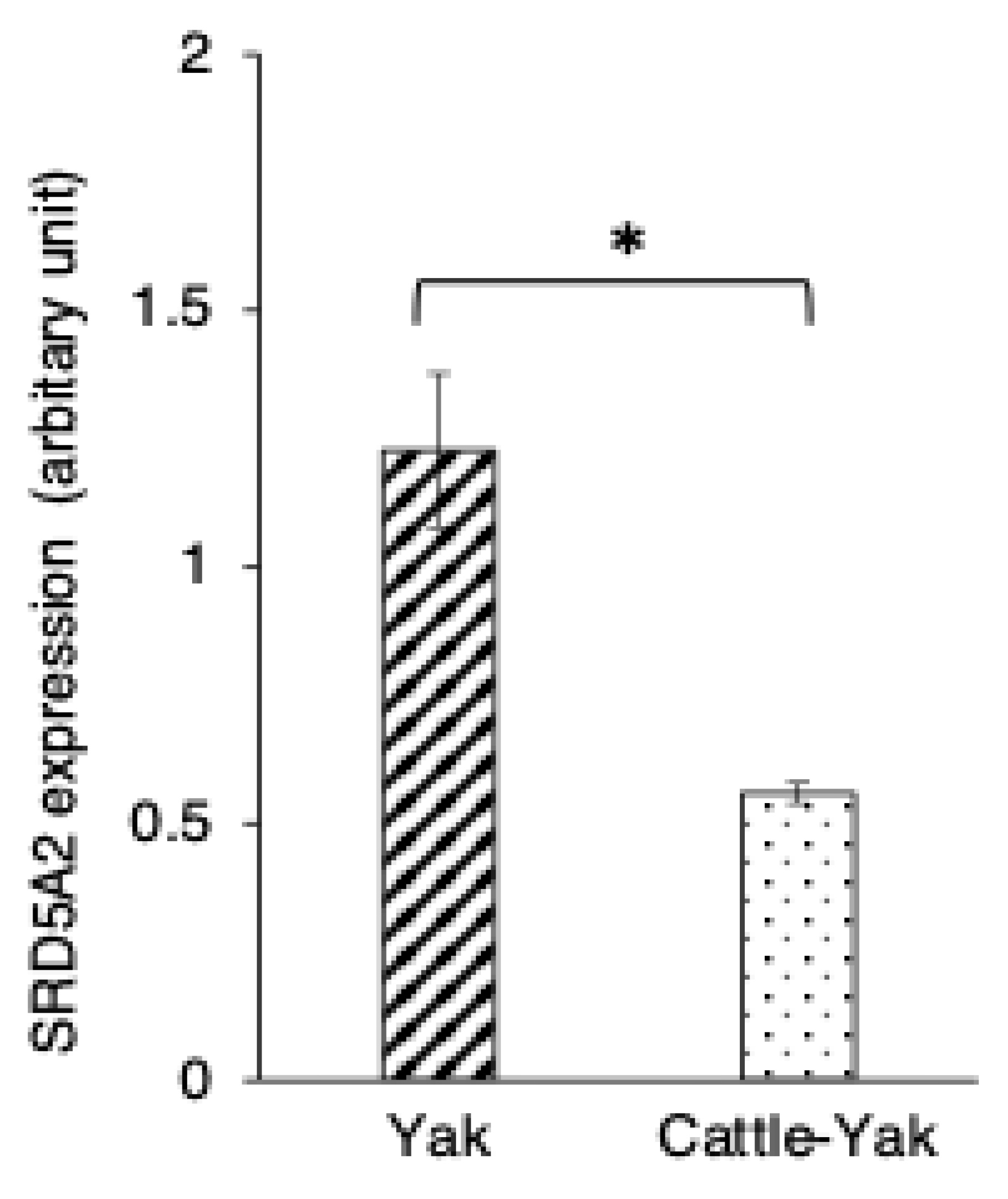
3.4. SRD5A2 Expression in Epididymal Epithelial Cells of Mature Yak and Cattle–Yak
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AR | androgen receptor |
| SRD5A1 | 5α-reductase isoform 1 |
| SRD5A2 | 5α-reductase isoform 2 |
| F1 | first generation |
| F2 | second generation |
| miRNAs | microRNAs |
| DHT | 5α-dihydroxytestosterone |
| H2O2 | hydrogen peroxide |
| PBS | phosphate-buffered saline |
| IgG | immunoglobulin G |
| BSA | bovine serum albumin |
| AEC | 3-amino-9-ethylcarbazol |
| ANOVA | analysis of variance |
References
- Wang, C.; Hussain Solangi, T.; Wang, H.; Yang, L.; Adjei, M.; Ahmed, S.; Shahzad, K.; Zhao, W.; Lang, X. High-throughput sequencing reveals differential expression of miRNAs in yak and cattleyak epididymis. Reprod. Domest. Anim. 2022, 57, 125–140. [Google Scholar] [CrossRef]
- Phakdeedindan, P.; Wittayarat, M.; Tharasanit, T.; Techakumphu, M.; Shimazaki, M.; Sambuu, R.; Hirata, M.; Tanihara, F.; Taniguchi, M.; Otoi, T. Aberrant levels of DNA methylation and H3K9 acetylation in the testicular cells of crossbred cattle–yak showing infertility. Reprod. Domest. Anim. 2022, 57, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Lang, X.; Adjei, M.; Wang, C.; Chen, X.; Li, C.; Wang, P.; Pan, M.; Li, K.; Shahzad, K.; Zhao, W. RNA-Seq reveals the functional specificity of epididymal caput, corpus, and cauda genes of cattleyak. Anim. Sci. J. 2022, 93, e13732. [Google Scholar] [CrossRef] [PubMed]
- Shimazaki, M.; Wittayarat, M.; Sambuu, R.; Sugita, A.; Kawaguchi, M.; Hirata, M.; Tanihara, F.; Takagi, M.; Taniguchi, M.; Otoi, T. Disruption of cell proliferation and apoptosis balance in the testes of crossbred cattle-yaks affects spermatogenic cell fate and sterility. Reprod. Domest. Anim. 2022, 57, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.L.; Song, H.F.; Guan, J.Q. Investigation on mechanism of sterility of male hybrids between yak and cattle. J. Appl. Anim. Res. 2014, 42, 395–399. [Google Scholar] [CrossRef]
- de Souza, A.P.B.; Schorr-Lenz, Â.M.; Lucca, F.; Bustamante-Filho, I.C. The epididymis and its role on sperm quality and male fertility. Anim. Reprod. (AR) 2018, 14, 1234–1244. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Q.; Zhang, W.; Li, J.; Li, Z.; Tang, Z.; Li, Y.; Han, C.; Hall, S.H.; Zhang, Y. Comparative profiling of genes and miRNAs expressed in the newborn, young adult, and aged human epididymides. Acta Biochim. Biophys. Sin. 2010, 42, 145–153. [Google Scholar] [CrossRef]
- Chu, C.; Zheng, G.; Hu, S.; Zhang, J.; Xie, S.; Ma, W.; Ni, M.; Tang, C.; Zhou, L.; Zhou, Y. Epididymal region-specific miRNA expression and DNA methylation and their roles in controlling gene expression in rats. PLoS ONE 2015, 10, e0124450. [Google Scholar] [CrossRef]
- Cornwall, G.A. New insights into epididymal biology and function. Hum. Reprod. Update 2009, 15, 213–227. [Google Scholar] [CrossRef]
- Zhao, W.; Mengal, K.; Yuan, M.; Quansah, E.; Li, P.; Wu, S.; Xu, C.; Yi, C.; Cai, X. Comparative RNA-Seq analysis of differentially expressed genes in the epididymides of Yak and cattleyak. Curr. Genom. 2019, 20, 293–305. [Google Scholar] [CrossRef]
- Ghadessy, F.J.; Lim, J.; Abdullah, A.A.; Panet-Raymond, V.; Choo, C.K.; Lumbroso, R.; Tut, T.G.; Gottlieb, B.; Pinsky, L.; Trifiro, M.A. Oligospermic infertility associated with an androgen receptor mutation that disrupts interdomain and coactivator (TIF2) interactions. J. Clin. Investig. 1999, 103, 1517–1525. [Google Scholar] [CrossRef] [PubMed]
- Yong, E.; Loy, C.; Sim, K. Androgen receptor gene and male infertility. Hum. Reprod. Update 2003, 9, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.J.; Hardy, M.P.; Inigo, I.V.; Huhtaniemi, I.; Bardin, C.W.; Moo-Young, A.J. Effects of androgen on androgen receptor expression in rat testicular and epididymal cells: A quantitative immunohistochemical study. Biol. Reprod. 2000, 63, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Kuriwaki, R.; Hagino, S.; Shimazaki, M.; Sambuu, R.; Hirata, M.; Tanihara, F.; Takagi, M.; Taniguchi, M.; Otoi, T. Abnormal functions of Leydig cells in crossbred cattle–yak showing infertility. Reprod. Domest. Anim. 2020, 55, 209–216. [Google Scholar] [CrossRef]
- Chengalvala, M.; Oh, T.; Roy, A.K. Selective androgen receptor modulators. Expert Opin. Ther. Pat. 2003, 13, 59–66. [Google Scholar] [CrossRef]
- Thigpen, A.E.; Silver, R.I.; Guileyardo, J.M.; Casey, M.L.; McConnell, J.; Russell, D.W. Tissue distribution and ontogeny of steroid 5 alpha-reductase isozyme expression. J. Clin. Investig. 1993, 92, 903–910. [Google Scholar] [CrossRef]
- Luo, X.; Zhang, Z.; Wei, Y.; Zhou, J.; Wu, K.; Zhao, X. Investigation on mechanism of sterility of male hybrids between yak and cattle. In Proceedings of the International Congress on Yak, Chengdu, China, 20–26 September 2004. [Google Scholar]
- Hou, Y.; Yuan, P.; Fu, Y.; Zhang, Q.; Wei, Y.; Gao, L.; Liu, L.; Wang, X.; Zheng, X.; Feng, W. Duzhong butiansu prescription improves heat stress-induced spermatogenic dysfunction by regulating sperm formation and heat stress pathway. Evid. -Based Complement. Altern. Med. 2020, 2020, 6723204. [Google Scholar] [CrossRef]
- Walters, K.; Simanainen, U.; Handelsman, D. Molecular insights into androgen actions in male and female reproductive function from androgen receptor knockout models. Hum. Reprod. Update 2010, 16, 543–558. [Google Scholar] [CrossRef]
- Aquila, S.; Montanaro, D.; Guido, C.; Santoro, M.; Perrotta, I.; Gervasi, S.; De Amicis, F.; Lanzino, M. Human sperm molecular anatomy: The enzyme 5α-reductase (SRD5A) is present in the sperm and may be involved in the varicocele-related infertility. Histochem. Cell Biol. 2015, 144, 67–76. [Google Scholar] [CrossRef]
- Zi, X.; Zhong, G.; Wen, Y.; Zhong, J.; Liu, C.; Ni, Y.; Yezi, Y.; Ashi, M. Growth performance, carcass composition and meat quality of Jiulong-yak (Bos grunniens). Asian-Australas. J. Anim. Sci. 2004, 17, 410–414. [Google Scholar] [CrossRef]
- Perobelli, J.E.; Patrão, M.T.; Fernandez, C.D.; Sanabria, M.; Klinefelter, G.R.; Avellar, M.C.W.; Kempinas, W.D. Androgen deprivation from pre-puberty to peripuberty interferes in proteins expression in pubertal and adult rat epididymis. Reprod. Toxicol. 2013, 38, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Sebo, Z.L.; Rodeheffer, M.S. Prepubertal androgen signaling is required to establish male fat distribution. Stem Cell Rep. 2022, 17, 1081–1088. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.B.; McEwan, I.J. Androgen Signaling in Other Body Systems. In Testosterone: From Basic Research to Clinical Applications; Springer: Berlin/Heidelberg, Germany, 2013; pp. 37–57. [Google Scholar]
- Zaya, R.; Hennick, C.; Pearl, C.A. In vitro expression of androgen and estrogen receptors in prepubertal and adult rat epididymis. Gen. Comp. Endocrinol. 2012, 178, 573–586. [Google Scholar] [CrossRef]
- Hazra, R.; Upton, D.; Desai, R.; Noori, O.; Jimenez, M.; Handelsman, D.J.; Allan, C.M. Elevated expression of the Sertoli cell androgen receptor disrupts male fertility. Am. J. Physiol. -Endocrinol. Metab. 2016, 311, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.-W.; Chu, D.; Yan, S.-N.; Yin, Y.-F.; Bian, Q.; Weng, B.; Chen, B.; Ran, M.-L. MiR-191 promotes the porcine immature Sertoli cell proliferation by targeting the BDNF gene through activating the PI3K/AKT signaling pathway. Yi Chuan= Hered. 2021, 43, 680–693. [Google Scholar]
- Saartok, T.; Dahlberg, E.; GUSTAFSSON, J.-Å. Relative binding affinity of anabolic-androgenic steroids: Comparison of the binding to the androgen receptors in skeletal muscle and in prostate, as well as to sex hormone-binding globulin. Endocrinology 1984, 114, 2100–2106. [Google Scholar] [CrossRef]
- Amory, J.K.; Wang, C.; Swerdloff, R.S.; Anawalt, B.D.; Matsumoto, A.M.; Bremner, W.J.; Walker, S.E.; Haberer, L.J.; Clark, R.V. The effect of 5α-reductase inhibition with dutasteride and finasteride on semen parameters and serum hormones in healthy men. J. Clin. Endocrinol. Metab. 2007, 92, 1659–1665. [Google Scholar] [CrossRef]
- Ueda, Y.; Suzuki, K.; Kajimoto, M.; Fujimoto, K.; Mahendroo, M.; Ema, M.; Yamada, G.; Hara, I. Possible testosterone redundancy for 5α-dihydrotestosterone in the masculinization of mouse external genitalia. Exp. Anim. 2022, 71, 451–459. [Google Scholar] [CrossRef]
- Yang, T.; Yang, Y.; Song, X.; Liu, L.; Yang, Y.; Xing, X.; Yang, F.; Peng, Y. Comparative studies on testis, epididymis and serum hormone concentrations in foxes, and hybrids during the pre-breeding period. Anim. Reprod. Sci. 2019, 203, 61–67. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wittayarat, M.; Kawanishi, K.; Ohata, H.; Nagahara, M.; Sambuu, R.; Sambuu, O.; Hirata, M.; Tanihara, F.; Taniguchi, M.; Otoi, T.; et al. Aberrant Expression Levels of Androgen Receptor and SRD5A2 in Epididymal Epithelial Cells of Crossbred Infertile Cattle–Yak. Animals 2025, 15, 660. https://doi.org/10.3390/ani15050660
Wittayarat M, Kawanishi K, Ohata H, Nagahara M, Sambuu R, Sambuu O, Hirata M, Tanihara F, Taniguchi M, Otoi T, et al. Aberrant Expression Levels of Androgen Receptor and SRD5A2 in Epididymal Epithelial Cells of Crossbred Infertile Cattle–Yak. Animals. 2025; 15(5):660. https://doi.org/10.3390/ani15050660
Chicago/Turabian StyleWittayarat, Manita, Kimika Kawanishi, Haruka Ohata, Megumi Nagahara, Rentsenkhand Sambuu, Otgonjargal Sambuu, Maki Hirata, Fuminori Tanihara, Masayasu Taniguchi, Takeshige Otoi, and et al. 2025. "Aberrant Expression Levels of Androgen Receptor and SRD5A2 in Epididymal Epithelial Cells of Crossbred Infertile Cattle–Yak" Animals 15, no. 5: 660. https://doi.org/10.3390/ani15050660
APA StyleWittayarat, M., Kawanishi, K., Ohata, H., Nagahara, M., Sambuu, R., Sambuu, O., Hirata, M., Tanihara, F., Taniguchi, M., Otoi, T., & Sato, Y. (2025). Aberrant Expression Levels of Androgen Receptor and SRD5A2 in Epididymal Epithelial Cells of Crossbred Infertile Cattle–Yak. Animals, 15(5), 660. https://doi.org/10.3390/ani15050660




